Summary
We have established and efficient system to specify NG2/PDGF-Rα/OLIG2+ oligodendrocyte precursor cells (OPCs) from human embryonic stem cells (hESCs) at low, physiological (3%) oxygen levels. This was achieved via both forebrain and spinal cord origins, with up to 98% of cells expressing NG2. Developmental insights reveal a critical role for fibroblast growth factor 2 (FGF-2) in OLIG2 induction via ventral forebrain pathways. The OPCs mature in vitro to express O4 (46%) and subsequently become galactocerebroside (GALC), O1, and myelin basic protein-positive (MBP+) multibranching oligodendrocytes. These were cultured alongside hESC-derived neurons. The electrophysiological properties of human OPCs are similar to those of rat OPCs, with large voltage-gated sodium currents and the ability to fire action potentials. Exposure to a selective retinoid X receptor agonist increased the proportion of O4+ oligodendrocytes that express MBP from 5% to 30%. Thus, we have established a developmentally engineered system to investigate the biological properties of human OPCs and test the effects of putative remyelinating agents prior to clinical application.
Highlights
-
•
Human OPCs and oligodendrocytes can be generated at physiological (3%) O2 tensions
-
•
hESC-derived OPCs can be specified via both spinal cord and ventral forebrain origins
-
•
Human OPCs have large voltage-gated sodium currents and can fire action potentials
-
•
RXR signaling is a relevant target for remyelinating therapies in humans
Stacpoole and colleagues established a system to specify human oligodendrocyte precursor cells (OPCs) and multibranching oligodendrocytes from embryonic stem cells at physiological (3%) oxygen, from both forebrain and spinal cord. These human OPCs can fire action potentials and respond to retinoid X receptor agonists, demonstrating the usefulness of this developmentally engineered system as a translational resource.
Introduction
The ability to generate human oligodendrocyte precursor cells (OPCs) and oligodendrocytes in vitro, and thereby study the signals that promote OPC differentiation, maturation, and myelination, could provide new insights into human demyelinating diseases such as multiple sclerosis (MS), as well as other neurological disorders in which oligodendrocyte lineage cells play a key role, including periventricular multifocal leukoencephalopathy, multiple system atrophy, and malignant gliomas (Liu et al., 2011; Papp and Lantos, 1994; Mázló and Tariska, 1980). Human embryonic stem cells (hESCs), by virtue of their dual characteristics of self-renewal and pluripotency, have the greatest potential to provide the large numbers of these cells that are required for such studies. However, techniques that were developed in mouse ESC-based systems (Billon et al., 2002; Brüstle et al., 1999; Glaser et al., 2005) have not readily translated to human cells in culture. Few studies have reported successful specification of human OPCs from hESCs (Nistor et al., 2005; Kang et al., 2007; Izrael et al., 2007; Hu et al., 2009; Sundberg et al., 2010; Wang et al., 2013), and still fewer have convincingly shown in vitro generation of mature human oligodendrocytes (and then only in small numbers; Izrael et al., 2007; Hu et al., 2009; Wang et al., 2013).
The difficulty of applying methods developed in mouse ESCs to hESCs likely reflects a critical difference in the default identity of NPCs generated from the two different species. Sonic hedgehog (Shh) signaling predominates in the mouse system, whereas WNT signaling predominates in human cells, resulting in NPCs with a default ventral (mouse) versus dorsal (human) phenotype (Gaspard et al., 2008; Li et al., 2009). Since the earliest OPCs are derived from ventral origins under the control of Shh (Kessaris et al., 2006; Lu et al., 2000), this indicates a requirement for ventralizing morphogens in human systems (Hu et al., 2009).
A further technical challenge has been the inability to maintain human OPCs in culture long enough for more than a minority of the cells to mature into multibranching oligodendrocytes (Hu et al., 2009; Wang et al., 2013). This may be due to the particular sensitivity of the oligodendrocyte lineage to oxidative stress (Casaccia-Bonnefil, 2000), as well as the universal use of a 20% oxygen (O2) environment in previous hESC-based studies. Oxygen levels in the brain are far removed from the 20% environment typically used for in vitro studies, with an average level of 3% (ranging from 2.5% to 5.3% in gray matter and 0.8% to 2.1% in white matter of the cortex; Erecińska and Silver, 2001). We previously demonstrated the beneficial effects of low, physiological oxygen (3%) on the survival and long-term culture of hESC-derived NPCs, and the directed differentiation of these cells into dopaminergic and motor neurones, using chemically defined, serum-free conditions (Stacpoole et al., 2011a). Notably, we found that OLIG2 induction was 2-fold greater at 3% O2 than at 20% O2. Additionally, evidence from studies of human, mouse, and rat cortical NPCs shows that culture at 2%–5% O2 significantly increases the number of O4+ oligodendrocytes generated (Pistollato et al., 2007; Chen et al., 2007; Stacpoole et al., 2013). Furthermore, maturation into myelin basic protein-positive (MBP+) oligodendrocytes is enhanced by culture at low, physiological O2 (Akundi and Rivkees, 2009; Stacpoole et al., 2013). Taken together, these observations provide a strong rationale for investigating hESC-derived NPC specification into the oligodendrocyte lineage at low, physiological oxygen levels.
Previous hESC-based studies have aimed to generate human OPCs for transplantation purposes. Although one study used an in vitro system to investigate the developmental pathways involved in OPC specification via the pMN domain of the spinal cord (Hu et al., 2009), there are no comparable reports of generating OPCs from a forebrain origin; of OPC specification at low, physiological O2 tensions; or of using these human OPCs to advance an understanding of their biological characteristics or as a translational resource.
We therefore set out to establish a reliable system for generating OPCs and oligodendrocytes from both forebrain and spinal cord origins using our previously established hESC-neuralizing system at 3% O2 (Stacpoole et al., 2011a). We find a distinct requirement for fibroblast growth factor 2 (FGF-2) in OLIG2 induction via the ventral forebrain route, in contrast to the ventral spinal cord, and report that the small-molecule agonist of SHH signaling (SAG) is an effective alternative to purmorphamine (PM) in this system. We show that human OPCs can mature into multibranching oligodendrocytes that form close associations with hESC-derived neurons. These human OPCs have large voltage-gated sodium currents, can fire action potentials, and respond to retinoid X receptor (RXR) signaling by significantly upregulating MBP in this physiologically relevant in vitro system.
Results
Two Distinct Routes to OPCs from hESC-NPCs
Previously, we demonstrated that NPCs can be derived efficiently from hESCs at 3% O2 (Stacpoole et al., 2011a). NPCs generated through this chemically defined system have a default dorsal and rostral identity, and therefore require redirection toward either ventral spinal cord or ventral forebrain fates in order to generate oligodendrocytes (Figure 1A). Directed differentiation toward the pMN domain of the spinal cord can be achieved by application of established protocols, involving retinoic acid (RA) to caudalize the NPCs and ventral differentiation using the SHH agonist PM, inducing expression of HOXB4, NKX6.1, and OLIG2 (Hu and Zhang, 2009; Stacpoole et al., 2011a). We previously reported that using this approach, OLIG2 induction at day 28 is 2-fold greater at 3% O2 than at 20% O2 (Stacpoole et al., 2011a). In the current study, the combination of RA and PM at 3% O2 achieved OLIG2 induction in 57.3% ± 2.8% of NPCs by day 24 (Figure 1B). Since OLIG2 is essential for oligodendrocyte specification throughout the entire neuraxis, except for the hindbrain (Lu et al., 2000; Zhou and Anderson, 2002; Zhou et al., 2000), OLIG2 expression is a useful early marker of successful respecification of hESC-NPCs toward OPC-generating domains of the forebrain and spinal cord.
