Abstract
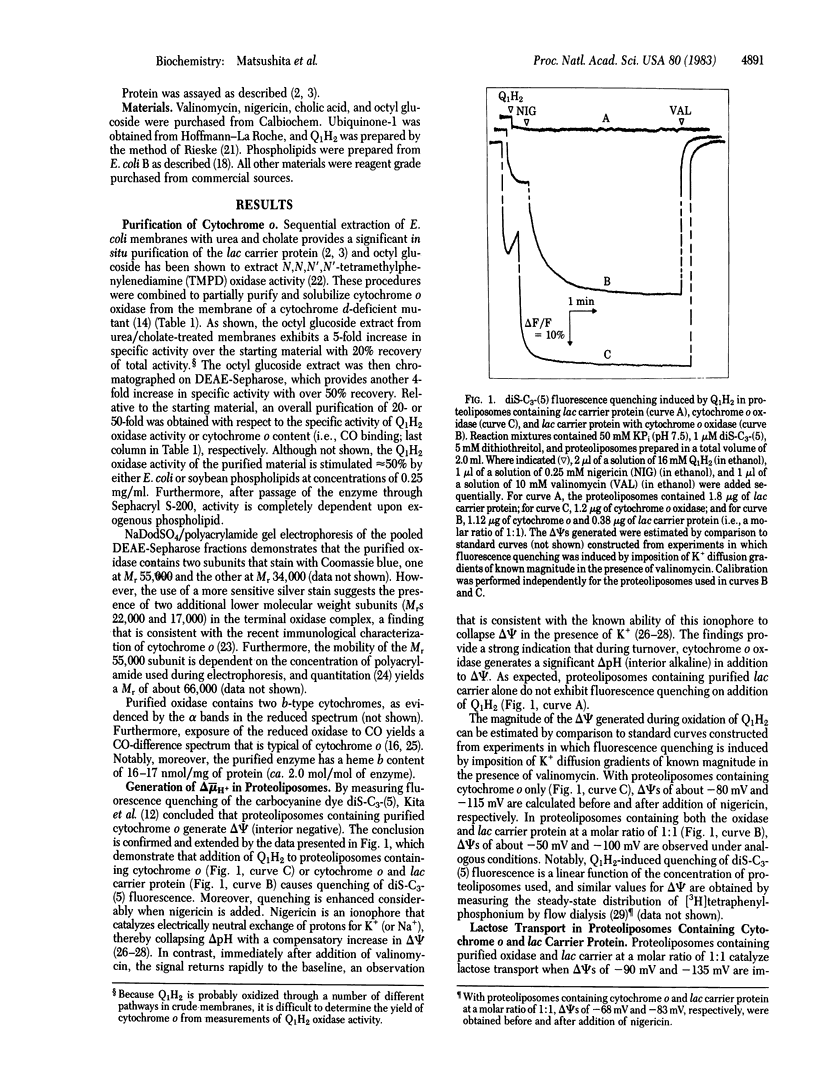
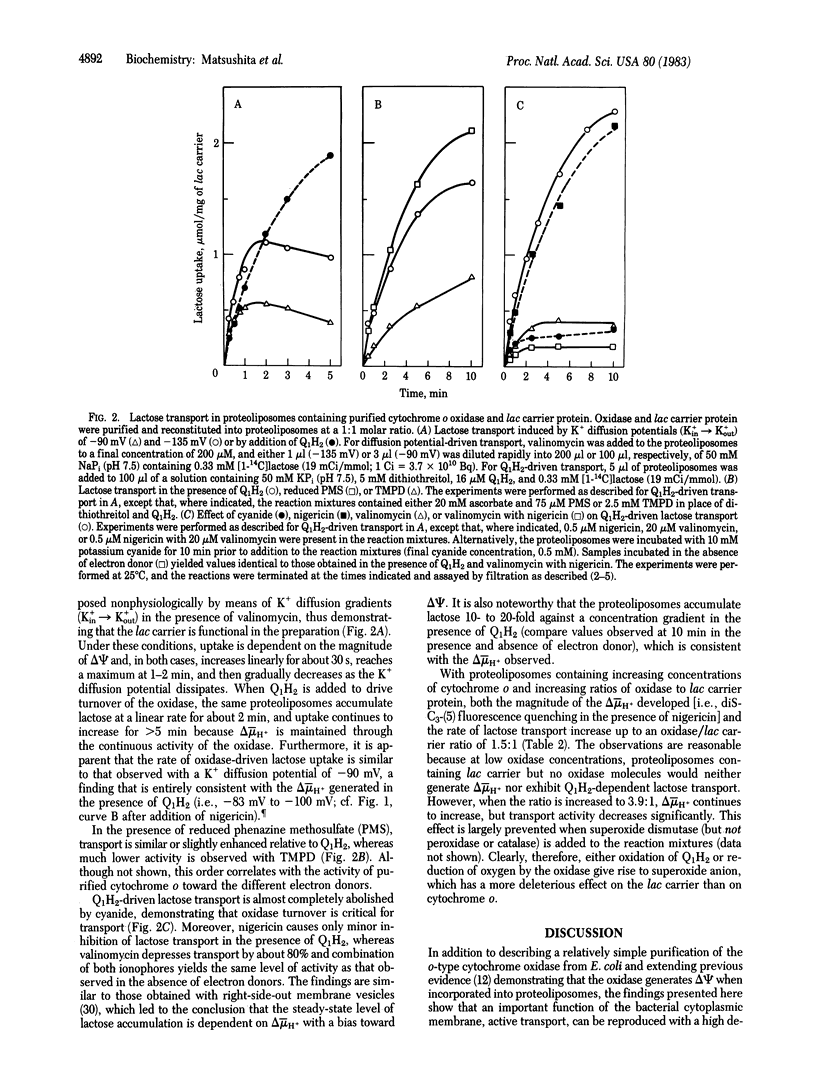
Most active transport across the bacterial cell membrane is driven by a proton electrochemical gradient (delta-muH+, interior negative and alkaline) generated via electron transfer through a membrane-bound respiratory chain. This phenomenon is now reproduced in vitro with proteoliposomes containing only two proteins purified from the membrane of Escherichia coli. An o-type cytochrome oxidase was extracted from membranes of a cytochrome d terminal oxidase mutant with octyl beta-D-glucopyranoside after sequential treatment with urea and cholate and was purified to homogeneity by ion-exchange chromatography. The purified oxidase contains four polypeptides (MrS 66,000, 35,000, 22,000, and 17,000), two b-type cytochromes (b558 and b563), and 16-17 nmol of heme b per mg of protein, and it catalyzes the oxidation of ubiquinol and other electron donors with specific activities 20- to 30-fold higher than crude membranes. The lac carrier protein was purified as described. Proteoliposomes were formed in the presence of the oxidase and lac carrier protein by detergent dilution, followed by freeze-thaw/sonication. The system generates a delta-muH+ (interior negative and alkaline) with ubiquinol as electron donor and the magnitude of delta-muH+ is dependent on the concentration of cytochrome o in the proteoliposomes. Furthermore, the proteoliposomes transport lactose against a concentration gradient to an extent that is commensurate with the magnitude of delta-muH+ generated. The results provide powerful additional support for the "chemiosmotic hypothesis" and demonstrate that purified lac carrier protein retains the ability to function in a physiological manner.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banker G. A., Cotman C. W. Measurement of free electrophoretic mobility and retardation coefficient of protein-sodium dodecyl sulfate complexes by gel electrophoresis. A method to validate molecular weight estimates. J Biol Chem. 1972 Sep 25;247(18):5856–5861. [PubMed] [Google Scholar]
