Abstract
Study Objective
Short sleep episodes are common in modern society. We recently demonstrated that short nights reduce phase advances to light. Here we show that short nights also reduce phase delays to light.
Design
Two weeks of 6-hour sleep episodes in the dark (short nights) and 2 weeks of long 9-hour sleep episodes (long nights) in counterbalanced order, separated by 7 days. Following each series of nights, there was a dim-light phase assessment to assess baseline phase. Three days later, subjects were exposed to a phase-delaying light stimulus for 2 days, followed by a final phase assessment.
Setting
Subjects slept at home in dark bedrooms but came to the laboratory for the phase assessments and light stimulus.
Participants
Seven young healthy subjects.
Interventions
The 3.5-hour light stimulus was four 30-minute pulses of bright light (∼5000 lux) separated by 30-minute intervals of room light. The stimulus began 2.5 hours after each subject's dim-light melatonin onset, followed by a 6- or 9-hour sleep episode. On the second night, the bright light and sleep episode began 1 hour later.
Measurements and Results
The dim-light melatonin onset and dim-light melatonin offset phase delayed 1.4 and 0.7 hours less in the short nights, respectively (both p ≤ .015).
Conclusions
These results indicate for the first time that short nights can reduce circadian phase delays, that long nights can increase phase delays to light, or both. People who curtail their sleep may inadvertently reduce their circadian responsiveness to evening light.
Keywords: Circadian, melatonin, phase response curve, phase shift, photoperiod, sleep deprivation
It is well recognized that much of modern society is experiencing short sleep episodes. The results of a recent national sleep foundation poll suggest that 31% of Americans regularly sleep 6 hours or less each weeknight and 17% of Americans do the same on weekends.1 Because we sleep with our eyes closed and are usually exposed to artificial or natural light when awake, the trend for short sleep durations is producing short night lengths and thus long perceived photoperiods.
Many studies in nonhuman animals have shown that night length (photoperiod) can greatly impact circadian phase shifts to light. In 1984, Pittendrigh and colleagues2 used the wheel-running activity patterns of free-running Syrian hamsters to generate phase response curves to 15 minute pulses of light. They found that the phase response curve to light was greatly reduced in both amplitude and range in hamsters who had previously experienced short nights (long photoperiod, light-dark (LD) 18:6) compared with long nights (short photoperiod, LD 10:14). Evans et al3 have since reported similar findings. In 1985, Illnerova and Vanecek4 exposed rats to a history of short nights (LD 18:6) or long nights (LD 6:18) and then measured phase shifts in the pineal N-acetyltransferase rhythm to 1-minute light pulses. They found that both phase advances and phase delays were attenuated after the short nights. More recently, it has been reported that phase delays in the mouse activity rhythm in response to light pulses progressively decrease in magnitude as prior night length decreases (LD 8:16 to 12:12 to 16:8).5
To determine if the effect of photoperiod on circadian phase shifts to light are similar in humans, we recently examined phase advances in humans who experienced 2 weeks of short (6-hour) nights and also 2 weeks of long (9-hour) nights. We found for the first time that, just as in other animals, humans phase advanced significantly less during short nights than during long nights.6 Here we report on a similar experiment in humans that showed that phase delays to bright light are also reduced after a series of short nights.
Methods
Subjects
Seven healthy volunteers (5 men, 2 women, mean age 30.4 years; mean ± SD: body mass index 22.7 ± 2.8 kg/m2) participated. All subjects were nonsmokers, medication free, consumed only moderate caffeine doses (<300 mg/day) and reported no medical, psychiatric, or sleep disorders, as assessed from in-person interviews and several screening questionnaires (Minnesota Multiphasic Personality Inventory-2,7 Beck Depression Inventory,8 Pittsburgh Sleep Quality Index,9 and part of a general health questionnaire).10 A urine drug screen confirmed that all subjects were free of common drugs of abuse. No subject was color blind, as determined by the Ishihara test. No subject had worked night shifts or flown across more than 2 time zones in the previous month. The self-reported mean (± SD) weekday sleep schedule in the week before the study was 00:03 ± 1.1 hours to 7:58 ± 1.0 hours and on the weekend it was 00:13 ± 1.0 hours to 8:16 ± 0.9 hours. In the 5 weekdays before starting the study, average bedtimes ranged between 10:20 pm and 1:32 am, and average wake times ranged between 6:40 am and 9:51 am. On the weekend, average bedtimes ranged between 10:30 pm and 1:30 am, and wake times ranged between 7:30 am and 9:43 am. Morningness-eveningness prior to the start of the study was assessed,11 and there was 1 moderate morning, 5 neither, and 1 moderate evening type. The protocol was approved by the Rush University Medical Center Institutional Review Board. All subjects gave written informed consent prior to participation.
