Abstract
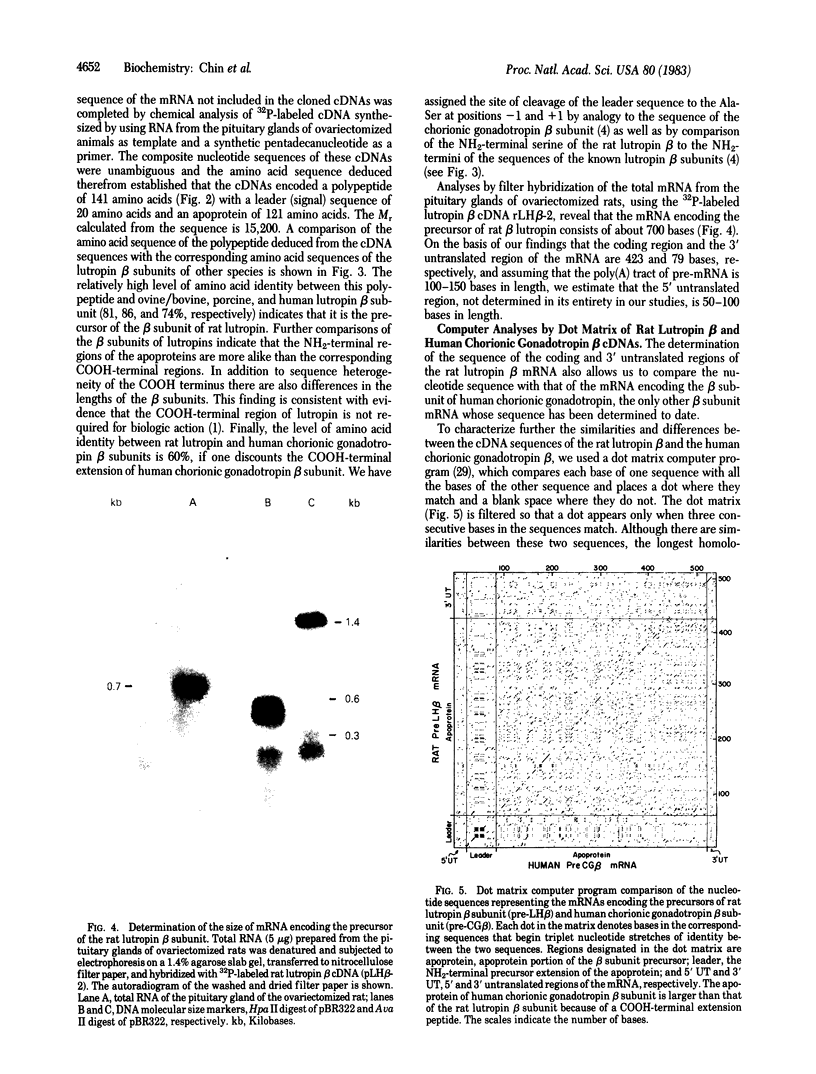
We have determined the nucleotide sequences of cDNAs encoding the precursor of the beta subunit of rat lutropin, a polypeptide hormone that regulates gonadal function, including the development of gametes and the production of steroid sex hormones. The cDNAs were prepared from poly(A)+ RNA derived from the pituitary glands of rats 4 weeks after ovariectomy and were cloned in bacterial plasmids. Bacterial colonies containing transfected plasmids were screened by hybridization with a 32P-labeled cDNA encoding the beta subunit of human chorionic gonadotropin, a protein that is related in structure to lutropin. Several recombinant plasmids were detected that by nucleotide sequence analyses contained coding sequences for the precursor of the beta subunit of lutropin. Complete determination of the nucleotide sequences of these cDNAs, as well as of cDNA reverse-transcribed from pituitary poly(A)+ RNA by using a synthetic pentadecanucleotide as a primer of RNA, provided the entire 141-codon sequence of the precursor of the beta subunit of rat lutropin. The precursor consists of a 20 amino acid leader (signal) peptide and an apoprotein of 121 amino acids. The amino acid sequence of the rat lutropin beta subunit shows similarity to the beta subunits of the ovine/bovine, porcine, and human lutropins (81, 86, and 74% of amino acids identical, respectively). Blot hybridization of pituitary RNAs separated by electrophoresis on agarose gels showed that the mRNA encoding the lutropin beta subunit consists of approximately 700 bases. The availability of cDNAs for both the alpha and beta subunits of lutropin will facilitate studies of the regulation of lutropin expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chan S. J., Noyes B. E., Agarwal K. L., Steiner D. F. Construction and selection of recombinant plasmids containing full-length complementary DNAs corresponding to rat insulins I and II. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5036–5040. doi: 10.1073/pnas.76.10.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin W. W., Habener J. F., Kieffer J. D., Maloof F. Cell-free translation of the messenger RNA coding for the alpha subunit of thyroid-stimulating hormone. J Biol Chem. 1978 Nov 25;253(22):7985–7988. [PubMed] [Google Scholar]
- Chin W. W., Kronenberg H. M., Dee P. C., Maloof F., Habener J. F. Nucleotide sequence of the mRNA encoding the pre-alpha-subunit of mouse thyrotropin. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5329–5333. doi: 10.1073/pnas.78.9.5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin W. W., Maloof F., Habener J. F. Thyroid-stimulating hormone biosynthesis. Cellular processing, assembly, and release of subunits. J Biol Chem. 1981 Mar 25;256(6):3059–3066. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cooke N. E., Coit D., Shine J., Baxter J. D., Martial J. A. Human prolactin. cDNA structural analysis and evolutionary comparisons. J Biol Chem. 1981 Apr 25;256(8):4007–4016. [PubMed] [Google Scholar]
- Counis R., Ribot G., Corbani M., Poissonnier M., Jutisz M. Cell-free translation of the rat pituitary messenger RNA coding for the precursors of alpha and beta subunits of lutropin. FEBS Lett. 1981 Jan 26;123(2):151–155. doi: 10.1016/0014-5793(81)80275-x. [DOI] [PubMed] [Google Scholar]