Figure 1.
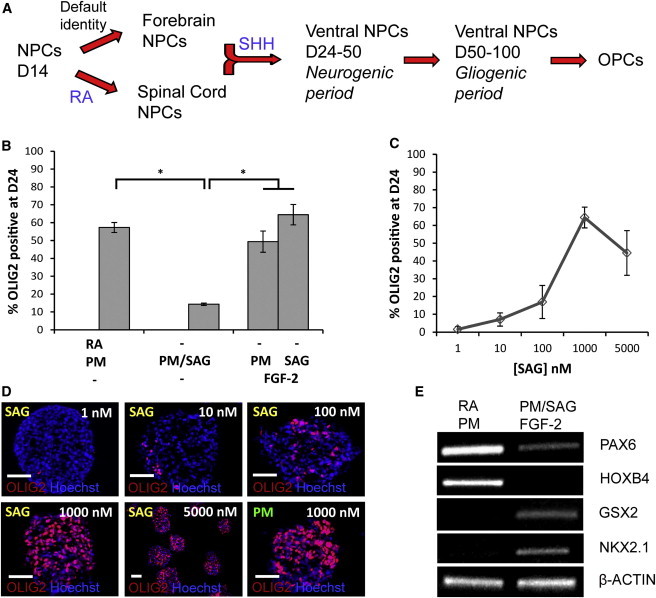
Efficient OLIG2 Induction Can Be Achieved with or without RA, Revealing a Distinct Role for FGF-2 in OLIG2 Induction from Human Forebrain Origins
(A) In order to direct differentiation of hESC-NPCs toward the OLIG2-defined ventral regions of the forebrain and spinal cord, from which the earliest OPCs arise, strategies both with and without the caudalizing morphogen RA were considered.
(B) OLIG2 expression was efficiently induced by a combination of RA and PM, with 57.3% ± 2.8% of NPCs expressing OLIG2 by D24. Removal of RA led to a significant reduction in the levels of OLIG2 expression to 14.3% ± 0.7% (p < 0.001), but the combination of FGF-2 with a SHH agonist restored OLIG2 induction to levels (58.0% ± 4.9%) similar to those obtained with the protocol including RA (p = 0.932).
(B–D) SHH is the key ventralizing morphogen, and the small-molecule hedgehog agonist SAG demonstrates a concentration-dependent ability to induce OLIG2, peaking at 1,000 nM, and demonstrating efficacy equivalent to that of PM for inducing OLIG2 expression in combination with 10 ng/ml FGF-2 (p = 0.13). OLIG2 staining is shown on 12-μm-thick neurosphere sections.
(E) RT-PCR analysis of positional identity markers showed that FGF-2 did not caudalize the NPCs because, in contrast to results from the RA-based spinal cord protocol, the caudal gene HOXB4 was not upregulated. Instead, ventral forebrain genes such as GSX2 and NKX2.1 were induced by the combination of SHH agonist and FGF-2, in contrast to the effects of RA and SHH signaling. β-actin was used as a loading control. Scale bar, 50 μm; percentages are given ± SEM; ∗p < 0.001.
See also Table S1.
Simple removal of RA from this technique, leaving SHH activation as the only exogenous signal, did not result in efficient specification toward ventral forebrain, as evidenced by low levels (14.3% ± 0.7%) of OLIG2 induction at day 24 (Figure 1B). We hypothesized that FGF-2 is an additional requirement for OLIG2 induction via the ventral forebrain route, following the recent report that it cooperates with Shh to specify OPCs from the murine ventral forebrain (Furusho et al., 2011). Application of 10 ng/ml FGF-2 in combination with a SHH agonist (1 μm) resulted in a 4.5-fold increase in OLIG2 induction in the absence of RA to 58.0% ± 4.9%, similar to levels achieved through the established RA- and PM-driven spinal cord route (p = 0.932; Figure 1B). Comparable efficiency was achieved with the HUES-9 line, with OLIG2 induction via the FGF-2/SHH agonist ventral forebrain route of 53.1% ± 4.2% (p = 0.57; Figure S1A available online). The SHH agonist used was either PM or SAG, both of which activate SHH signaling by binding directly to smoothened (unlike the endogenous ligand, which binds to patched, releasing its inhibition of smoothened and thus activating the signaling pathway; Chen et al., 2002; Sinha and Chen, 2006). SAG was used because it has a higher potency than PM, with an EC50 of 3 nM compared with 1 μM in Shh-LIGHT2 cells, and PM is difficult to use in protracted protocols because of its narrow working range and toxicity above 1.5 μM (Li et al., 2008; Hu and Zhang, 2009). We found that SAG had efficacy equivalent to that of PM at 1 μM (p = 0.13), but a wider working range up to 5 μM (Figures 1B–D).
One possible explanation for the efficacy in inducing OLIG2 expression using the combination of FGF-2 and SHH signaling is that FGF-2 caudalizes the cells. However, expression of caudal genes such as HOXB4 was not observed, in contrast to the effect of RA plus PM. Instead, the ventral forebrain markers GSX2 and NKX2.1 were upregulated alongside OLIG2, specifically in the FGF-2/SHH agonist condition (Figure 1E). Thus, both FGF-2 and SHH signaling pathways are required to direct differentiation of hESC-NPCs toward the ventral forebrain, whereas RA and SHH are sufficient to specify hESC-NPCs toward the pMN domain of the spinal cord.
OLIG2+ NPCs Generate Large Numbers of Human OPCs
hESC-NPCs that were regionally specified to OLIG2-expressing cells by day 24 (D24), either with or without RA, were expanded with 1 μM PM or SAG in addition to 10 ng/ml FGF-2 between days 24 and 50 (Figure 2A). The purpose of the FGF-2 at this stage is to prevent differentiation into neuronal precursors, based on the observation both in vitro and in vivo that at early time points NPCs give rise to neurons, whereas at later time points they become gliogenic (Bouhon et al., 2006; Hu et al., 2009; Joannides et al., 2007; Krencik et al., 2011). At D50, the FGF-2 was replaced with 10 ng/ml platelet-derived growth factor (PDGF) and 40 ng/ml T3 to promote the survival and proliferation of OPCs. Spheres were plated for terminal differentiation at D100 on pdl-laminin-coated glass coverslips in Dulbecco’s modified Eagle’s medium (DMEM) 3:1 F12, supplemented with 1% N2, 1% penicillin streptomycin fungizone (PSF), 10 ng/ml PDGF, 10 ng/ml insulin growth factor 1 (IGF-1), 10 ng/ml NT3, 40 ng/ml T3, and 1 μM cyclic AMP (cAMP). We added 10 ng/ml brain-derived neurotrophic factor (BDNF) after 7 days, because BDNF secreted by active neurons is thought to promote OPC maturation into oligodendrocytes (Du et al., 2003).
Figure 2.