Protocol
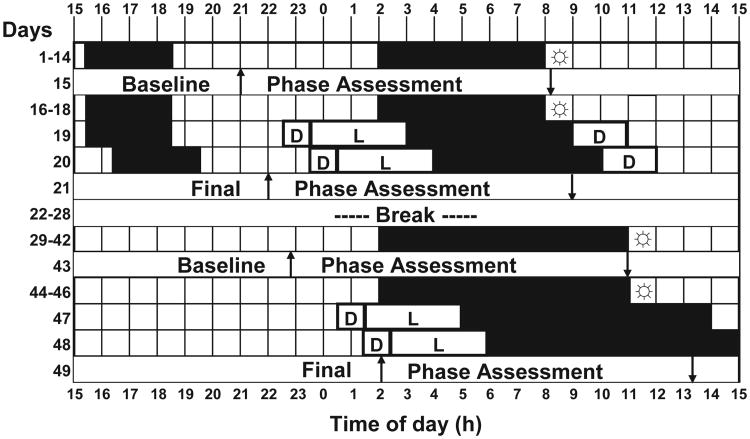
As part of a within-subjects repeated-measures design, each subject experienced 14 six-hour nights and 14 nine-hour nights (Figure 1), except for 1 subject who experienced only 12 nine-hour nights. Each series of nights was followed by a phase assessment, 3 more days on 6- or 9-hour nights, a 2-day phase-delaying bright-light stimulus, and then another phase assessment. There was a 7-day break between the short- and long-night conditions, during which subjects returned to their prestudy sleep times. Four subjects completed the short nights first, and 3 subjects completed the long nights first.
Figure 1.
An experimental protocol for an individual subject who experienced the short nights first. Black shading indicates the sleep/dark episodes at night (6 or 9 hours) and optional 3-hour nap zone in the afternoon during the short-nights condition. L refers to delaying bright-light stimulus: four 30-minute bright-light pulses alternating with ordinary room light, starting 2.5 hours after the baseline dimlight melatonin onset and delaying by 1 hour on the second night; D, dim light (< 60 lux) in the laboratory; ↑, the time of dim-light melatonin onset; ↓, the time of dim-light melatonin offset;
 = at least 10 minutes of outdoor light. For clarity, the phase assessments are shown as starting and ending at 15:00.
= at least 10 minutes of outdoor light. For clarity, the phase assessments are shown as starting and ending at 15:00.
Sleep
All subjects slept at home except during the 2 days of the delaying bright-light stimulus when they slept in the laboratory. At home, they slept in dark bedrooms (< 1 lux, bedroom windows were covered with black plastic), and the laboratory bedrooms were also completely dark. During the short nights, subjects woke at their habitual weekday wake time and went to bed 6 hours before wake time. During the long nights, subjects woke 3 hours later than their habitual weekday wake time and went to bed at the same time as on the short nights. During their scheduled dark-sleep episodes, the subjects were instructed to lie in bed and try to sleep. All subjects slept alone. They were not permitted to read, watch TV, listen to music, or talk on the telephone at this time. Napping was also permitted during the short nights, within a 3-hour window, centered 12 hours from the midpoint of the nighttime dark period. Subjects were required to try and nap for at least 1 hour on at least 9 of the 19 days with short nights. It is unlikely that these naps significantly altered circadian phase because they occurred at a time when neither light nor dark phase shift circadian rhythms.12,13 Even if the naps did slightly alter baseline circadian phase, the bright-light stimulus began at the same circadian phase in each condition (see below).
