Abstract
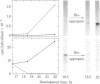
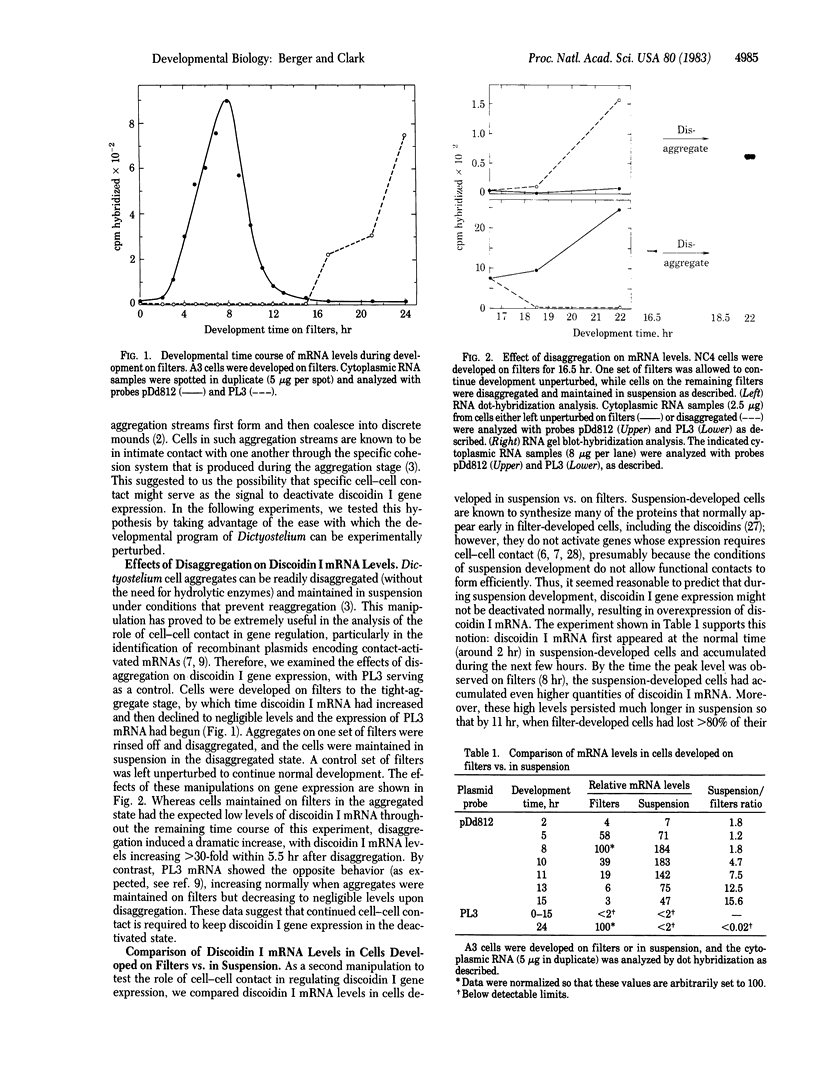
Specific cell-cell contact is a major regulatory signal controlling cell differentiation in Dictyostelium discoideum, causing dramatic changes in the developmental program of gene expression. In this report, we focus on the relationships between specific cell-cell contact and the activity of the genes for discoidin I, an endogenous lectin that has been implicated in the cell-cell cohesion process. By performing quantitative RNA dot-hybridization assays and RNA gel blot-hybridization analyses, using as a probe a recombinant plasmid containing a discoidin I cDNA insert, we have measured changes in discoiding I mRNA levels during normal development and in response to specific manipulations of the state of cellular aggregation. Our major findings are as follows. (i) During normal development on filters, there is a close temporal correspondence between the establishment of specific cell-cell contacts and the decline in discoidin I mRNA levels. By the tight-aggregate stage, discoidin I mRNA is barely detectable. (ii) When tight aggregates are disaggregated and the cells are maintained in the disaggregated state, there is a dramatic rise in discoidin I mRNA content. (iii) When cells are developed in suspension (conditions that interfere with the establishment of tight cell-cell contacts), discoidin I mRNA accumulates to abnormally high levels, and these persist well after the levels in filter-developed cells have declined. Taken together, these results strongly suggest that cell-cell contact is the normal developmental signal to deactivate discoidin I gene expression; thus, a contact-deactivated gene for which a recombinant DNA probe is available has now been identified. Furthermore, we demonstrate that exogenous cAMP almost completely blocks the disaggregation-induced reactivation of discoidin I gene expression. Possible mechanistic relationships between specific cell-cell contact, intracellular cAMP levels, and developmental gene expression are discussed.
