Abstract
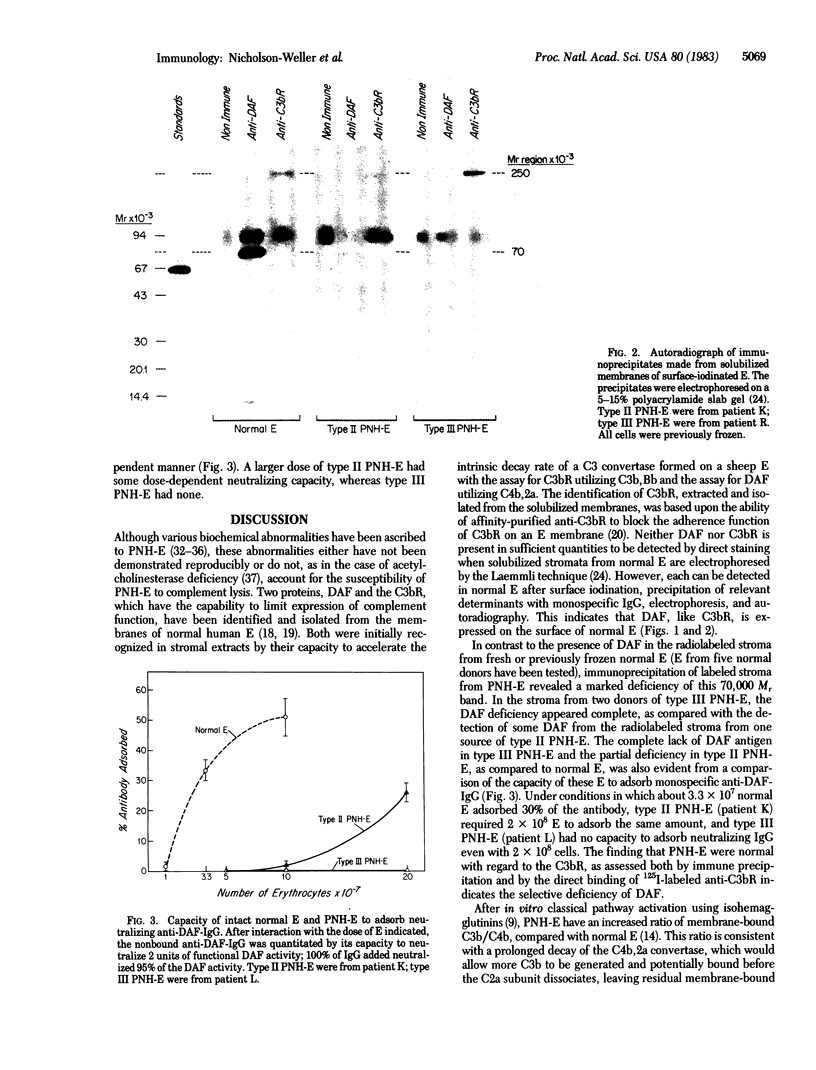
Paroxysmal nocturnal hemoglobinuria (PNH) is an acquired defect of bone marrow stem cells in which the affected clones produce erythrocytes (also granulocytes and platelets) with membranes that are abnormally sensitive to complement-mediated lysis. Abnormal erythrocytes (E) from patients with PNH (PNH-E) are 3-5 times more sensitive (type II PNH-E) or 15-25 times more sensitive (type III PNH-E) to lysis in vitro by human complement than normal E from unaffected individuals and the functionally normal E that arise from unaffected clones and the functionally normal E that arise from unaffected clones in PNH patients (type I PNH-E). After complement activation by either the classical or alternative pathway, abnormal amounts of C3b are deposited on the membranes of PNH-E compared with normal E, suggesting that the PNH-E membrane cannot regulate the events responsible for C3b deposition. Two proteins that decrease the stability of the classical and alternative pathway C3 convertases on target cells have been isolated from normal human E stroma: the 70,000 Mr decay accelerating factor of stroma (DAF) and the 250,000 Mr C3b receptor (C3bR). Specific immune precipitates of solubilized membranes from 125I-surface-labeled normal E demonstrate both proteins. In contrast, specific immune precipitates of PNH-E from three patients show C3bR but are deficient in DAF; type II PNH-E are relatively deficient and type III PNH-E are totally deficient in DAF. Antibody that neutralizes the activity of isolated DAF is adsorbed by intact normal E under conditions in which it is weakly adsorbed by type II PNH-E and not adsorbed by type III PNH-E. The deficiency of DAF antigen in PNH-E, as assessed by lack of immunoprecipitation and antibody adsorption, could explain the abnormal sensitivity of PNH-E to complement-mediated lysis and suggests that DAF may protect the membranes of normal E from damage resulting from autologous complement activation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aster R. H., Enright S. E. A platelet and granulocyte membrane defect in paroxysmal nocturnal hemoglobinuria: usefulness for the detection of platelet antibodies. J Clin Invest. 1969 Jul;48(7):1199–1210. doi: 10.1172/JCI106084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas S. J., Shapiro B., Green J. W. Surface properties of erythrocytes: normal, paroxysmal nocturnal hemoglobinuria and glutathione-treated cells. Biochim Biophys Acta. 1973 Oct 11;323(2):194–206. doi: 10.1016/0005-2736(73)90144-2. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- CROSBY W. H. Paroxysmal nocturnal hemoglobinuria; a classic description by Paul Strübling in 1882, and a bibliography of the disease. Blood. 1951 Mar;6(3):270–284. [PubMed] [Google Scholar]
- Chua C., Hoffmann E. M., Adams J. P., Rosse W. F. Inhibitors of complement derived from the erythrocyte membrane in paroxysmal nocturnal hemoglobinuria. Blood. 1980 May;55(5):772–776. [PubMed] [Google Scholar]
- Cotmore S. F., Furthmayr H., Marchesi V. T. Immunochemical evidence for the transmembrane orientation of glycophorin A. Localization of ferritin-antibody conjugates in intact cells. J Mol Biol. 1977 Jul 5;113(3):539–553. doi: 10.1016/0022-2836(77)90237-6. [DOI] [PubMed] [Google Scholar]