- Daniels-McQueen S., McWillians D., Birken S., Canfield R., Landefeld T., Boime I. Identification of mRNAs encoding the alpha and beta subunits of human choriogonadotropin. J Biol Chem. 1978 Oct 10;253(19):7109–7114. [PubMed] [Google Scholar]
- Fiddes J. C., Goodman H. M. The cDNA for the beta-subunit of human chorionic gonadotropin suggests evolution of a gene by readthrough into the 3'-untranslated region. Nature. 1980 Aug 14;286(5774):684–687. doi: 10.1038/286684a0. [DOI] [PubMed] [Google Scholar]
- Fontaine Y. A., Burzawa-Gerard E. Esquisse de l'evolution des hormones gonadotropes et thyreotropes des vertébrés. Gen Comp Endocrinol. 1977 Jul;32(3):341–347. doi: 10.1016/0016-6480(77)90213-1. [DOI] [PubMed] [Google Scholar]
- Glenn S. D., Nahm H. S., Greenwald G. S., Ward D. N. Isolation and characterization of hamster luteinizing hormone. Endocrinology. 1982 Oct;111(4):1263–1269. doi: 10.1210/endo-111-4-1263. [DOI] [PubMed] [Google Scholar]
- Godine J. E., Chin W. W., Habener J. F. Cell-free synthesis and processing of the precursors to the subunits of luteinizing hormone. J Biol Chem. 1981 Mar 10;256(5):2475–2479. [PubMed] [Google Scholar]
- Godine J. E., Chin W. W., Habener J. F. Luteinizing and follicle-stimulating hormones. Cell-free translations of messenger RNAs coding for subunit precursors. J Biol Chem. 1980 Sep 25;255(18):8780–8783. [PubMed] [Google Scholar]
- Godine J. E., Chin W. W., Habener J. F. alpha Subunit of rat pituitary glycoprotein hormones. Primary structure of the precursor determined from the nucleotide sequence of cloned cDNAs. J Biol Chem. 1982 Jul 25;257(14):8368–8371. [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Ike Y., Ikuta S., Itakura K. Solid phase synthesis of polynucleotides. VI. Further studies on polystyrene copolymers for the solid support. Nucleic Acids Res. 1982 Mar 11;10(5):1755–1769. doi: 10.1093/nar/10.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller D., Fetherston J., Boime I. Isolation of mRNA from bovine pituitary. The cell-free synthesis of the alpha and beta subunits of luteinizing hormone. Eur J Biochem. 1980 Jul;108(2):367–372. doi: 10.1111/j.1432-1033.1980.tb04731.x. [DOI] [PubMed] [Google Scholar]
- Keutmann H. T., Williams R. M., Ryan R. J. Structure of human luteinizing hormone beta subunit: evidence for a related carboxyl-terminal sequence among certain peptide hormones. Biochem Biophys Res Commun. 1979 Oct 12;90(3):842–848. doi: 10.1016/0006-291x(79)91904-1. [DOI] [PubMed] [Google Scholar]
- Kourides I. A., Weintraub B. D. mRNA-directed biosynthesis of alpha subunit of thyrotropin: translation in cell-free and whole-cell systems. Proc Natl Acad Sci U S A. 1979 Jan;76(1):298–302. doi: 10.1073/pnas.76.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landefeld T. D. Identification of in vitro synthesized pituitary glycoprotein alpha subunit. Translation of a possible precursor. J Biol Chem. 1979 May 25;254(10):3685–3688. [PubMed] [Google Scholar]
- Landefeld T. D., Kepa J. The cell free synthesis of bovine lutropin beta subunit. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1111–1118. doi: 10.1016/0006-291x(79)91150-1. [DOI] [PubMed] [Google Scholar]
- Landefeld T., Boguslawski S., Corash L., Boime I. The cell-free synthesis of the alpha subunit of human chorionic gonadotropin. Endocrinology. 1976 May;98(5):1220–1227. doi: 10.1210/endo-98-5-1220. [DOI] [PubMed] [Google Scholar]