When they slept at home, the subjects were required to go outside to receive a minimum of 10 minutes of light in the first 1.5 hours after their scheduled wake time, to mimic the morning light that many people receive every day. To ensure compliance with the study requirements, all subjects were required to call the lab voice mail (time and date of call was recorded) before turning out their lights at night and at their wake time in the morning each day. Subjects also wore a wrist actigraphy monitor with photosensor (Actiwatch-L, Mini-Mitter, Bend, OR) on their nondominant wrist and around their necks as a medallion during the entire study. This equipment recorded activity and light exposure every minute. Subjects also completed daily sleep logs, noting lights-off time at night, estimated sleep-onset time, any awakenings during the night, time of final awakening, and lights-on time in the morning. These times were verified with the wrist actigraphy and photosensor recordings.
Because there are safety issues associated with sleep depriving subjects outside of the laboratory,14 subjects were provided with the emergency contact phone numbers of both investigators. Subjects came to the laboratory every 1 to 3 days to meet with HJB, and the sleep logs and activity data from the wrist monitor and light data from the medallion were inspected. Subjects were also questioned about adverse events at these times, especially any related to sleepiness during the short nights. All subjects were strongly reminded to try and nap every day and to spend more time in bed during the 3-hour nap window. Some subjects napped in the laboratory, as this was more convenient for them. Not a single subject reported an adverse event.
Phase Assessment
Each subject experienced 4 dim-light phase assessments to determine their endogenous melatonin profiles (Figure 1). Melatonin is a hormone synthesized and released from the pineal gland15 and, in dim light, is a reliable marker of the circadian clock.16,17 The phase assessments were 20 to 24 hours long and began between 4:00 pm and 9:00 pm, depending on the condition. Subjects were reimbursed for a taxi cab ride home and so never drove themselves home. During the phase assessments, subjects remained awake and seated in recliners in dim light (< 5 lux, at the level of the subjects' eyes, in the direction of gaze, Minolta TL-1 light meter, Ramsey, NJ). Subjects gave a saliva sample every 30 minutes using Salivettes (Sarstedt, Newton, NC). Subjects were not permitted to consume any alcohol or caffeine in the 48 hours prior to the start of a phase assessment and were breathalyzed on arrival. Nonsteroidal anti-inflammatory drugs were not permitted during the entire study, as these drugs have been shown to suppress melatonin.18 Toothpaste or mouthwash was not allowed during the phase assessments. Small snacks and fluids were permitted, except in the 10 minutes before each sample, and subjects were required to rinse and brush their teeth with water while remaining seated 10 minutes before each sample if they had consumed food or drink. The samples were later radioimmunoassayed (single samples) for melatonin by Pharmasan Laboratories (Osceola, WI). The sensitivity of the assay was 0.7 pg/mL, and intraassay and interassay coefficient of variances were 12.1% and 13.2%, respectively.
Bright-Light Stimulus
During the delaying bright-light stimulus, each subject was exposed to bright intermittent light spanning 3.5 hours (mean intensity 5171 ± 671 lux), 30 minutes alternating with 30 minutes ordinary, dim room light < 60 lux (measured periodically at angle of gaze with Extech 401025 light meter, Waltham, MA). The bright light was produced by a single light box (61 × 61 × 10 cm, Enviro-Med, Vancouver, WA) placed on a desk about 40 cm in front of the subject's eyes. Each light box had a diffuser screen and contained four 54-cm long 40-watt fluorescent horizontal tubes (Philips PL-L40W/41/RS/IS, 4100K). The room light was produced by a ceiling fixture containing 3 fluorescent tubes. On the first night, the bright light began 2.5 hours after each subject's baseline dim-light melatonin onset (DLMO) (Figure 1). Immediately following the bright-light stimulus, subjects were put to bed in a dark individual temperature-controlled bedroom and remained in the dark for 6 or 9 hours, depending on the condition. Subjects arrived at the laboratory 1 hour before the bright-light exposure began and were permitted to leave the laboratory at the end of the 9-hour sleep episodes and 2 hours after the end of the 6-hour sleep episodes. In this way, each subject's light exposure was controlled for at least the first 14 hours after their DLMO. On the second night of the bright-light stimulus, subjects experienced the same procedure, except the timing of their sleep and bright-light exposure was 1 hour later. Following each 2-day delaying bright-light stimulus, subjects had a final phase assessment.