- Carrasco N., Tahara S. M., Patel L., Goldkorn T., Kaback H. R. Preparation, characterization, and properties of monoclonal antibodies against the lac carrier protein from Escherichia coli. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6894–6898. doi: 10.1073/pnas.79.22.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. L., Boublik M., Kaback H. R. Structure of the lac carrier protein of Escherichia coli. J Biol Chem. 1983 Jan 10;258(1):31–34. [PubMed] [Google Scholar]
- Foster D. L., Garcia M. L., Newman M. J., Patel L., Kaback H. R. Lactose-proton symport by purified lac carrier protein. Biochemistry. 1982 Oct 26;21(22):5634–5638. doi: 10.1021/bi00265a038. [DOI] [PubMed] [Google Scholar]
- Garcia M. L., Viitanen P., Foster D. L., Kaback H. R. Mechanism of lactose translocation in proteoliposomes reconstituted with lac carrier protein purified from Escherichia coli. 1. Effect of pH and imposed membrane potential on efflux, exchange, and counterflow. Biochemistry. 1983 May 10;22(10):2524–2531. doi: 10.1021/bi00279a033. [DOI] [PubMed] [Google Scholar]
- Goldkorn T., Rimon G., Kaback H. R. Topology of the lac carrier protein in the membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3322–3326. doi: 10.1073/pnas.80.11.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. N., Gennis R. B. Isolation and characterization of an Escherichia coli mutant lacking cytochrome d terminal oxidase. J Bacteriol. 1983 Jun;154(3):1269–1275. doi: 10.1128/jb.154.3.1269-1275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. S. An ecf mutation in Escherichia coli pleiotropically affecting energy coupling in active transport but not generation or maintenance of membrane potential. J Biol Chem. 1977 Dec 10;252(23):8582–8588. [PubMed] [Google Scholar]
- Kita K., Kasahara M., Anraku Y. Formation of a membrane potential by reconstructed liposomes made with cytochrome b562-o complex, a terminal oxidase of Escherichia coli K12. J Biol Chem. 1982 Jul 25;257(14):7933–7935. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mitchell P. The Ninth Sir Hans Krebs Lecture. Compartmentation and communication in living systems. Ligand conduction: a general catalytic principle in chemical, osmotic and chemiosmotic reaction systems. Eur J Biochem. 1979 Mar 15;95(1):1–20. doi: 10.1111/j.1432-1033.1979.tb12934.x. [DOI] [PubMed] [Google Scholar]
