Abstract
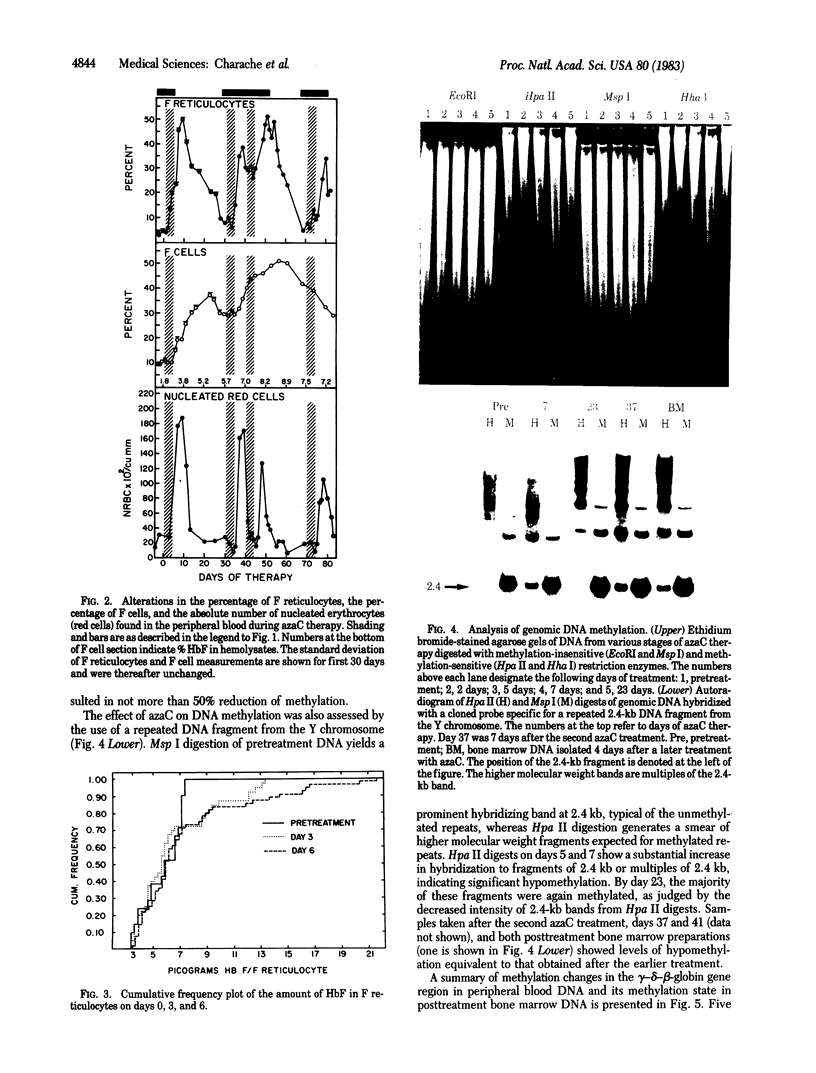
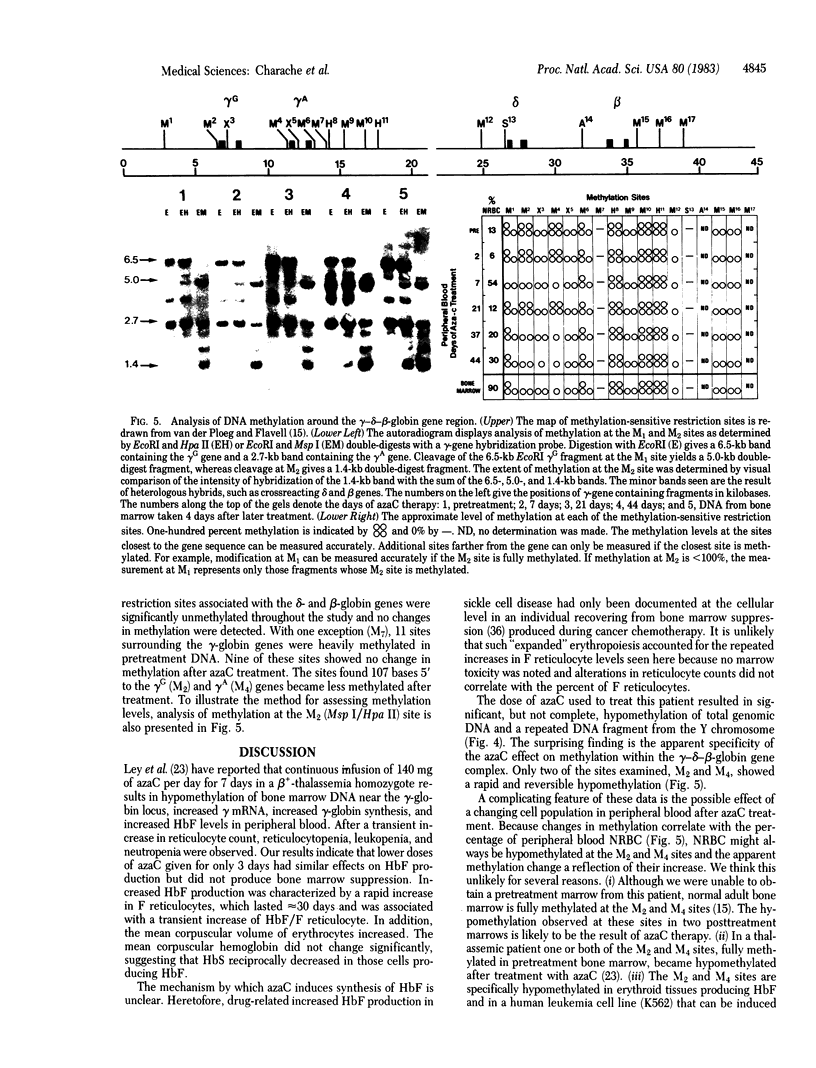
Increased production of fetal hemoglobin (HbF) was observed in a patient with sickle cell anemia treated with 5-azacytidine. Each of four courses of therapy resulted in a rapid and prolonged increase in the percentage of HbF containing reticulocytes (F reticulocytes) and HbF containing erythrocytes (F cells). The percentage of HbF in peripheral blood rose from 1.8 to 8.9%. The rise in HbF production was accompanied by an increase in peripheral blood hemoglobin concentration from 8 to 12 g/dl and an increase in mean erythrocyte volume. Treatment with 5-azacytidine resulted in hypomethylation of total genomic and a Y-chromosome-specific DNA fragment isolated from both peripheral blood and bone marrow. Of 15 restriction enzyme sites around the gamma-delta-beta-globin gene complex, only 2 became hypomethylated: one 107 bases 5' to the gamma G and the other 107 bases 5' to the gamma A globin genes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedict W. F., Banerjee A., Gardner A., Jones P. A. Induction of morphological transformation in mouse C3H/10T1/2 clone 8 cells and chromosomal damage in hamster A(T1)C1-3 cells by cancer chemotherapeutic agents. Cancer Res. 1977 Jul;37(7 Pt 1):2202–2208. [PubMed] [Google Scholar]
- Bertles J. F., Rabinowitz R., Döbler J. Hemoglobin interaction: modification of solid phase composition in the sickling phenomenon. Science. 1970 Jul 24;169(3943):375–377. doi: 10.1126/science.169.3943.375. [DOI] [PubMed] [Google Scholar]
- Bird A., Taggart M., Macleod D. Loss of rDNA methylation accompanies the onset of ribosomal gene activity in early development of X. laevis. Cell. 1981 Nov;26(3 Pt 1):381–390. doi: 10.1016/0092-8674(81)90207-5. [DOI] [PubMed] [Google Scholar]
- Charache S., Duffy T. P., Jander N., Scott J. C., Bedine M., Morrell R. Toxic-therapeutic ratio of sodium cyanate. Arch Intern Med. 1975 Aug;135(8):1043–1047. [PubMed] [Google Scholar]
- Charache S., Fox J., McCurdy P., Kazazian H., Jr, Winslow R., Hathaway P., van Beneden R., Jessop M. Postsynthetic deamidation of hemoglobin Providence (beta 82 Lys replaced by Asn, Asp) and its effect on oxygen transport. J Clin Invest. 1977 Apr;59(4):652–658. doi: 10.1172/JCI108683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charache S., Walker W. G. Failure of desmopressin to lower serum sodium or prevent crisis in patients with sickle cell anemia. Blood. 1981 Nov;58(5):892–896. [PubMed] [Google Scholar]