Data Analysis
Three phase markers were derived from each melatonin profile, the dim-light melatonin onset, dim-light melatonin offset (DL-MOff), and the midpoint, halfway in time between the DLMO and DLMOff. For each subject's melatonin profile, a threshold was calculated as the mean of 3 low consecutive daytime values plus twice the standard deviation.19 Each subject's DLMO was the point in time (as determined with linear interpolation) when the melatonin concentration exceeded the threshold. The DLMOff was the point in time when melatonin levels fell below the threshold. The mean (± SD) threshold was 1.5 ± 0.5 pg/mL. Total sleep time was calculated from the sleep logs as the time from sleep onset to final wake time minus any awakenings (> 5 minutes) during the night, plus any naps taken during the day.
Statistical Analysis
The phase delays in the DLMO, midpoint, and DLMOff were each analyzed with a 2-way univariate analysis of variance with a within-subjects factor NIGHT (short vs long) and a between-subjects factor ORDER (short nights first vs long nights first). A Bonferroni correction resulted in statistical significance occurring at p < .017. Differences in phase shifts in the DLMO versus DL-MOff and total sleep time in the short and long nights were compared with paired t-tests. Statistical significance of the t-tests was determined at p < .05. Data are presented as means ± SDs unless otherwise specified.
Results
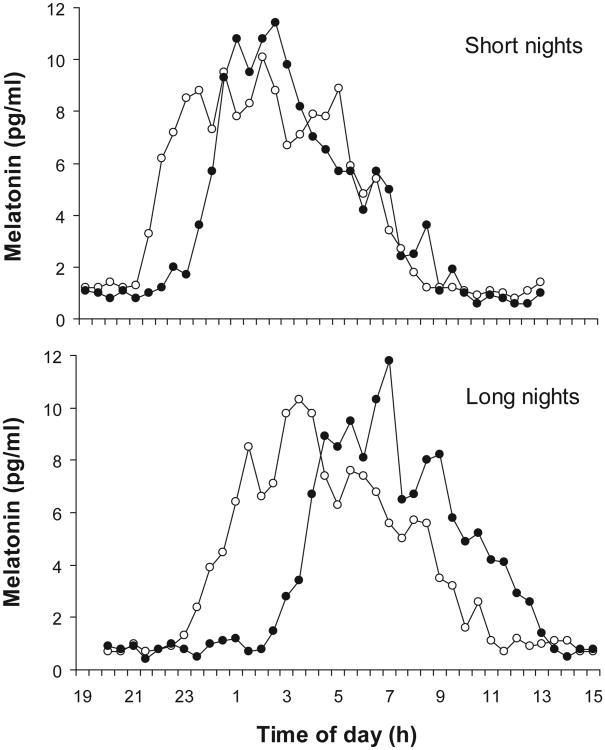
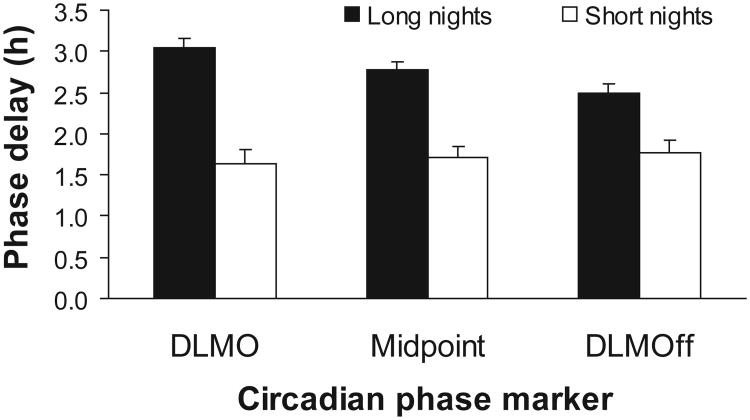
Dim-light melatonin profiles from each of the 4 phase assessments for an individual subject are shown in Figure 2. The melatonin rhythm phase delayed in response to the bright-light stimulus during both short and long nights, but the delay was markedly attenuated during the short nights. The same pattern can be seen in the average phase delays of all 7 subjects (Figure 3). The analyses of variance revealed a significant main effect of NIGHT for the DLMO, midpoint, and DLMOff (F1,5 = 16.57, p = .01; F1,5 = 32.59, p = .002; F1,5 = 13.12, p = .015, respectively), such that all 3 circadian markers phase delayed significantly less during the short nights. There were no significant main effects for ORDER or any significant interaction between ORDER and NIGHT (all p ≥ .10).