Keywords: cell-cell recognition, endogenous lectin, mRNA levels, developmental manipulations, cAMP
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton T. H., Lodish H. F. Synthesis of developmentally regulated proteins in Dictyostelium discoideum which are dependent on continued cell-cell interaction. Dev Biol. 1977 Oct 1;60(1):207–216. doi: 10.1016/0012-1606(77)90119-1. [DOI] [PubMed] [Google Scholar]
- Bartles J. R., Frazier W. A., Rosen S. D. Slime mold lectins. Int Rev Cytol. 1982;75:61–99. doi: 10.1016/s0074-7696(08)61002-5. [DOI] [PubMed] [Google Scholar]
- Blumberg D. D., Lodish H. F. Complexity of nuclear and polysomal RNAs in growing Dictyostelium discoideum cells. Dev Biol. 1980 Aug;78(2):268–284. doi: 10.1016/0012-1606(80)90336-x. [DOI] [PubMed] [Google Scholar]
- Blumberg D. D., Margolskee J. P., Barklis E., Chung S. N., Cohen N. S., Lodish H. F. Specific cell-cell contacts are essential for induction of gene expression during differentiation of Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1982 Jan;79(1):127–131. doi: 10.1073/pnas.79.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S., Landfear S. M., Blumberg D. D., Cohen N. S., Lodish H. F. Synthesis and stability of developmentally regulated dictyostelium mRNAs are affected by cell--cell contact and cAMP. Cell. 1981 Jun;24(3):785–797. doi: 10.1016/0092-8674(81)90104-5. [DOI] [PubMed] [Google Scholar]
- Devine J. M., Tsang A. S., Williams J. G. Differential expression of the members of the discoidin I multigene family during growth and development of Dictyostelium discoideum. Cell. 1982 Apr;28(4):793–800. doi: 10.1016/0092-8674(82)90058-7. [DOI] [PubMed] [Google Scholar]
- Devine J. M., Williams J. G. Characterization of sequence elements at the 5' end of a discoidin I gene isolated from Dictyostelium discoideum. Nucleic Acids Res. 1982 Feb 25;10(4):1231–1241. doi: 10.1093/nar/10.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier W. A., Rosen S. D., Reitherman R. W., Barondes S. H. Purification and comparison of two developmentally regulated lectins from Dictyostelium discoideum. Discoidin I and II. J Biol Chem. 1975 Oct 10;250(19):7714–7721. [PubMed] [Google Scholar]
- Frazier W., Glaser L. Surface components and cell recognition. Annu Rev Biochem. 1979;48:491–523. doi: 10.1146/annurev.bi.48.070179.002423. [DOI] [PubMed] [Google Scholar]
- Gerisch G. Univalent antibody fragments as tools for the analysis of cell interactions in Dictyostelium. Curr Top Dev Biol. 1980;14(Pt 2):243–270. doi: 10.1016/s0070-2153(08)60197-0. [DOI] [PubMed] [Google Scholar]
- Henderson E. J. The cyclic adenosine 3':5'-monophosphate receptor of Dictyostelium discoideum. Binding characteristics of aggregation-competent cells and variation of binding levels during the life cycle. J Biol Chem. 1975 Jun 25;250(12):4730–4736. [PubMed] [Google Scholar]
- Kaleko M., Rothman F. G. Membrane sites regulating developmental gene expression in Dictyostelium discoideum. Cell. 1982 Apr;28(4):801–811. doi: 10.1016/0092-8674(82)90059-9. [DOI] [PubMed] [Google Scholar]
- Landfear S. M., Lodish H. F. A role for cyclic AMP in expression of developmentally regulated genes in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1044–1048. doi: 10.1073/pnas.77.2.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G. C., Firtel R. A. Regulation of the synthesis of two carbohydrate-binding proteins in Dictyostelium discoideum. J Biol Chem. 1978 Jun 10;253(11):3924–3932. [PubMed] [Google Scholar]