- Liu W. K., Nahm H. S., Sweeney C. M., Holcomb G. N., Ward D. N. The primary structure of ovine luteinizing hormone. II. The amino acid sequence of the reduced, S-carboxymethylated A-subunit (LH- ). J Biol Chem. 1972 Jul 10;247(13):4365–4381. [PubMed] [Google Scholar]
- Maghuin-Rogister G., Combarnous Y., Hennen G. The primary structure of the porcine luteinizing-hormone alpha-subunit. Eur J Biochem. 1973 Nov 1;39(1):255–263. doi: 10.1111/j.1432-1033.1973.tb03122.x. [DOI] [PubMed] [Google Scholar]
- Maghuin-Rogister G., Hennen G. Luteinizing hormone. The primary structures of the beta-subunit from bovine and porcine species. Eur J Biochem. 1973 Nov 1;39(1):235–253. doi: 10.1111/j.1432-1033.1973.tb03121.x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny J. Matrix program to analyze primary structure homology. Nucleic Acids Res. 1982 Jan 11;10(1):127–131. doi: 10.1093/nar/10.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. G., Liao T., Howard S. M., Shome B., Cornell J. S. Studies on the structure of thyrotropin: its relationship to luteinizing hormone. Recent Prog Horm Res. 1971;27:165–212. doi: 10.1016/b978-0-12-571127-2.50029-8. [DOI] [PubMed] [Google Scholar]
- Pierce J. G., Parsons T. F. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ruddon R. W., Hanson C. A., Bryan A. H., Putterman G. J., White E. L., Perini F., Meade K. S., Aldenderfer P. H. Synthesis and secretion of human chorionic gonadotropin subunits by cultured human malignant cells. J Biol Chem. 1980 Feb 10;255(3):1000–1007. [PubMed] [Google Scholar]
- Sairam M. R., Samy T. S., Papkoff H., Li C. H. The primary structure of ovine interstitial cell-stimulating hormone. II. The -subunit. Arch Biochem Biophys. 1972 Dec;153(2):572–586. doi: 10.1016/0003-9861(72)90375-x. [DOI] [PubMed] [Google Scholar]
- Shome B., Parlow A. F. The primary structure of the hormone-specific, beta subunit of human pituitary luteinizing hormone (hLH). J Clin Endocrinol Metab. 1973 Mar;36(3):618–621. doi: 10.1210/jcem-36-3-618. [DOI] [PubMed] [Google Scholar]
- Stewart M., Stewart F. Constant and variable regions in glycoprotein hormone beta subunit sequences: implications for receptor binding specificity. J Mol Biol. 1977 Oct 15;116(1):175–179. doi: 10.1016/0022-2836(77)90126-7. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitukaitis J. L., Ross G. T., Braunstein G. D., Rayford P. L. Gonadotropins and their subunits: basic and clinical studies. Recent Prog Horm Res. 1976;32:289–331. doi: 10.1016/b978-0-12-571132-6.50019-1. [DOI] [PubMed] [Google Scholar]
- Vamvakopoulos N. C., Kourides I. A. Identification of separate mRNAs coding for the alpha and beta subunits of thyrotropin. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3809–3813. doi: 10.1073/pnas.76.8.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward D. N., Reichert L. E., Jr, Liu W. K., Nahm H. S., Hsia J., Lamkin W. M., Jones N. Chemical studies of luteinizing hormone from human and ovine pituitaries. Recent Prog Horm Res. 1973;29:533–561. doi: 10.1016/b978-0-12-571129-6.50018-x. [DOI] [PubMed] [Google Scholar]
- Weintraub B. D., Stannard B. S., Linnekin D., Marshall M. Relationship of glycosylation to de novo thyroid-stimulating hormone biosynthesis and secretion by mouse pituitary tumor cells. J Biol Chem. 1980 Jun 25;255(12):5715–5723. [PubMed] [Google Scholar]