Figure 2.
An individual subject's melatonin profiles during the baseline (open circles) and final phase assessment (closed circles) during the short and long nights. The melatonin levels were determined from saliva samples collected every 30 minutes in dim light. The protocol for this individual is shown in Figure 1.
Figure 3.
The mean phase delays (N = 7) observed in the dim-light melatonin onset (DLMO), midpoint, and dim-light melatonin offset (DLMOff) due to the delaying bright-light stimulus during the long and short nights. Error bars represent SEM. There was a significant reduction in the phase delay in all 3 circadian phase markers during the short nights, as compared with during the long nights.
In the short nights, the phase delays in the DLMO and DL-MOff were of similar magnitude (Figure 3). However, in the long nights, 5 subjects phase delayed their DLMO more than their DLMOff, resulting in a close to significant larger average phase delay in the DLMO than in the DLMOff (paired t6 = 2.24, p = .066).
As expected, the actigraphy-verified sleep logs showed that the average hours of sleep per day (nighttime sleep plus naps) in the 14 days before the bright-light stimulus were significantly shorter in the short nights than in the long nights (6.6 ± 0.4 vs 8.3 ± 0.4 hours, paired t6 = 11.19, p < .001).
Discussion
This study is the first to show that, in humans, circadian phase delays in response to evening light markedly decrease with short nights, increase with long nights, or both. These results cannot be due to differences in the timing of the bright-light stimulus between the 2 conditions because subjects were exposed to the bright-light stimulus at the same circadian phase, starting 2.5 hours after and ending 6 hours after the baseline DLMO. Because the DLMO occurs approximately 7 hours before the core body temperature minimum, 20-22 and approximately 8 hours before the crossover point of the light phase response curve,23 we estimate that the bright-light stimulus occurred at a time that produces large phase delays. Of course, the delaying bright-light stimulus in both conditions included a delaying sleep schedule, which even without bright light can induce phase delays due to associated changes in light exposure.24-26
A possible cause of the reduction in phase delays during short nights compared with those of long nights could be the chronic sleep deprivation that occurred during the short nights. Not a single subject napped every day during the short nights, thereby greatly reducing their sleep opportunity. Consequently, during the short nights, the subjects slept, on average, 1.7 hours less per day than during the long nights. The degree of sleep deprivation in the short nights, an average of 6.6 hours per night, is equivalent to that experienced by an individual who sleeps 6 hours per night during the week and 8.1 hours per night on the weekend. To date, there has been no investigation of the effects of sleep deprivation on phase shifts to bright light in humans. Research in Syrian hamsters has indicated that, after as little as 6 hours of sleep deprivation, phase delays in response to 30 minutes of 50 lux of light decrease to less than 15% of the phase delays in non–sleep-deprived hamsters.27 Likewise, phase delays in response to a 10-minute pulse of 100 lux in mice sleep deprived for 8 hours are reduced by 30% when compared to non–sleep-deprived mice.28 These reductions in phase shifts in sleep-deprived animals may be due to alterations in serotonergic activity.27,28
Another reason for the reduced phase delays during short nights could be the associated photoperiodic history. As detailed in the introduction, a study of Syrian hamsters found that a photoperi-odic history of short nights reduced the range and amplitude of the light phase response curve.2 We have recently shown in humans that phase advances also reduce during short nights,6 although the reduction in phase advances was greater than the reduction in phase delays we observed here. Together, these results suggest that the human phase response curve to light may also be reduced during short nights. Further work is required to test this possibility.
