Abstract
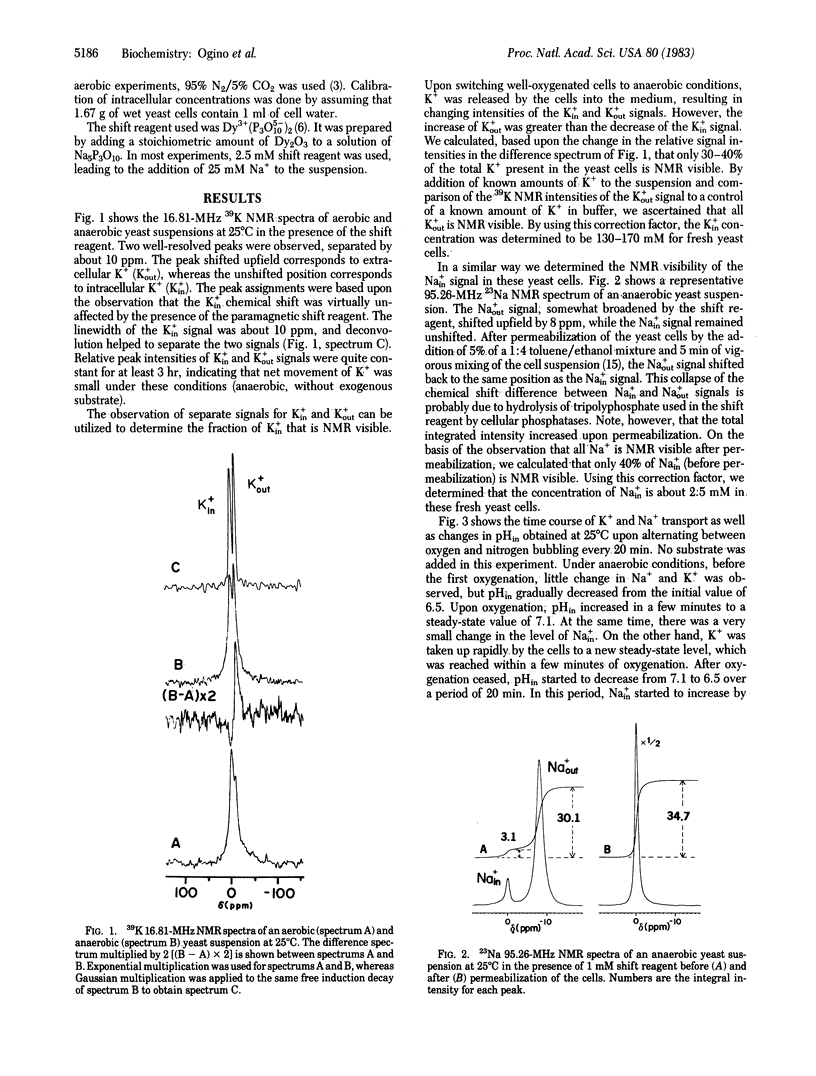
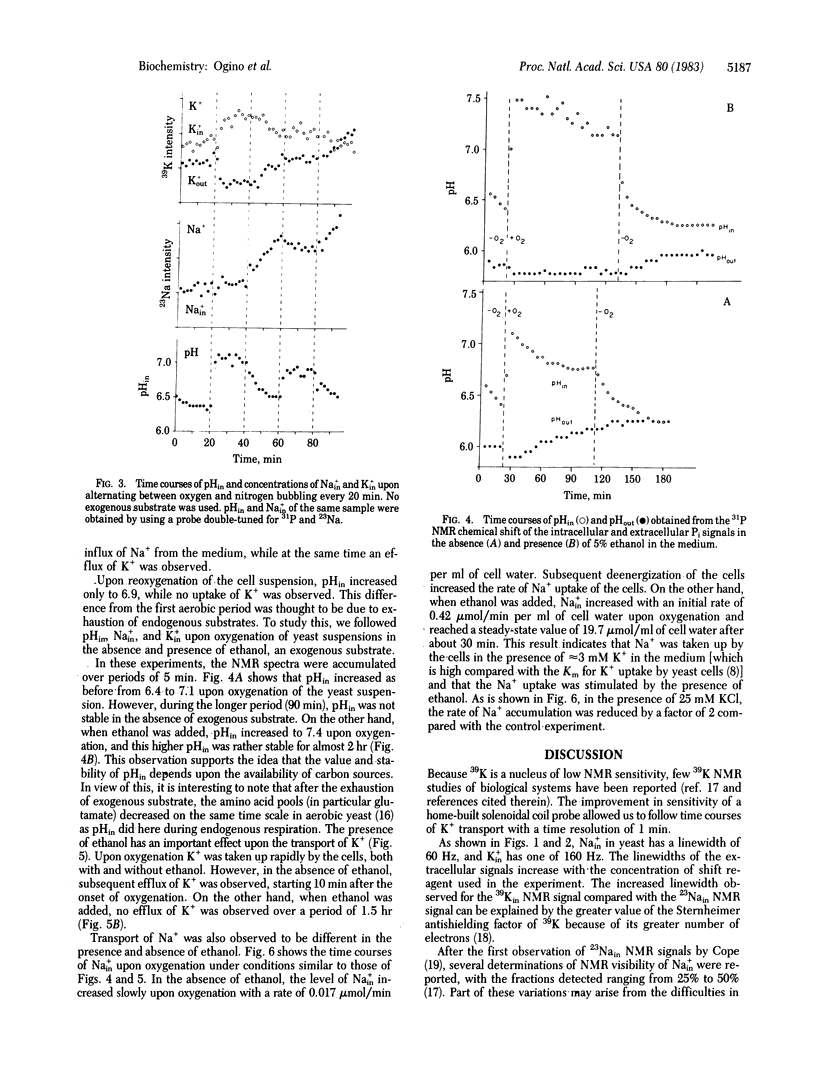
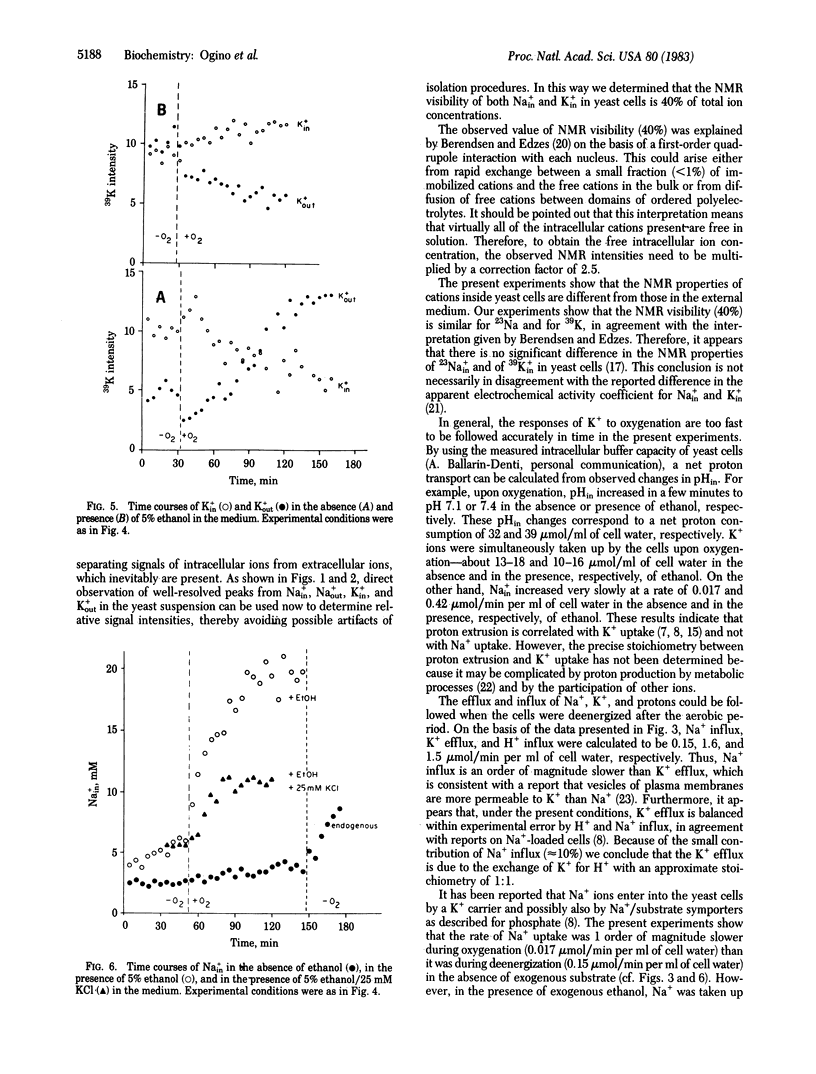
The relationship between efflux and influx of K+, Na+, and intracellular pH (pHin) in yeast cells upon energizing by oxygenation was studied by using the noninvasive technique of 39K, 23Na, and 31P NMR spectroscopy. By introducing an anionic paramagnetic shift reagent, Dy3+(P3O5(-10))2, into the medium, NMR signals of intra- and extracellular K+ and Na+ could be resolved, enabling us to study ion transport processes by NMR. Measurements showed that 40% of the intracellular K+ and Na+ in yeast cells contributed to the NMR intensities. By applying this correction factor, the intracellular ion concentrations were determined to be 130-170 mM K+ and 2.5 mM Na+ for fresh yeast cells. With the aid of a home-built solenoidal coil probe for 39K and a double-tuned probe for 23Na and 31P, we could follow time courses of K+ and Na+ transport and of pHin with a time resolution of 1 min. It was shown that H+ extrusion is correlated with K+ uptake and not with Na+ uptake upon energizing yeast cells