- Christy B., Scangos G. Expression of transferred thymidine kinase genes is controlled by methylation. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6299–6303. doi: 10.1073/pnas.79.20.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compere S. J., Palmiter R. D. DNA methylation controls the inducibility of the mouse metallothionein-I gene lymphoid cells. Cell. 1981 Jul;25(1):233–240. doi: 10.1016/0092-8674(81)90248-8. [DOI] [PubMed] [Google Scholar]
- Constantinides P. G., Jones P. A., Gevers W. Functional striated muscle cells from non-myoblast precursors following 5-azacytidine treatment. Nature. 1977 May 26;267(5609):364–366. doi: 10.1038/267364a0. [DOI] [PubMed] [Google Scholar]
- Creusot F., Acs G., Christman J. K. Inhibition of DNA methyltransferase and induction of Friend erythroleukemia cell differentiation by 5-azacytidine and 5-aza-2'-deoxycytidine. J Biol Chem. 1982 Feb 25;257(4):2041–2048. [PubMed] [Google Scholar]
- DeSimone J., Heller P., Hall L., Zwiers D. 5-Azacytidine stimulates fetal hemoglobin synthesis in anemic baboons. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4428–4431. doi: 10.1073/pnas.79.14.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dover G. J., Boyer S. H., Bell W. R. Microscopic method for assaying F cell production: illustrative changes during infancy and in aplastic anemia. Blood. 1978 Oct;52(4):664–672. [PubMed] [Google Scholar]
- Dover G. J., Boyer S. H., Charache S., Heintzelman K. Individual variation in the production and survival of F cells in sickle-cell disease. N Engl J Med. 1978 Dec 28;299(26):1428–1435. doi: 10.1056/NEJM197812282992603. [DOI] [PubMed] [Google Scholar]
- Dover G. J., Boyer S. H., Pembrey M. E. F-cell production in sickle cell anemia: regulation by genes linked to beta-hemoglobin locus. Science. 1981 Mar 27;211(4489):1441–1444. doi: 10.1126/science.6162200. [DOI] [PubMed] [Google Scholar]