- Daha M. R., Fearon D. T., Austen K. F. C3 requirements for formation of alternative pathway C5 convertase. J Immunol. 1976 Aug;117(2):630–634. [PubMed] [Google Scholar]
- Dalmasso A. P., Pizzimenti M. C., Vacs E., Diaz A. Abnormal solubilization by triton X-100 of erythrocyte membranes from patients with paroxysmal nocturnal hemoglobinuria. Proc Soc Exp Biol Med. 1974 Oct;147(1):273–279. doi: 10.3181/00379727-147-38325. [DOI] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F., Ruddy S. Formation of a hemolytically active cellular intermediate by the interaction between properdin factors B and D and the activated third component of complement. J Exp Med. 1973 Dec 1;138(6):1305–1313. doi: 10.1084/jem.138.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J Exp Med. 1980 Jul 1;152(1):20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Regulation of the amplification C3 convertase of human complement by an inhibitory protein isolated from human erythrocyte membrane. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5867–5871. doi: 10.1073/pnas.76.11.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Gigli I., Nussenzweig V. Human C4-binding protein. II. Role in proteolysis of C4b by C3b-inactivator. J Exp Med. 1978 Oct 1;148(4):1044–1051. doi: 10.1084/jem.148.4.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furthmayr H., Marchesi V. T. Subunit structure of human erythrocyte glycophorin A. Biochemistry. 1976 Mar 9;15(5):1137–1144. doi: 10.1021/bi00650a028. [DOI] [PubMed] [Google Scholar]
- Gockerman J. P. The lipids of the erythrocyte in paroxysmal nocturnal hemoglobinuria. Am J Hematol. 1978;5(4):323–333. doi: 10.1002/ajh.2830050407. [DOI] [PubMed] [Google Scholar]
- Goldlust M. B., Shin H. S., Hammer C. H., Mayer M. M. Studies of complement complex C5b,6 eluted from--EAC-6: reaction of C5b,6 with EAC4b,3b and evidence on the role of C2a and C3b in the activation of C5. J Immunol. 1974 Sep;113(3):998–1007. [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. Paroxysmal nocturnal hemoglobinuria. Hemolysis initiated by the C3 activator system. N Engl J Med. 1972 Jan 27;286(4):180–184. doi: 10.1056/NEJM197201272860403. [DOI] [PubMed] [Google Scholar]
- HINZ C. F., Jr, JORDAN W. S., Jr, PILLEMER L. The properdin system and immunity. IV. The hemolysis of erythrocytes from patients with paroxysmal nocturnal hemoglobinuria. J Clin Invest. 1956 May;35(5):453–457. doi: 10.1172/JCI103296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham T. H., Dingle J. H. STUDIES ON DESTRUCTION OF RED BLOOD CELLS. II. CHRONIC HEMOLYTIC ANEMIA WITH PAROXYSMAL NOCTURNAL HEMOGLOBINURIA: CERTAIN IMMUNOLOGICAL ASPECTS OF THE HEMOLYTIC MECHANISM WITH SPECIAL REFERENCE TO SERUM COMPLEMENT. J Clin Invest. 1939 Nov;18(6):657–672. doi: 10.1172/JCI101081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer C. H., Abramovitz A. S., Mayer M. M. A new activity of complement component C3: cell-bound C3b potentiates lysis of erythrocytes by C5b,6 and terminal components. J Immunol. 1976 Sep;117(3):830–834. [PubMed] [Google Scholar]