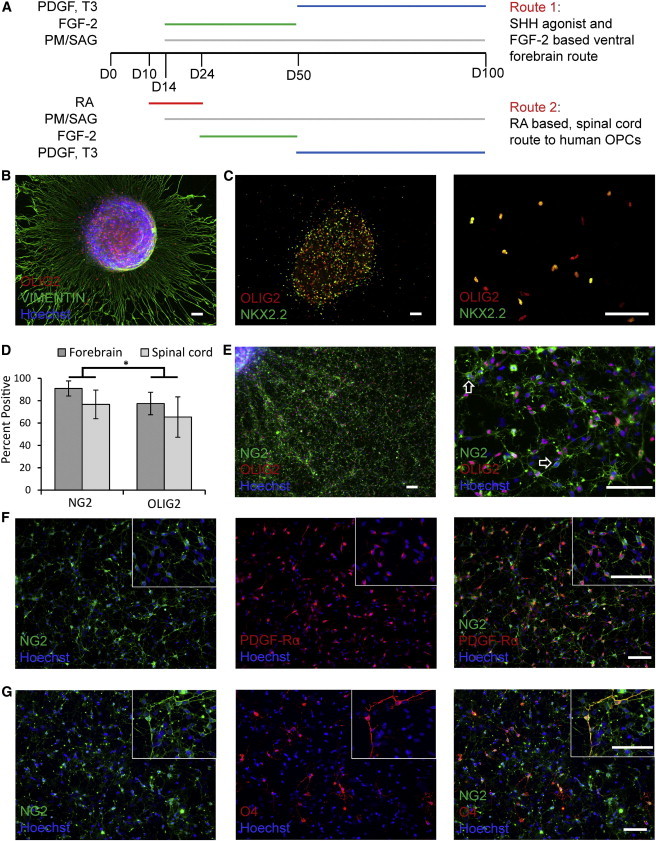
OPCs Can Be Efficiently Generated from hESC-NPCs Specified to OLIG2-Expressing Regions of the Ventral Forebrain or Ventral Spinal Cord
(A) A schematic illustrates two alternative routes to generate human OPCs from ESCs: with RA (via the pMN route) or without RA (ventral forebrain).
(B) Seven days after regionally specified D100 hESC-NPCs were plated for terminal differentiation, vimentin+ processes radiated out from the spheres. OLIG2-expressing cells appeared to migrate along these processes.
(C) Coexpression of NKX2.2 and OLIG2 also occurred at this time point.
(D and E) Three weeks after differentiation of D100 hESC-NPCs, up to 91% ± 7% of cells expressed NG2 and up to 77% ± 10% expressed OLIG2. There was no significant difference in the efficiency of specification of NG2+ precursor cells from either route, but although most cells expressed both markers, significantly more cells (p = 0.03) expressed NG2 than OLIG2 (arrows indicate NG2 single positive cells).
(F and G) Most NG2 cells also labeled with PDGF-Rα, and at this early stage a few cells also expressed the more mature oligodendrocyte lineage marker O4. Scale bar = 100 μm; percentages are given ± SEM.
See also Figures S1 and S2.
After 1 week, vimentin+ processes, suggestive of a radial glial identity, projected tangentially from differentiating spheres. On closer inspection, OLIG2+ cells could be seen, apparently migrating along these processes (Figure 2B). Coexpression of OLIG2 and NKX2.2 was also observed at this time point (Figure 2C). At D100 plus 3 weeks, the vast majority of cells (up to 98%) migrating out from the spheres labeled with NG2. A high proportion (up to 87%) of these NG2+ cells, but not all, also expressed OLIG2 (p = 0.03; Figures 2D and 2E). Both forebrain and spinalcorddirected differentiation protocols gave rise to human OPCs with comparable efficiency: the relative proportions of cells expressing NG2 were 91% ± 7% from the forebrain route and 77% ± 13% from the spinal cord route (p = 0.12), whereas for OLIG2 the relative proportions were 77% ± 10% and 65% ± 18% (p = 0.35; Figure 2D).
Further immunocytochemical characterization demonstrated that most NG2+ cells coexpressed PDGF-Rα (Figure 2F). At this relatively early stage, only a few cells expressed O4, with a typical bipolar or minimally branching morphology, overlapping in expression with NG2 (Figure 2G). There was no coexpression between O4 and β-III TUBULIN or NG2 and glial fibrillary acidic protein (GFAP) within this precursor population, indicating that these markers are selectively expressed by oligodendrocyte lineage cells rather than neurons or astrocytes (Figures S2A and S2B). NESTIN, expressed in both NPCs and immature astrocytes, generally overlapped with NG2 expression, except in cells that also expressed GFAP (Figures S2B and S2C).
Taken together, these results show that after 3 weeks of differentiation of D100 NPCs, the majority of cells generated through either route were OPCs.
Human OPCs Mature into O4-, O1-, Gal-C-, and MBP-Expressing Oligodendrocytes
After 4 weeks of terminal differentiation of D100 hESC-NPCs, large numbers of O4- and OLIG2-copositive oligodendrocyte lineage cells emerged with a bipolar or sparsely branching morphology (Figure 3A) and matured into multibranching oligodendrocytes over a further 1–2 weeks (Figure 3B). Up to 43% ± 5% of cells derived from H9 ESCs expressed O4 (Figure 3C). Comparable efficiency (46% ± 6%; p = 0.71) was achieved from the HUES-9 line (Figure S1B). Multibranching oligodendrocytes also labeled with GAL-C, O1, and MBP (Figures 3D–3F and S1C). We observed no significant difference in efficiency of oligodendrocyte generation between the two different developmental pathways (Figure 3C).
Figure 3.
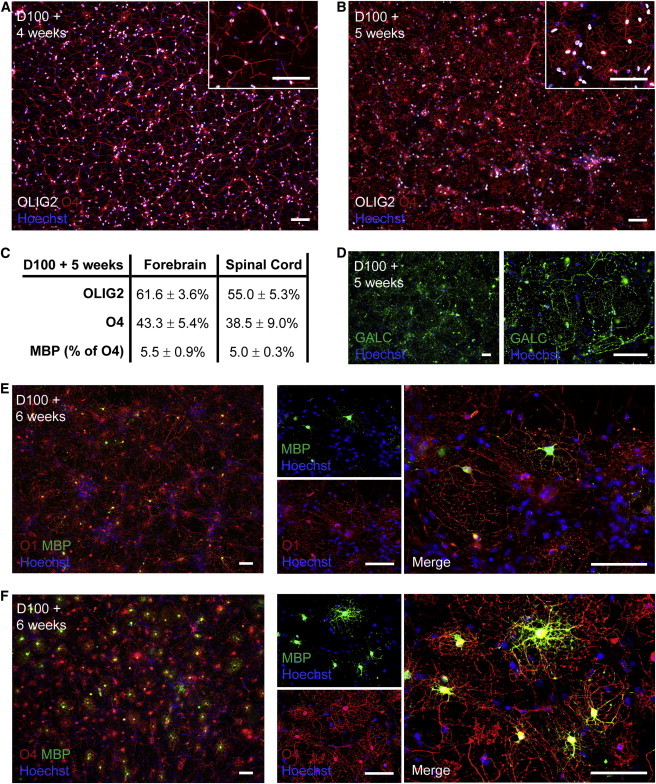
Oligodendrocytes Can Be Generated from hESC-NPCs at 3% O2
(A) After 4 weeks of terminal differentiation of D100 hESC-NPCs at 3% O2, O4+ oligodendrocytes with a bipolar or sparsely branching morphology emerged.
(B) Over a further 7–14 days, these O4+ cells took on a multibranching morphology more typical of mature oligodendrocytes.