A third possible explanation for the smaller phase delays during the short nights is the difference in the duration of total light exposure between the conditions. If subjects had napped for 3 hours every day during the short nights, then their total light exposure duration would have been approximately the same in both the short and long nights (15 hours/day). However, on average, subjects only napped 57 minutes per day, resulting in about 2 hours of additional daily light exposure during the short nights. This additional daily light exposure may have reduced the phase delays by decreasing photosensitivity. Hebert et al29 found that approximately 4 hours of bright light a day for a week compared with a week with no bright light reduced photosensitivity, as indexed by melatonin suppression to light. However, despite the 4-hour difference in exposure to bright light, only a modest effect was observed. Thus, it appears unlikely that reduced photosensitivity could fully account for the reduced phase delays during the short nights.
Finally, it is also worth noting the time of the start of the ambient morning light exposure upon waking during the short nights versus long nights in the laboratory. During the short nights, it started 12 hours after the baseline DLMO (at 9:00 am in Figure 1, day 19), whereas during the long nights, it started 15 hours after the DLMO (at about 2:00 pm on day 47). This slightly earlier ambient morning light during the short nights may have induced more of a phase advance, thereby partially counteracting the phase-delaying bright-light stimulus. However, because the ambient morning light in the laboratory was relatively dim (< 60 lux), it appears unlikely that this light could account for the observed reduction in phase delays (as seen in Figure 3). Also note that subjects were free to leave the laboratory 14 hours after the DLMO in the short nights, but they could not leave the laboratory until at least 15 hours after the DLMO in the long nights. Thus, the subjects may have received bright outdoor light at an earlier circadian phase during the short nights. However, we do not believe this difference in light exposure could account for our results.
Because some have theorized that the circadian clock is composed of morning and evening oscillators, which respond to dawn and dusk respectively,30,31 we might expect to see the DLMO, rather than the DLMOff, delay more in response to evening bright light. Indeed, on average, in the long nights, the DLMO did delay more than the DLMOff, and most individual subjects showed this pattern. However, in the short nights, there was little difference between the phase delay in the DLMO and DLMOff. It remains unclear why this difference between conditions exists. In our previous study,6 a phase-advancing stimulus with morning bright light resulted in the DLMO phase advancing slightly more than the DLMOff in both short and long nights. This is contrary to expectations that the DLMOff would advance more. In both studies, the DLMO usually phase shifted more than the DLMOff.
Our results clearly demonstrate that, when people curtail their sleep, thus creating a long perceived photoperiod, they phase delay less in response to evening light. These results readily generalize to modern society in 3 ways. Our protocol with 6- and 9-hour night lengths represents realistic variations in sleep length. Additionally, longer nights are often due to later wake times, as in our protocol. Finally, our subjects regularly experienced 10 minutes of sunlight every morning, similar to the morning light exposure many people receive every day. An attenuation in light-induced circadian phase delays during short nights has serious implications for the sleep-deprived general population, jet travelers, night workers, and patients with advanced sleep phase syndrome. Experimental studies of reentrainment have shown that the human circadian clock already phase shifts quite slowly in response to bright light, typically delaying less than 3 hours per day.32,33 Jet travelers who travel west require phase delays in order to entrain to destination time as quickly as possible, thereby minimizing jet lag.23,34 Night workers also require phase delays in order to remain alert at night and sleep well during the day.23,35 Patients with advanced sleep phase syndrome require phase delays in order that they can sleep and wake at socially acceptable times.36 In all of these cases, our results suggest that the phase delays necessary for optimal circadian entrainment to the environment will occur at an even slower rate if people truncate their sleep.
To conclude, we have demonstrated for the first time that short nocturnal sleep episodes in humans, which create a long perceived photoperiod, reduce circadian phase delays to light. Indeed, it may be that voluntary human behavior, such as choosing sleep times, can alter the shape of the light phase response curve. Future research needs to test the effect of different night lengths on circadian phase shifts to light, determine how many consecutive short nights are required to observe reduced phase shifts to light, if sleep deprivation in humans reduces circadian phase shifts independent of photoperiodic changes in light or dark exposure, and if phase shifts to exogenous melatonin are also reduced following short nights.
Acknowledgments
This work was made possible by grants from the National Heart Lung and Blood Institute (R01 HL72408) and the American Sleep Medicine Foundation, a foundation of the American Academy of Sleep Medicine. We thank Young Cho, Erin Cullnan, Meredith Durkin, Valerie Ellios, Clara Lee, Victoria Revell, PhD, Mark Smith, and Jonathan Swisher for their assistance with data collection; Dr Louis Fogg for his statistical advice; and our Medical Director, Keith L. Callahan, MD. Enviro-Med donated the light boxes.