- Hänsch G. M., Hammer C. H., Mayer M. M., Shin M. L. Activation of the fifth and sixth component of the complement system: similarities between C5b6 and C(56)a with respect to lytic enhancement by cell-bound C3b or A2C, and species preferences of target cell. J Immunol. 1981 Sep;127(3):999–1002. [PubMed] [Google Scholar]
- Hänsch G., Hammer C., Jiji R., Rother U., Shin M. Lysis of paroxysmal nocturnal hemoglobinuria erythrocytes by acid-activated serum. Immunobiology. 1983 Mar;164(2):118–126. doi: 10.1016/S0171-2985(83)80003-5. [DOI] [PubMed] [Google Scholar]
- Jones C. M., Shin M. L., Mayer M. M. On the lysis of paroxysmal nocturnal hemoglobinuria erythrocytes by complement: dual role of C3b. Blut. 1982 Oct;45(4):249–259. doi: 10.1007/BF00320192. [DOI] [PubMed] [Google Scholar]
- Kotsifopoulos P. N. Red cell membrane proteins abnormality in paroxysmal nocturnal hemoglobinuria and in in vitro induced PNH-like erythrocytes. Acta Haematol. 1976;56(6):328–333. doi: 10.1159/000207958. [DOI] [PubMed] [Google Scholar]
- Kunstling T. R., Rosse W. F. Erythrocyte acetylcholinesterase deficiency in paroxysmal nocturnal hemoglobinuria (PNH). A comparison of the complement-sensitive and insensitive populations. Blood. 1969 Apr;33(4):607–616. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Andrews E. P. Glycoproteins: isolation from cellmembranes with lithium diiodosalicylate. Science. 1971 Dec 17;174(4015):1247–1248. doi: 10.1126/science.174.4015.1247. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- Meryman H. T., Hornblower M. A method for freezing and washing red blood cells using a high glycerol concentration. Transfusion. 1972 May-Jun;12(3):145–156. doi: 10.1111/j.1537-2995.1972.tb00001.x. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Polley M. J., Calcott M. A. Formation and functional significance of a molecular complex derived from the second and the fourth component of human complement. J Exp Med. 1967 Feb 1;125(2):359–380. doi: 10.1084/jem.125.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa S., Stroud R. M. Mechanism of action of the C3b inactivator: requirement for a high molecular weight cofactor (C3b-C4bINA cofactor) and production of a new C3b derivative (C3b'). Immunochemistry. 1977 Nov-Dec;14(11-12):749–756. doi: 10.1016/0019-2791(77)90345-7. [DOI] [PubMed] [Google Scholar]
- Nicholson-Weller A., Burge J., Austen K. F. Purification from guinea pig erythrocyte stroma of a decay-accelerating factor for the classical c3 convertase, C4b,2a. J Immunol. 1981 Nov;127(5):2035–2039. [PubMed] [Google Scholar]
- Nicholson-Weller A., Burge J., Fearon D. T., Weller P. F., Austen K. F. Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertases of the complement system. J Immunol. 1982 Jul;129(1):184–189. [PubMed] [Google Scholar]
- Nicholson A., Brade V., Lee G. D., Shin H. S., Mayer M. M. Kinetic studies of the formation of the properdin system enzymes on zymosan: evidence that nascent C3b controls the rate of assembly. J Immunol. 1974 Mar;112(3):1115–1123. [PubMed] [Google Scholar]