(C–F) Multibranching oligodendrocytes also expressed GALC, O1, and MBP. Quantification from the forebrain and spinal cord pathways of the percentage of cells expressing the pan-lineage marker OLIG2 (p = 0.32), the oligodendrocyte marker O4 (p = 0.63), and the mature oligodendrocyte marker MBP (p = 0.90) after 5 weeks of terminal differentiation is shown. Scale bar = 100 μm; percentages are given ± SEM.
See also Figure S1.
Reminiscent of the behavior of rodent OPCs in vitro (Raff et al., 1983; Franklin et al., 1995), human OPCs also generated GFAP+ cells (23% ± 6%). Although the vast majority of NG2+ cells differentiated into glia, small numbers of β-III+ neurons (10% ± 3%) were also observed after 5 weeks of terminal differentiation of D100 NPCs.
Toward a Human Myelination Assay
The generation of large numbers of not only OPCs but also mature, multibranching human oligodendrocytes represents a significant advance and led us to consider the possibility of establishing myelinating cocultures of hESC-NPC derived neurons and oligodendrocytes. The ability to generate such myelinating cultures would allow investigation of the signaling molecules involved in human myelination, providing a platform to evaluate the efficacy of putative remyelinating agents.
The observation that differentiating cultures of hESC-NPCs can be maintained long-term at 3% O2 (at least 3 months), even in the absence of exogenous growth factors, highlights a useful feature of this more physiologically relevant cell culture system (Stacpoole et al., 2011a). Furthermore, the finding that early ESC-NPCs typically differentiate into neurons, whereas later ESC-NPCs become glia, recapitulating the situation in development, should help to specify these different populations from hESC-NPCs (Joannides et al., 2007; Bouhon et al., 2006; Krencik et al., 2011; Liu and Zhang, 2011). Consistent with these observations, and using the RA-based pathway, D50 hESC-NPCs differentiated for 5 weeks showed no difference in the number of OLIG2+ (p = 0.20) and O4+ (p = 0.44) oligodendrocyte lineage cells generated compared with D100 hESC-NPCs differentiated for 5 weeks (Figure 4C). However, whereas D100 hESC-NPCs generated astrocytes alongside oligodendrocyte lineage cells after 5 weeks of differentiation, oligodendrocytes from D50 hESC-NPCs developed among an extensive network of hESC-NPC-derived neurons (Figures 4A and 4B).
Figure 4.
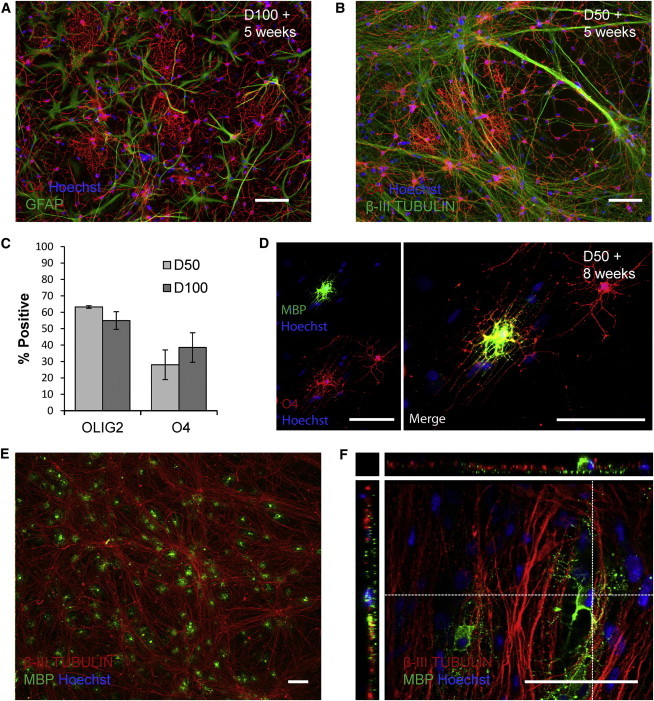
Toward In Vitro Myelination of Human Neurons
(A) After 5 weeks of terminal differentiation at D100, hESC-NPCs generated mainly O4+ oligodendrocytes, but also GFAP+ astrocytes.
(B and C) At the same time point following differentiation of D50 hESC-NPCs, there was no difference in the number of OLIG2 (p = 0.20) or O4+ (p = 0.44) cells generated, but at this earlier stage, oligodendrocyte lineage cells developed alongside hESC-NPC-derived neurons.
(D and E) After 8 weeks of terminal differentiation of D50 NPCs, MBP-expressing oligodendrocytes emerged among these neural networks.
(E and F) The pattern of MBP expression was suggestive of appropriate engagement with hESC-NPC-derived neurons. Scale bar = 100 μm in (A)–(E) and 50 μm in (F); percentages are given ± SEM.
MBP+ oligodendrocytes emerged after 6–8 weeks of terminal differentiation of D50 hESC-NPCs, typically with well-aligned processes, appropriately oriented along the axons (Figures 4D–4F). Confocal imaging showed a close approximation of MBP+ processes with β-III TUBULIN-labeled neurons, revealing engagement of axons by oligodendrocytes (Figure 4F). Beyond 10 weeks, fewer O4+ cells remained, suggesting a time window between 5 and 10 weeks in which further manipulation of the culture environment might allow myelinating human cocultures to be established.
Spiking and Nonspiking Human OPCs
The ability to reliably generate large numbers of human oligodendrocyte lineage cells from hESCs provides a valuable resource for addressing important biological and translational questions.
Studies in rats showed that some OPCs fire action potentials, whereas others do not (Káradóttir et al., 2008); however, this is a controversial finding that has not been replicated in mice (Ziskin et al., 2007). We therefore investigated the electrophysiological properties of human OPCs. We performed whole-cell patch-clamp recordings on human OPCs that were initially selected on the basis of morphology, with postfixation staining for O4 and either PDGF-Rα or NG2 used to confirm the OPC identity of lucifer yellow (LY)-filled cells (Figure 5A). Although 100% of the cells that were located following immunolabeling were positive for either or both of the OPC markers applied, the overall low yield of recovery of LY-filled cells (19%) led us to develop an O4 prestaining protocol (used in conjunction with morphology-based selection) to obtain real-time confirmation that the cells selected for electrophysiological recordings were of the oligodendrocyte lineage (Figure 5B, top row). O4 was chosen because it is the first definitive marker of the oligodendrocyte lineage expressed on the cell surface (labeling cells ranging from late-stage OPCs to mature, multibranching MBP+ oligodendrocytes), whereas NG2 and PDGF-Rα labeling, taken alone, could potentially identify a wider range of cells in this ESC-based system. Recordings were obtained from cells (n = 41) after O4 prelabeling, and postfixation immunocytochemistry was also performed to validate the technique (Figure 5B, bottom row; 32 of 33 O4 prelabeled and LY-filled cells that were retrieved costained with PDGF-Rα or NG2, and the remaining O4+ cell had the multibranching appearance of a mature oligodendrocyte).
Figure 5.