- Mangiarotti G., Lefebvre P., Lodish H. F. Differences in the stability of developmentally regulated mRNAs in aggregated and disaggregated Dictyostelium discoideum cells. Dev Biol. 1982 Jan;89(1):82–91. doi: 10.1016/0012-1606(82)90296-2. [DOI] [PubMed] [Google Scholar]
- Newell P. C., Franke J., Sussman M. Regulation of four functionally related enzymes during shifts in the developmental program of Dictyostelium discoideum. J Mol Biol. 1972 Feb 14;63(3):373–382. doi: 10.1016/0022-2836(72)90434-2. [DOI] [PubMed] [Google Scholar]
- Okamoto K. Induction of cyclic AMP phosphodiesterase by disaggregation of the multicellular complexes of Dictyostelium discoideum. Eur J Biochem. 1979 Jan 15;93(2):221–227. doi: 10.1111/j.1432-1033.1979.tb12814.x. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Takeuchi I. Changes in activities of two developmentally regulated enzymes induced by disaggregation of the pseudoplasmodia of Dictyostelium discoideum. Biochem Biophys Res Commun. 1976 Sep 20;72(2):739–746. doi: 10.1016/s0006-291x(76)80101-5. [DOI] [PubMed] [Google Scholar]
- Parish R. W. Cyclic AMP induces the synthesis of developmentally regulated plasma membrane proteins in Dictyostelium. Biochim Biophys Acta. 1979 May 3;553(1):179–182. doi: 10.1016/0005-2736(79)90040-3. [DOI] [PubMed] [Google Scholar]
- Poole S., Firtel R. A., Lamar E., Rowekamp W. Sequence and expression of the discoidin I gene family in Dictyostelium discoideum. J Mol Biol. 1981 Dec 5;153(2):273–289. doi: 10.1016/0022-2836(81)90278-3. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Biochemical properties of hormone-sensitive adenylate cyclase. Annu Rev Biochem. 1980;49:533–564. doi: 10.1146/annurev.bi.49.070180.002533. [DOI] [PubMed] [Google Scholar]
- Rowekamp W., Poole S., Firtel R. A. Analysis of the multigene family coding the developmentally regulated carbohydrate-binding protein discoidin-I in D. discoideum. Cell. 1980 Jun;20(2):495–505. doi: 10.1016/0092-8674(80)90636-4. [DOI] [PubMed] [Google Scholar]
- Springer W. R., Barondes S. H. Evidence for another cell-adhesion molecule in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6561–6565. doi: 10.1073/pnas.79.21.6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto S., Okamoto K., Takeuchi I. The effects of cyclic AMP on disaggregation-induced changes in activities of developmentally regulated enzymes in Dictyostelium discoideum. Biochem Biophys Res Commun. 1978 Feb 28;80(4):858–865. doi: 10.1016/0006-291x(78)91323-2. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town C., Gross J. The role of cyclic nucleotides and cell agglomeration in postaggregative enzyme synthesis in Dictyostelium discoideum. Dev Biol. 1978 Apr;63(2):412–420. doi: 10.1016/0012-1606(78)90145-8. [DOI] [PubMed] [Google Scholar]
- Tsang A. S., Devine J. M., Williams J. G. The multiple subunits of discoidin I are encoded by different genes. Dev Biol. 1981 May;84(1):212–217. doi: 10.1016/0012-1606(81)90385-7. [DOI] [PubMed] [Google Scholar]
- Wieben E. D. Regulation of the synthesis of lactate dehydrogenase-X during spermatogenesis in the mouse. J Cell Biol. 1981 Mar;88(3):492–498. doi: 10.1083/jcb.88.3.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. G., Lloyd M. M., Devine J. M. Characterization and transcription analysis of a cloned sequence derived from a major developmentally regulated mRNA of D. discoideum. Cell. 1979 Aug;17(4):903–913. doi: 10.1016/0092-8674(79)90330-1. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Tsang A. S., Mahbubani H. A change in the rate of transcription of a eukaryotic gene in response to cyclic AMP. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7171–7175. doi: 10.1073/pnas.77.12.7171. [DOI] [PMC free article] [PubMed] [Google Scholar]