- Dover G. J., Boyer S. H. Quantitation of hemoglobins within individual red cells: asynchronous biosynthesis of fetal and adult hemoglobin during erythroid maturation in normal subjects. Blood. 1980 Dec;56(6):1082–1091. [PubMed] [Google Scholar]
- Dover G. J., Boyer S. H. The cellular distribution of fetal hemoglobin: normal adults and hemoglobinopathies. Tex Rep Biol Med. 1980;40:43–54. [PubMed] [Google Scholar]
- Dover G. J., Boyer S. H., Zinkham W. H. Production of erythrocytes that contain fetal hemoglobin in anemia. Transient in vivo changes. J Clin Invest. 1979 Feb;63(2):173–176. doi: 10.1172/JCI109286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenfeld G., McGhee J. Methylation and gene control. Nature. 1982 Apr 15;296(5858):602–603. doi: 10.1038/296602a0. [DOI] [PubMed] [Google Scholar]
- Friedman S. The effect of 5-azacytidine on E. coli DNA methylase. Biochem Biophys Res Commun. 1979 Aug 28;89(4):1328–1333. doi: 10.1016/0006-291x(79)92154-5. [DOI] [PubMed] [Google Scholar]
- Groudine M., Eisenman R., Weintraub H. Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature. 1981 Jul 23;292(5821):311–317. doi: 10.1038/292311a0. [DOI] [PubMed] [Google Scholar]
- HERMAN E. C., Jr, CONLEY C. L. Hereditary persistence of fetal hemoglobin. A family study. Am J Med. 1960 Jul;29:9–17. doi: 10.1016/0002-9343(60)90003-6. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980 May;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- Kunkel L. M., Smith K. D., Boyer S. H., Borgaonkar D. S., Wachtel S. S., Miller O. J., Breg W. R., Jones H. W., Jr, Rary J. M. Analysis of human Y-chromosome-specific reiterated DNA in chromosome variants. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1245–1249. doi: 10.1073/pnas.74.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley T. J., DeSimone J., Anagnou N. P., Keller G. H., Humphries R. K., Turner P. H., Young N. S., Keller P., Nienhuis A. W. 5-azacytidine selectively increases gamma-globin synthesis in a patient with beta+ thalassemia. N Engl J Med. 1982 Dec 9;307(24):1469–1475. doi: 10.1056/NEJM198212093072401. [DOI] [PubMed] [Google Scholar]
- Mandel J. L., Chambon P. DNA methylation: organ specific variations in the methylation pattern within and around ovalbumin and other chicken genes. Nucleic Acids Res. 1979 Dec 20;7(8):2081–2103. doi: 10.1093/nar/7.8.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. D., Ginder G. D. Specific DNA methylation sites in the vicinity of the chicken beta-globin genes. Nature. 1979 Aug 2;280(5721):419–420. doi: 10.1038/280419a0. [DOI] [PubMed] [Google Scholar]