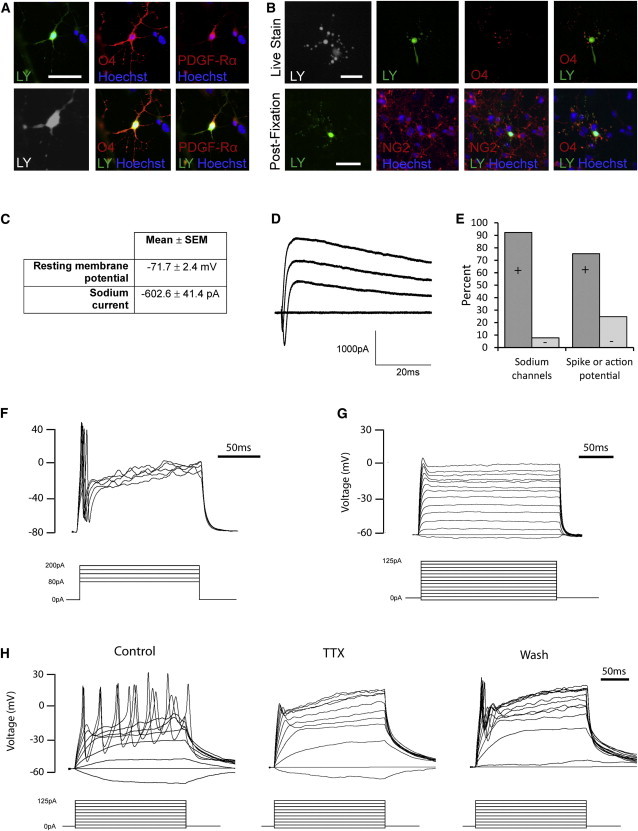
Electrophysiological Investigation Reveals Spiking and Nonspiking Groups of Human OPCs
(A) LY-filled cells were identified after electrophysiological recordings were performed and colabeled with O4 and either PDGF-Rα or NG2, confirming their identity as OPCs.
(B) Low recovery rates of LY-filled cells led to the development of an O4 prestaining protocol (top row), which was validated by postfixation immunocytochemistry (bottom row).
(C–E) Characterization of the electrophysiological properties of human OPCs revealed that they have an average resting membrane potential of −71.7 mV and the majority (n = 107/116) have voltage-gated sodium channels and outward rectifying potassium channels, with an average sodium current of −602.6 pA in response to a depolarizing voltage step.
(D) Amplitude trace for an example OPC, demonstrating an inward sodium current with a maximum amplitude of 1,092 pA when depolarized to 0 mV.
(E–G) The majority (75.3%) of OPCs with sodium channels (n = 70/93) fired a spike or action potential in response to current injection, but 24.7% did not, even when depolarized to 0 mV.
(H) Occasional cells (n = 3/70) fired trains of action potentials that were abolished by application of the voltage-gated sodium channel blocker TTX and returned following its removal. Scale bar = 50 μm.
Electrophysiological investigation showed that the average resting membrane potential of human OPCs was −71.7 ± 2.4 mV. The majority of human OPCs (92%; n = 116) had sodium channels and outward rectifying potassium currents, with an average sodium current of −602.6 ± 41.4 pA in response to depolarization by a voltage step (Figures 5C–5E). Consistent with these large inward sodium currents, approximately three-quarters of the OPCs fired a spike or action potential in response to current injection (n = 70/93); a smaller proportion of OPCs (24.7%) did not respond, even when depolarized above 0 mV (Figures 5E–5G). Occasional cells (n = 3/70) fired trains of action potentials that were blocked by the application of tetrodotoxin (TTX) and returned after it was removed (Figure 5H). TTX was also applied to OPCs that fired spikes or single action potentials, blocking this activity (n = 47/54) and confirming that it represents action potentials driven by activation of voltage-gated sodium channels (and not other voltage-gated channels such as calcium channels). Although spikes or single action potentials were a typical feature of the majority (75%) of OPCs from which recordings were obtained, the infrequent finding of trains of action potentials suggests that this particular feature is not typical of human OPCs, and it is possible that these cells (which were recorded before the O4 prestaining protocol was instituted) may in fact be neuronal.
Human OPCs Respond to RXR Signaling
These human oligodendrocyte lineage cells also provide a valuable resource for assessing the effects of putative remyelinating agents on human cells prior to clinical translation. RXRγ signaling was recently identified as a key pathway involved in OPC maturation and remyelination (Huang et al., 2011). We investigated whether human OPCs can respond to RXR signaling by applying the selective RXR agonist PA0124 to D100 NPC cultures after 3 weeks of terminal differentiation into OPCs, as characterized in Figure 2 (Nishimaki-Mogami et al., 2008). After 2 weeks, the number of O4+ oligodendrocytes that also expressed MBP increased by 6-fold, from 4.7% ± 2.7% to 29.9% ± 5.5% (p = 0.006; Figures 6A, 6B, and 6D). Application of the selective antagonist HX331 did not significantly affect maturation (Takahashi et al., 2002), indicating that RXR signaling is not a major contributor to OPC maturation under basal conditions in this human system (Figures 6C and 6D). These results show that activation of RXR signaling in human OPCs promotes differentiation into MBP+ oligodendrocytes, establishing this in vitro system as a useful model for investigating the activity of putative remyelinating agents in human cells.
Figure 6.
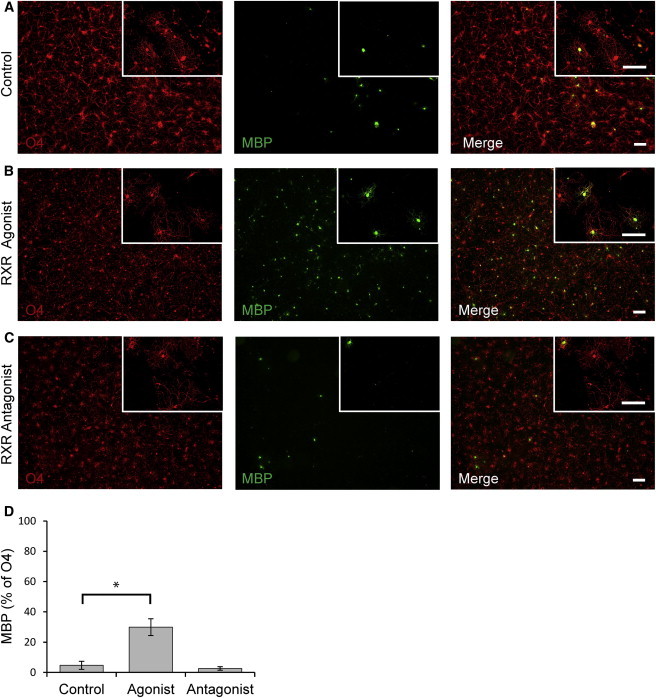
RXR Signaling Promotes the Maturation of hESC-NPC-Derived Human Oligodendrocytes
(A and B) Application of the RXR-selective agonist PA024 (0.75 μM) to the NG2+ OPC population generated after 3 weeks of terminal differentiation of D100 hESC-NPCs promoted their maturation into MBP+ oligodendrocytes over the subsequent 2 weeks.
(C) The antagonist HX331 (0.75 μM) did not significantly affect human OPC maturation (p = 0.50).
(D) Quantification of the percentage of O4+ oligodendrocytes expressing MBP after 5 weeks of terminal differentiation of D100 hESC-NPCs, with or without RXR signaling modulation, shows a significant increase in MBP expression among the O4+ oligodendrocytes following activation of RXR signaling (p = 0.006). Scale bar = 100 μm; percentages are given ± SEM.
Discussion
We have established a system for directing differentiation of hESCs toward the oligodendrocyte lineage, via both ventral forebrain and ventral spinal cord origins, under physiologically relevant 3% O2 conditions. This in vitro resource provides a powerful tool for investigating developmental pathways, the efficacy of small molecules in achieving in vitro specification of OPCs, the basic biological characteristics of these human cells, and the effects of putative remyelinating agents on human OPC maturation.