Footnotes
Disclosure Statement: This was not an industry supported study. Dr. Burgess is a consultant for Respironics. Dr. Eastman has indicated no financial conflict of interest.
References
- 1.National Sleep Foundation. Less fun, less sleep, more work: an American portrait. [Accessed on: 08/15/05];A National Sleep Foundation Poll. 2001 Available at: www.sleepfoundation.org/publications/2001poll.html.
- 2.Pittendrigh CS, Elliott J, Takamura T. Ciba Foundation Symposium 104 Photoperiodic Regulation of Insect and Molluscan Hormones. London: Pitman; 1984. The circadian component in photoperiodic induction; pp. 26–47. [PubMed] [Google Scholar]
- 3.Evans JA, Elliott JA, Gorman MR. Photoperiod differentially modulates photic and nonphotic phase response curves of hamsters. Am J Physiol. 2004;286:R539–46. doi: 10.1152/ajpregu.00456.2003. [DOI] [PubMed] [Google Scholar]
- 4.Illnerova H, Vanecek J. Entrainment of the circadian rhythm in rat pineal N-acetyltransferase activity under extremely long and short photoperiods. J Pineal Res. 1985;2:67–78. doi: 10.1111/j.1600-079x.1985.tb00628.x. [DOI] [PubMed] [Google Scholar]
- 5.Refinetti R. Compression and expansion of circadian rhythm in mice under long and short photoperiods. Integr Physiol Behav Sci. 2002;37:114–27. doi: 10.1007/BF02688824. [DOI] [PubMed] [Google Scholar]
- 6.Burgess HJ, Eastman CI. Short nights attenuate light-induced circadian phase advances in humans. J Clin Endocrinol Metab. 2005;90:4437–40. doi: 10.1210/jc.2005-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butcher JN, Dahlstrom WG, Graham JR, Tellegen A, Kaemmer B. MMPI-2 (Minnesota Multiphasic Personality Inventory-2): Manual for Administration and Scoring. Minneapolis: University of Minnesota Press; 1989. [Google Scholar]
- 8.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:53–63. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 9.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 10.Tasto DL, Colligan MJ, Skjei EW, Polly SJ. Health Consequences of Shift Work. Cincinnati: NIOSH Publication #78-154; 1978. [Google Scholar]
- 11.Horne JA, Ostberg O. Self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 12.Buxton OM, L'Hermite-Baleriaux M, Turek FW, Van Cauter E. Daytime naps in darkness phase shift the human circadian rhythms of melatonin and thyrotropin secretion. Am J Physiol. 2000;278:R373–82. doi: 10.1152/ajpregu.2000.278.2.R373. [DOI] [PubMed] [Google Scholar]
- 13.Dumont M, Carrier J. Daytime sleep propensity after moderate circadian phase shifts induced with bright light exposure. Sleep. 1997;20:11–7. doi: 10.1093/sleep/20.1.11. [DOI] [PubMed] [Google Scholar]
- 14.Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–33. [PubMed] [Google Scholar]
- 15.Moore RY. The innervation of the mammalian pineal gland. Prog Reprod Biol. 1978;4:1–29. [Google Scholar]
- 16.Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms. 2002;17:181–93. doi: 10.1177/074873002129002474. [DOI] [PubMed] [Google Scholar]
- 17.Lewy AJ, Cutler NL, Sack RL. The endogenous melatonin profile as a marker of circadian phase position. J Biol Rhythms. 1999;14:227–36. doi: 10.1177/074873099129000641. [DOI] [PubMed] [Google Scholar]
- 18.Murphy PJ, Myers BL, Badia P. Nonsteroidal anti-inflammatory drugs alter body temperature and suppress melatonin in humans. Physiol Behav. 1996;59:133–9. doi: 10.1016/0031-9384(95)02036-5. [DOI] [PubMed] [Google Scholar]