- McKeon C., Ohkubo H., Pastan I., de Crombrugghe B. Unusual methylation pattern of the alpha 2 (l) collagen gene. Cell. 1982 May;29(1):203–210. doi: 10.1016/0092-8674(82)90104-0. [DOI] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Shapiro L. J. Reactivation of an inactive human X chromosome: evidence for X inactivation by DNA methylation. Science. 1981 Jan 23;211(4480):393–396. doi: 10.1126/science.6164095. [DOI] [PubMed] [Google Scholar]
- Noguchi C. T., Schechter A. N. The intracellular polymerization of sickle hemoglobin and its relevance to sickle cell disease. Blood. 1981 Dec;58(6):1057–1068. [PubMed] [Google Scholar]
- Pembrey M. E., Wood W. G., Weatherall D. J., Perrine R. P. Fetal haemoglobin production and the sickle gene in the oases of Eastern Saudi Arabia. Br J Haematol. 1978 Nov;40(3):415–429. doi: 10.1111/j.1365-2141.1978.tb05813.x. [DOI] [PubMed] [Google Scholar]
- Riggs A. D. X inactivation, differentiation, and DNA methylation. Cytogenet Cell Genet. 1975;14(1):9–25. doi: 10.1159/000130315. [DOI] [PubMed] [Google Scholar]
- SINGER K., CHERNOFF A. I., SINGER L. Studies on abnormal hemoglobins. I. Their demonstration in sickle cell anemia and other hematologic disorders by means of alkali denaturation. Blood. 1951 May;6(5):413–428. [PubMed] [Google Scholar]
- Schmeckpeper B. J., Willard H. F., Smith K. D. Isolation and characterization of cloned human DNA fragments carrying reiterated sequences common to both autosomes and the X chromosome. Nucleic Acids Res. 1981 Apr 24;9(8):1853–1872. doi: 10.1093/nar/9.8.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wilson J. T., Wilson L. B., deRiel J. K., Villa-komaroff L., Efstratiadis A., Forget B. G., Weissman S. M. Insertion of synthetic copies of human globin genes into bacterial plasmids. Nucleic Acids Res. 1978 Feb;5(2):563–581. doi: 10.1093/nar/5.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ploeg L. H., Flavell R. A. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980 Apr;19(4):947–958. doi: 10.1016/0092-8674(80)90086-0. [DOI] [PubMed] [Google Scholar]