The critical and hitherto unrecognized role of FGF-2 in the specification of human OPCs via ventral forebrain origins has been clearly demonstrated in this in vitro system. Similarly, we were able to show that SAG is an effective alternative small-molecule agonist of SHH signaling to PM in this system, establishing an optimal working concentration for OLIG2 induction of 1 μM SAG in the presence of 10 ng/ml FGF-2. Robust generation of both OPCs and oligodendrocytes will facilitate the development of an in vitro human myelination assay. Our electrophysiological studies reveal that the majority of human OPCs have sodium channels and can fire action potentials, which is consistent with observations in their rat counterparts but in contrast to results in mice. Our system provides a platform for extending such electrophysiological studies to compare OPCs derived from different developmental origins and investigate responses to neurotransmitters such as glutamate and NMDA receptor signaling (Káradóttir et al., 2005).
The observation that human OPCs respond to RXR signaling is exciting, especially when it considered alongside the observation that the borders of chronically active MS plaques contain OPCs expressing RXRγ (Huang et al., 2011). These same areas were previously reported to contain so-called premyelinating oligodendrocytes (Wolswijk, 1998; Chang et al., 2002), and it is thought that their failure to differentiate into myelinating oligodendrocytes is a critical factor in the development of progressive disease. Furthermore, it has been proposed that the key factor influencing this failure of remyelination is age (Franklin, 2002). Intraperitoneal delivery of 9-cis RA (a ligand for RXR activation) to aged rats with a focal area of demyelination was shown to enhance remyelination (Huang et al., 2011). Thus, our data provide further evidence to support a clinical trial of an RXR agonist in patients with secondary progressive MS.
Taken together, our findings indicate that this developmentally engineered, physiologically relevant system for generating human OPCs and oligodendrocytes from hESCs has the potential to provide further insights into pathways involved in human myelination and remyelination, and thus may ultimately lead to new therapeutic approaches in the treatment of MS.
Experimental Procedures
Cell Culture
H9 hESCs were maintained under feeder-free conditions at 20% O2 in chemically defined medium (CDM; 50% IMDM [Invitrogen], 50% F12 [Invitrogen], 7 μg/ml insulin [Roche], 30 μg/ml transferrin [Roche], 5 mg/ml BSA [Sigma], 1% lipid 100× [Invitrogen], and 450 μM monothioglycerol [Sigma]) (Brons et al., 2007), supplemented with 12 ng/ml FGF-2 (R&D Systems) and 10 ng/ml activin, between passages 56 and 85. All cultures were supplemented with 1% penicillin and streptomycin (Invitrogen). Six-well plates (Nunc) were coated overnight with mouse embryonic fibroblast (MEF) medium (advanced DMEM F12 [Invitrogen], 10% fetal bovine serum [Biosera], 1% L-glutamine [Invitrogen], and 0.1 mM β-mercaptoethanol [Sigma]) and colonies were passaged with 1 mg/ml collagenase (Invitrogen) every 3–5 days. HUES-9 cells (hES Facility, Harvard University), between passages 30 and 42, were grown on irradiated MEF feeders supplemented with 10 ng/ml FGF-2, 10 ng/ml activin, and 10 ng/ml insulin.
For neural conversion, colonies were lifted off with liberase 125 μg/ml (Roche) after incubation for 15–20 min, allowed to settle in 15 ml tubes, and rinsed with CDM before they were chopped with a McIlwain Tissue Chopper (Mickle Engineering) at 120 μm distances in two directions perpendicular to each other (Joannides et al., 2006). The resulting cellular aggregates were then suspended in repellent tissue culture flasks (Nunc) at a density of approximately 200,000 cells/ml of CDM in the absence of growth factors. From this point onward, cells were cultured in a 3% O2 and 5% CO2 incubator, with oxygen displaced by nitrogen. The resultant spheres were fed every other day (50% media change) and chopped again at day 10 before they were transferred to an orbital shaker to prevent aggregation (Stacpoole et al., 2011b).
From D10 onward, morphogens were applied as described in the text (Figure 2A). In the FGF-2-based ventral forebrain protocol, we added 10 ng/ml FGF-2 plus 5 mg/ml heparin (Sigma) between D14 and D50, 1 μM PM (Calbiochem) or 1 μM SAG (Smoothened agonist; Enzo Life Sciences) between D14 and D100, and 10 ng/ml PDGF plus 40 ng/ml T3 between D50 and D100. For the RA-based, spinal cord method, we added 0.1 μM RA between D10 and D24, 1 μM of PM between D14 and D100, 10 ng/ml FGF-2 plus 5 mg/ml heparin between D24 and D50, and 10 ng/ml PDGF plus 40 ng/ml T3 between D50 and D100. A 50% change of medium was performed every 2–3 days and spheres were passaged by mechanical chopping every 2 weeks.
For terminal differentiation, NPCs were plated onto coverslips coated with 10 μg/ml poly-D-lysine/laminin (Sigma), and cultured in DMEM 3:1 F12/1% N2 (Invitrogen)/1% penicillin-streptomycin supplemented with 10 ng/ml PDGF (Peprotech), 10 ng/ml IGF-1 (Peprotech), 10 ng/ml NT3 (Peprotech), 40 ng/ml T3 (Sigma), and 1 μM cAMP (Sigma), with a 50% medium change every 2–3 days. After 7 days, 10 ng/ml BDNF (Peprotech) was added. A 50% media change was performed every 2–3 days.
Between weeks 3 and 5, we investigated the role of RXR signaling in OPC maturation by adding 0.75 μM of the agonist PA024 or 0.75 μM of the antagonist HX331 to the differentiation media of D100 hESC-NPCs that had been plated for terminal differentiation.
RNA Isolation and RT-PCR
Total cellular RNA was extracted using the RNeasy Mini isolation kit (QIAGEN). RNA samples (2 μg) treated with RNase-free-DNase (New England Biolabs) were reverse transcribed in 100 μl with random hexamers using MMLV RT (Invitrogen) according to the manufacturer’s protocol. PCR was conducted in a 25 μl reaction volume using 2 μl cDNA with BioTaq polymerase (BioLine). Primer sequences are shown in Table S1. β-actin was used as a housekeeping control. The amplification products were analyzed by agarose gel electrophoresis.
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde (Sigma) for 15 min at room temperature and rinsed three times with PBS. Spheres were fixed for 45 min, rinsed three times with PBS, and cryoprotected with 30% sucrose overnight before they were embedded in optimal cutting temperature medium. Blocks were cut on a cryostat at 12 μm intervals and collected on Superfrost Plus-charged slides. Samples were blocked for 1 hr at room temperature with 5% goat serum/0.2% Triton X-100 (Sigma)/PBS and incubated overnight at 4°C with primary antibodies (see Table S2 for a complete list) in 2% goat serum/0.2% Triton X-100/PBS. (Triton was omitted throughout for NG2, PDGF-Rα, galactocerebroside [GALC], O4, O1, and MBP staining). After three rinses in PBS, Alexa Fluor secondary antibodies (Invitrogen 488, 555, or 647) were applied 1:1,000 in 1% goat serum/0.2% Triton X-100/PBS/Hoescht 1:4,000 for 1 hr at room temperature. Sphere sections and coverslips were mounted with FluorSave (Calbiochem).