- Packman C. H., Rosenfeld S. I., Jenkins D. E., Jr, Thiem P. A., Leddy J. P. Complement lysis of human erythrocytes. Differeing susceptibility of two types of paroxysmal nocturnal hemoglobinuria cells to C5b-9. J Clin Invest. 1979 Aug;64(2):428–433. doi: 10.1172/JCI109479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J Exp Med. 1977 Jul 1;146(1):257–270. doi: 10.1084/jem.146.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. J., Baker P. J., Rosse W. F. Increased enzymatic activity of the alternative pathway convertase when bound to the erythrocytes of paroxysmal nocturnal hemoglobinuria. J Clin Invest. 1982 Feb;69(2):337–346. doi: 10.1172/JCI110457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righetti P. G., Perrella M., Zanella A., Sirchia G. The membrane abnormality of the red cell in paroxysmal nocturnal haemoglobinuria. Nat New Biol. 1973 Oct 31;245(148):273–276. doi: 10.1038/newbio245273a0. [DOI] [PubMed] [Google Scholar]
- Rosse W. F., Dacie J. V. Immune lysis of normal human and paroxysmal nocturnal hemoglobinuria (PNH) red blood cells. I. The sensitivity of PNH red cells to lysis by complement and specific antibody. J Clin Invest. 1966 May;45(5):736–748. doi: 10.1172/JCI105388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse W. F., Dacie J. V. Immune lysis of normal human and paroxysmal nocturnal hemoglobinuria (PNH) red blood cells. II. The role of complement components in the increased sensitivity of PNH red cells to immune lysis. J Clin Invest. 1966 May;45(5):749–757. doi: 10.1172/JCI105389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse W. F., Logue G. L., Adams J., Crookston J. H. Mechanisms of immune lysis of the red cells in hereditary erythroblastic multinuclearity with a positive acidified serum test and paroxysmal nocturnal hemoglobinuria. J Clin Invest. 1974 Jan;53(1):31–43. doi: 10.1172/JCI107551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse W. F. Variations in the red cells in paroxysmal nocturnal haemoglobinuria. Br J Haematol. 1973 Mar;24(3):327–342. doi: 10.1111/j.1365-2141.1973.tb01657.x. [DOI] [PubMed] [Google Scholar]
- Weiler J. M., Daha M. R., Austen K. F., Fearon D. T. Control of the amplification convertase of complement by the plasma protein beta1H. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3268–3272. doi: 10.1073/pnas.73.9.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaley K., Ruddy S. Modulation of the alternative complement pathways by beta 1 H globulin. J Exp Med. 1976 Nov 2;144(5):1147–1163. doi: 10.1084/jem.144.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. G., Wong W. W., Schur P. H., Fearon D. T. Mode of inheritance of decreased C3b receptors on erythrocytes of patients with systemic lupus erythematosus. N Engl J Med. 1982 Oct 14;307(16):981–986. doi: 10.1056/NEJM198210143071604. [DOI] [PubMed] [Google Scholar]
- YACHNIN S., RUTHENBERG J. M. THE INITIATION AND ENHANCEMENT OF HUMAN RED CELL LYSIS BY ACTIVATORS OF THE FIRST COMPONENT OF COMPLEMENT AND BY FIRST COMPONENT ESTERASE; STUDIES USING NORMAL RED CELLS AND RED CELLS FROM PATIENTS WITH PAROXYSMAL NOCTURNAL HEMOGLOBINURIA. J Clin Invest. 1965 Apr;44:518–534. doi: 10.1172/JCI105165. [DOI] [PMC free article] [PubMed] [Google Scholar]