- Newman M. J., Foster D. L., Wilson T. H., Kaback H. R. Purification and reconstitution of functional lactose carrier from Escherichia coli. J Biol Chem. 1981 Nov 25;256(22):11804–11808. [PubMed] [Google Scholar]
- Newman M. J., Wilson T. H. Solubilization and reconstitution of the lactose transport system from Escherichia coli. J Biol Chem. 1980 Nov 25;255(22):10583–10586. [PubMed] [Google Scholar]
- Plate C. A., Suit J. L. The eup genetic locus of Escherichia coli and its role in H+/solute symport. J Biol Chem. 1981 Dec 25;256(24):12974–12980. [PubMed] [Google Scholar]
- Racker E., Stoeckenius W. Reconstitution of purple membrane vesicles catalyzing light-driven proton uptake and adenosine triphosphate formation. J Biol Chem. 1974 Jan 25;249(2):662–663. [PubMed] [Google Scholar]
- Ramos S., Kaback H. R. The electrochemical proton gradient in Escherichia coli membrane vesicles. Biochemistry. 1977 Mar 8;16(5):848–854. doi: 10.1021/bi00624a006. [DOI] [PubMed] [Google Scholar]
- Ramos S., Kaback H. R. The relationship between the electrochemical proton gradient and active transport in Escherichia coli membrane vesicles. Biochemistry. 1977 Mar 8;16(5):854–859. doi: 10.1021/bi00624a007. [DOI] [PubMed] [Google Scholar]
- Ramos S., Schuldiner S., Kaback H. R. The electrochemical gradient of protons and its relationship to active transport in Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1892–1896. doi: 10.1073/pnas.73.6.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos S., Schuldiner S., Kaback H. R. The use of flow dialysis for determinations of deltapH and active transport. Methods Enzymol. 1979;55:680–688. doi: 10.1016/0076-6879(79)55076-9. [DOI] [PubMed] [Google Scholar]
- Reenstra W. W., Patel L., Rottenberg H., Kaback H. R. Electrochemical proton gradient in inverted membrane vesicles from Escherichia coli. Biochemistry. 1980 Jan 8;19(1):1–9. doi: 10.1021/bi00542a001. [DOI] [PubMed] [Google Scholar]
- Solioz M., Carafoli E., Ludwig B. The cytochrome c oxidase of Paracoccus denitrificans pumps protons in a reconstituted system. J Biol Chem. 1982 Feb 25;257(4):1579–1582. [PubMed] [Google Scholar]