Cells and sections were viewed under a Leica microscope (AF-6000) with appropriate filters for cell identification and counting. Confocal imaging using a scanning laser confocal microscope (TCS-NT-UV; Leica) was also performed; typical stacks were composed of 10–20 optical sections of 0.5–1.0 μm thickness.
Electrophysiology
O4 prelabeling was used as an adjunct to morphology-based selection of OPCs for electrophysiological investigation. Live staining at 37°C and 3% O2 involved 30 min incubation with O4 primary antibody in terminal differentiation medium (1 in 200), followed by 3 × 5 min rinses with warm DMEM, then 30 min incubation with IgM-555 secondary (also in differentiation medium [1 in 400]), and finally three further rinses with warm DMEM before replacement with differentiation medium. Two to three coverslips were stained in tandem and were used for recordings within 6 hr. Whole-cell current clamping of OPCs was performed at room temperature using glass microelectrodes of 5.5–9 MΩ resistance containing internal solution (130 mM potassium gluconate, 4 mM NaCl, 10 mM HEPES, 10 mM BAPTA, 4 mM MgATP, 0.5 mM Na2GTP, 0.5 mM CaCl2, and 2 mM K-LY, pH adjusted to 7.3 with KOH). Series resistance was 19 ± 1.25 MΩ. Cultures were continuously perfused with external solution (144 mM NaCl, 2.5 mM KCl, 10 mM HEPES, 10 mM NaH2PO4, 2.5 mM CaCl2, 10 mM glucose, 2 m MgCl2, buffered with HEPES, pH adjusted to 7.35 with NaOH), which also contained glycine (100 mM) and strychnine (5 μM). The voltage-gated sodium channel blocker TTX (1 μM) was applied as described in the text. Recordings were corrected for −14 mV junctional potential and data were analyzed using Clampfit 10.2. After recordings were completed, coverslips were fixed with 4% paraformaldehyde for 15 min at room temperature. OPCs were further stained for PDGF-Rα or NG2 using the methods described above.
Quantification and Statistical Analysis
All experiments were performed at least three times unless otherwise stated. Student’s unpaired t test was used for statistical analysis; p values < 0.05 were considered significant and data are presented as mean ± SEM.
Acknowledgments
This work was supported by the Multiple Sclerosis Society UK, the Evelyn Trust, the Medical Research Council, the Wellcome Trust, and the National Institute for Health Research (Cambridge Biomedical Research Centre). S.R.L.S. was supported by a Sir David Walker Fellowship, a joint Medical Research Council and Multiple Sclerosis Society Clinical Research Training Fellowship (No. G0800487), and a Raymond and Beverly Sackler Studentship.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplemental Information
References
- Akundi R.S., Rivkees S.A. Hypoxia alters cell cycle regulatory protein expression and induces premature maturation of oligodendrocyte precursor cells. PLoS ONE. 2009;4:e4739. doi: 10.1371/journal.pone.0004739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billon N., Jolicoeur C., Ying Q.L., Smith A., Raff M. Normal timing of oligodendrocyte development from genetically engineered, lineage-selectable mouse ES cells. J. Cell Sci. 2002;115:3657–3665. doi: 10.1242/jcs.00049. [DOI] [PubMed] [Google Scholar]
- Bouhon I.A., Joannides A., Kato H., Chandran S., Allen N.D. Embryonic stem cell-derived neural progenitors display temporal restriction to neural patterning. Stem Cells. 2006;24:1908–1913. doi: 10.1634/stemcells.2006-0031. [DOI] [PubMed] [Google Scholar]
- Brons I.G., Smithers L.E., Trotter M.W., Rugg-Gunn P., Sun B., Chuva de Sousa Lopes S.M., Howlett S.K., Clarkson A., Ahrlund-Richter L., Pedersen R.A., Vallier L. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- Brüstle O., Jones K.N., Learish R.D., Karram K., Choudhary K., Wiestler O.D., Duncan I.D., McKay R.D. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999;285:754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- Casaccia-Bonnefil P. Cell death in the oligodendrocyte lineage: a molecular perspective of life/death decisions in development and disease. Glia. 2000;29:124–135. doi: 10.1002/(sici)1098-1136(20000115)29:2<124::aid-glia5>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Chang A., Tourtellotte W.W., Rudick R., Trapp B.D. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N. Engl. J. Med. 2002;346:165–173. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- Chen J.K., Taipale J., Young K.E., Maiti T., Beachy P.A. Small molecule modulation of Smoothened activity. Proc. Natl. Acad. Sci. USA. 2002;99:14071–14076. doi: 10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.L., Pistollato F., Hoeppner D.J., Ni H.T., McKay R.D., Panchision D.M. Oxygen tension regulates survival and fate of mouse central nervous system precursors at multiple levels. Stem Cells. 2007;25:2291–2301. doi: 10.1634/stemcells.2006-0609. [DOI] [PubMed] [Google Scholar]
- Du Y., Fischer T.Z., Lee L.N., Lercher L.D., Dreyfus C.F. Regionally specific effects of BDNF on oligodendrocytes. Dev. Neurosci. 2003;25:116–126. doi: 10.1159/000072261. [DOI] [PubMed] [Google Scholar]
- Erecińska M., Silver I.A. Tissue oxygen tension and brain sensitivity to hypoxia. Respir. Physiol. 2001;128:263–276. doi: 10.1016/s0034-5687(01)00306-1. [DOI] [PubMed] [Google Scholar]
- Franklin R.J.M. Why does remyelination fail in multiple sclerosis? Nat. Rev. Neurosci. 2002;3:705–714. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- Franklin R.J.M., Bayley S.A., Milner R., Ffrench-Constant C., Blakemore W.F. Differentiation of the O-2A progenitor cell line CG-4 into oligodendrocytes and astrocytes following transplantation into glia-deficient areas of CNS white matter. Glia. 1995;13:39–44. doi: 10.1002/glia.440130105. [DOI] [PubMed] [Google Scholar]
- Furusho M., Kaga Y., Ishii A., Hébert J.M., Bansal R. Fibroblast growth factor signaling is required for the generation of oligodendrocyte progenitors from the embryonic forebrain. J. Neurosci. 2011;31:5055–5066. doi: 10.1523/JNEUROSCI.4800-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspard N., Bouschet T., Hourez R., Dimidschstein J., Naeije G., van den Ameele J., Espuny-Camacho I., Herpoel A., Passante L., Schiffmann S.N. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature. 2008;455:351–357. doi: 10.1038/nature07287. [DOI] [PubMed] [Google Scholar]
- Glaser T., Perez-Bouza A., Klein K., Brüstle O. Generation of purified oligodendrocyte progenitors from embryonic stem cells. FASEB J. 2005;19:112–114. doi: 10.1096/fj.04-1931fje. [DOI] [PubMed] [Google Scholar]
- Hu B.Y., Zhang S.C. Differentiation of spinal motor neurons from pluripotent human stem cells. Nat. Protoc. 2009;4:1295–1304. doi: 10.1038/nprot.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B.Y., Du Z.W., Li X.J., Ayala M., Zhang S.C. Human oligodendrocytes from embryonic stem cells: conserved SHH signaling networks and divergent FGF effects. Development. 2009;136:1443–1452. doi: 10.1242/dev.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J.K., Jarjour A.A., Nait Oumesmar B., Kerninon C., Williams A., Krezel W., Kagechika H., Bauer J., Zhao C., Baron-Van Evercooren A. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat. Neurosci. 2011;14:45–53. doi: 10.1038/nn.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izrael M., Zhang P., Kaufman R., Shinder V., Ella R., Amit M., Itskovitz-Eldor J., Chebath J., Revel M. Human oligodendrocytes derived from embryonic stem cells: effect of noggin on phenotypic differentiation in vitro and on myelination in vivo. Mol. Cell. Neurosci. 2007;34:310–323. doi: 10.1016/j.mcn.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Joannides A., Fiore-Hériché C., Westmore K., Caldwell M., Compston A., Allen N., Chandran S. Automated mechanical passaging: a novel and efficient method for human embryonic stem cell expansion. Stem Cells. 2006;24:230–235. doi: 10.1634/stemcells.2005-0243. [DOI] [PubMed] [Google Scholar]