- 19.Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12:457–66. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- 20.Sharkey KM, Eastman CI. Melatonin phase shifts human circadian rhythms in a placebo-controlled simulated night-work study. Am J Physiol. 2002;282:R454–63. doi: 10.1152/ajpregu.00135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown EN, Choe Y, Shanahan TL, Czeisler CA. A mathematical model of diurnal variations in human plasma melatonin levels. Am J Physiol. 1997;272:E506–16. doi: 10.1152/ajpendo.1997.272.3.E506. [DOI] [PubMed] [Google Scholar]
- 22.Cagnacci A, Soldani R, Laughlin GA, Yen SSC. Modification of circadian body temperature rhythm during the luteal menstrual phase: role of melatonin. J Appl Physiol. 1996;80:25–9. doi: 10.1152/jappl.1996.80.1.25. [DOI] [PubMed] [Google Scholar]
- 23.Revell VL, Eastman CI. How to trick mother nature into letting you fly around or stay up all night. J Biol Rhythms. 2005;20:353–65. doi: 10.1177/0748730405277233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samkova L, Vondrasova D, Hajek I, Illnerova H. A fixed morning awakening coupled with a low intensity light maintains a phase advance of the human circadian system. Neurosci Lett. 1997;224:21–4. doi: 10.1016/s0304-3940(97)13460-7. [DOI] [PubMed] [Google Scholar]
- 25.Jelinkova-Vondrasova D, Hajek I, Illnerova H. Adjustment of the human circadian system to changes of the sleep schedule under dim light at home. Neurosci Lett. 1999;265:111–4. doi: 10.1016/s0304-3940(99)00220-7. [DOI] [PubMed] [Google Scholar]
- 26.Gordijn MCM, Beersma DGM, Korte HJ, Van den Hoofdakker RH. Effects of light exposure and sleep displacement on dim light melatonin onset. J Sleep Res. 1999;8:163–74. doi: 10.1046/j.1365-2869.1999.00156.x. [DOI] [PubMed] [Google Scholar]
- 27.Mistlberger RE, Landry GL, Marchant EG. Sleep deprivation can attenuate light-induced phase shifts of circadian rhythms in hamsters. Neurosci Lett. 1997;238:5–8. doi: 10.1016/s0304-3940(97)00815-x. [DOI] [PubMed] [Google Scholar]
- 28.Challet E, Turek FW, Laute M, Van Reeth O. Sleep deprivation decreases phase-shift responses of circadian rhythms to light in the mouse: role of serotonergic and metabolic signals. Brain Res. 2001;909:81–91. doi: 10.1016/s0006-8993(01)02625-7. [DOI] [PubMed] [Google Scholar]
- 29.Hebert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33:198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. V. Pacemaker Structure: A clock for all seasons. J Comp Physiol. 1976;106:333–55. [Google Scholar]
- 31.Illnerova H, Vanecek J. Two-oscillator structure of the pacemaker controlling the circadian rhythm of N-acetyltransferase in the rat pineal gland. J Comp Physiol. 1982;145:539–48. [Google Scholar]
- 32.Eastman CI, Martin SK. How to use light and dark to produce circadian adaptation to night shift work. Ann Med. 1999;31:87–98. doi: 10.3109/07853899908998783. [DOI] [PubMed] [Google Scholar]
- 33.Shanahan TL, Kronauer RE, Duffy JF, Williams GH, Czeisler CA. Melatonin rhythm observed throughout a three-cycle bright-light stimulus designed to reset the human circadian pacemaker. J Biol Rhythms. 1999;14:237–53. doi: 10.1177/074873099129000560. [DOI] [PubMed] [Google Scholar]
- 34.Waterhouse J, Reilly T, Atkinson G. Jet-lag. Lancet. 1997;350:1611–6. doi: 10.1016/S0140-6736(97)07569-7. [DOI] [PubMed] [Google Scholar]
- 35.Burgess HJ, Sharkey KM, Eastman CI. Bright light, dark and melatonin can promote circadian adaptation in night shift workers. Sleep Med Rev. 2002;6:407–20. [PubMed] [Google Scholar]
- 36.Reid KJ, Burgess HJ. Circadian rhythm sleep disorders. Prim Care. 2005;32:449–73. doi: 10.1016/j.pop.2005.02.002. [DOI] [PubMed] [Google Scholar]