- Sone N., Hinkle P. C. Proton transport by cytochrome c oxidase from the thermophilic bacterium PS3 reconstituted in liposomes. J Biol Chem. 1982 Nov 10;257(21):12600–12604. [PubMed] [Google Scholar]
- Tsuchiya T., Misawa A., Miyake Y., Yamasaki K., Niiya S. Solubilization and reconstitution of membrane energy-transducing systems of Escherichia coli. FEBS Lett. 1982 Jun 7;142(2):231–234. doi: 10.1016/0014-5793(82)80141-5. [DOI] [PubMed] [Google Scholar]
- Viitanen P., Garcia M. L., Foster D. L., Kaczorowski G. J., Kaback H. R. Mechanism of lactose translocation in proteoliposomes reconstituted with lac carrier protein purified from Escherichia coli. 2. Deuterium solvent isotope effects. Biochemistry. 1983 May 10;22(10):2531–2536. doi: 10.1021/bi00279a034. [DOI] [PubMed] [Google Scholar]
- Villarejo M. Evidence for two lac Y gene derived protein products in the E. coli membrane. Biochem Biophys Res Commun. 1980 Mar 13;93(1):16–23. doi: 10.1016/s0006-291x(80)80239-7. [DOI] [PubMed] [Google Scholar]
- Villarejo M., Ping C. Localization of the lactose permease protein(s) in the E. coli envelope. Biochem Biophys Res Commun. 1978 Jun 14;82(3):935–942. doi: 10.1016/0006-291x(78)90873-2. [DOI] [PubMed] [Google Scholar]
- Wallace B. J., Young I. G. Role of quinones in electron transport to oxygen and nitrate in Escherichia coli. Studies with a ubiA- menA- double quinone mutant. Biochim Biophys Acta. 1977 Jul 7;461(1):84–100. doi: 10.1016/0005-2728(77)90071-8. [DOI] [PubMed] [Google Scholar]
- Wikström M., Krab K. Proton-pumping cytochrome c oxidase. Biochim Biophys Acta. 1979 Aug 17;549(2):177–122. doi: 10.1016/0304-4173(79)90014-4. [DOI] [PubMed] [Google Scholar]
- Wright J. K., Schwarz H., Straub E., Overath P., Bieseler B., Beyreuther K. Lactose carrier protein of Escherichia coli. Reconstitution of galactoside binding and countertransport. Eur J Biochem. 1982 Jun;124(3):545–552. doi: 10.1111/j.1432-1033.1982.tb06628.x. [DOI] [PubMed] [Google Scholar]
- Yang T. Y., Jurtshuk P., Jr Studies on the red oxidase (cytochrome o) of Azotobacter vinelandii. Biochem Biophys Res Commun. 1978 Apr 14;81(3):1032–1039. doi: 10.1016/0006-291x(78)91454-7. [DOI] [PubMed] [Google Scholar]