- Joannides A.J., Webber D.J., Raineteau O., Kelly C., Irvine K.A., Watts C., Rosser A.E., Kemp P.J., Blakemore W.F., Compston A. Environmental signals regulate lineage choice and temporal maturation of neural stem cells from human embryonic stem cells. Brain. 2007;130:1263–1275. doi: 10.1093/brain/awm070. [DOI] [PubMed] [Google Scholar]
- Kang S.M., Cho M.S., Seo H., Yoon C.J., Oh S.K., Choi Y.M., Kim D.W. Efficient induction of oligodendrocytes from human embryonic stem cells. Stem Cells. 2007;25:419–424. doi: 10.1634/stemcells.2005-0482. [DOI] [PubMed] [Google Scholar]
- Káradóttir R., Cavelier P., Bergersen L.H., Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–1166. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Káradóttir R., Hamilton N.B., Bakiri Y., Attwell D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat. Neurosci. 2008;11:450–456. doi: 10.1038/nn2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessaris N., Fogarty M., Iannarelli P., Grist M., Wegner M., Richardson W.D. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci. 2006;9:173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krencik R., Weick J.P., Liu Y., Zhang Z.J., Zhang S.C. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat. Biotechnol. 2011;29:528–534. doi: 10.1038/nbt.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.J., Hu B.Y., Jones S.A., Zhang Y.S., Lavaute T., Du Z.W., Zhang S.C. Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules. Stem Cells. 2008;26:886–893. doi: 10.1634/stemcells.2007-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.J., Zhang X., Johnson M.A., Wang Z.B., Lavaute T., Zhang S.C. Coordination of sonic hedgehog and Wnt signaling determines ventral and dorsal telencephalic neuron types from human embryonic stem cells. Development. 2009;136:4055–4063. doi: 10.1242/dev.036624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Zhang S.C. Specification of neuronal and glial subtypes from human pluripotent stem cells. Cell. Mol. Life Sci. 2011;68:3995–4008. doi: 10.1007/s00018-011-0770-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Sage J.C., Miller M.R., Verhaak R.G., Hippenmeyer S., Vogel H., Foreman O., Bronson R.T., Nishiyama A., Luo L., Zong H. Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell. 2011;146:209–221. doi: 10.1016/j.cell.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q.R., Yuk D., Alberta J.A., Zhu Z., Pawlitzky I., Chan J., McMahon A.P., Stiles C.D., Rowitch D.H. Sonic hedgehog—regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 2000;25:317–329. doi: 10.1016/s0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- Mázló M., Tariska I. Morphological demonstration of the first phase of polyomavirus replication in oligodendroglia cells of human brain in progressive multifocal leukoencephalopathy (PML) Acta Neuropathol. 1980;49:133–143. doi: 10.1007/BF00690753. [DOI] [PubMed] [Google Scholar]
- Nishimaki-Mogami T., Tamehiro N., Sato Y., Okuhira K., Sai K., Kagechika H., Shudo K., Abe-Dohmae S., Yokoyama S., Ohno Y. The RXR agonists PA024 and HX630 have different abilities to activate LXR/RXR and to induce ABCA1 expression in macrophage cell lines. Biochem. Pharmacol. 2008;76:1006–1013. doi: 10.1016/j.bcp.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Nistor G.I., Totoiu M.O., Haque N., Carpenter M.K., Keirstead H.S. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;49:385–396. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- Papp M.I., Lantos P.L. The distribution of oligodendroglial inclusions in multiple system atrophy and its relevance to clinical symptomatology. Brain. 1994;117:235–243. doi: 10.1093/brain/117.2.235. [DOI] [PubMed] [Google Scholar]
- Pistollato F., Chen H.L., Schwartz P.H., Basso G., Panchision D.M. Oxygen tension controls the expansion of human CNS precursors and the generation of astrocytes and oligodendrocytes. Mol. Cell. Neurosci. 2007;35:424–435. doi: 10.1016/j.mcn.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Raff M.C., Miller R.H., Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Sinha S., Chen J.K. Purmorphamine activates the Hedgehog pathway by targeting Smoothened. Nat. Chem. Biol. 2006;2:29–30. doi: 10.1038/nchembio753. [DOI] [PubMed] [Google Scholar]
- Stacpoole S.R., Bilican B., Webber D.J., Luzhynskaya A., He X.L., Compston A., Karadottir R., Franklin R.J., Chandran S. Derivation of neural precursor cells from human ES cells at 3% O(2) is efficient, enhances survival and presents no barrier to regional specification and functional differentiation. Cell Death Differ. 2011;18:1016–1023. doi: 10.1038/cdd.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacpoole S.R., Bilican B., Webber D.J., Luzhynskaya A., He X.L., Compston A., Karadottir R., Franklin R.J.M., Chandran S. Efficient derivation of NPCs, spinal motor neurons and midbrain dopaminergic neurons from hESCs at 3% oxygen. Nat. Protoc. 2011;6:1229–1240. doi: 10.1038/nprot.2011.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacpoole S.R., Webber D.J., Bilican B., Compston A., Chandran S., Franklin R.J. Neural precursor cells cultured at physiologically relevant oxygen tensions have a survival advantage following transplantation. Stem Cells Transl. Med. 2013;2:464–472. doi: 10.5966/sctm.2012-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg M., Skottman H., Suuronen R., Narkilahti S. Production and isolation of NG2+ oligodendrocyte precursors from human embryonic stem cells in defined serum-free medium. Stem Cell Res. (Amst.) 2010;5:91–103. doi: 10.1016/j.scr.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Takahashi B., Ohta K., Kawachi E., Fukasawa H., Hashimoto Y., Kagechika H. Novel retinoid X receptor antagonists: specific inhibition of retinoid synergism in RXR-RAR heterodimer actions. J. Med. Chem. 2002;45:3327–3330. doi: 10.1021/jm0255320. [DOI] [PubMed] [Google Scholar]
- Wang S., Bates J., Li X., Schanz S., Chandler-Militello D., Levine C., Maherali N., Studer L., Hochedlinger K., Windrem M., Goldman S.A. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell. 2013;12:252–264. doi: 10.1016/j.stem.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolswijk G. Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J. Neurosci. 1998;18:601–609. doi: 10.1523/JNEUROSCI.18-02-00601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Anderson D.J. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109:61–73. doi: 10.1016/s0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Wang S., Anderson D.J. Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron. 2000;25:331–343. doi: 10.1016/s0896-6273(00)80898-3. [DOI] [PubMed] [Google Scholar]
- Ziskin J.L., Nishiyama A., Rubio M., Fukaya M., Bergles D.E. Vesicular release of glutamate from unmyelinated axons in white matter. Nat. Neurosci. 2007;10:321–330. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