by oxygenation. When the cells were deenergized after the aerobic period, K+ efflux, H+ influx, and Na+ influx were calculated to be 1.6, 1.5, and 0.15 mumol/min per ml of cell water, respectively. Therefore, under the present conditions, K+ efflux is balanced by exchange for H+ with an approximate stoichiometry of 1:1.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balschi J. A., Cirillo V. P., Springer C. S., Jr Direct high-resolution nuclear magnetic resonance studies of cation transport in vivo, Na+ transport in yeast cells. Biophys J. 1982 Jun;38(3):323–326. doi: 10.1016/S0006-3495(82)84566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bañuelos M., Gancedo C., Gancedo J. M. Activation by phosphate of yeast phosphofructokinase. J Biol Chem. 1977 Sep 25;252(18):6394–6398. [PubMed] [Google Scholar]
- Berendsen H. J., Edzes H. T. The observation and general interpretation of sodium magnetic resonance in biological material. Ann N Y Acad Sci. 1973 Mar 30;204:459–485. doi: 10.1111/j.1749-6632.1973.tb30799.x. [DOI] [PubMed] [Google Scholar]
- Borst-Pauwels G. W. Ion transport in yeast. Biochim Biophys Acta. 1981 Dec;650(2-3):88–127. doi: 10.1016/0304-4157(81)90002-2. [DOI] [PubMed] [Google Scholar]
- CONWAY E. J., RYAN H., CARTON E. Active transport of sodium ions from the yeast cell. Biochem J. 1954 Sep;58(1):158–167. doi: 10.1042/bj0580158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope F. W. Nuclear magnetic resonance evidence for complexing of sodium ions in muscle. Proc Natl Acad Sci U S A. 1965 Jul;54(1):225–227. doi: 10.1073/pnas.54.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann G. F., Boehm C., Theuvenet A. P. Sugar transport and potassium permeability in yeast plasma membrane vesicles. Biochim Biophys Acta. 1976 May 21;433(3):583–596. doi: 10.1016/0005-2736(76)90283-2. [DOI] [PubMed] [Google Scholar]
- Gadian D. G., Radda G. K. NMR studies of tissue metabolism. Annu Rev Biochem. 1981;50:69–83. doi: 10.1146/annurev.bi.50.070181.000441. [DOI] [PubMed] [Google Scholar]
- Goffeau A., Slayman C. W. The proton-translocating ATPase of the fungal plasma membrane. Biochim Biophys Acta. 1981 Dec 30;639(3-4):197–223. doi: 10.1016/0304-4173(81)90010-0. [DOI] [PubMed] [Google Scholar]
- Kakinuma Y., Ohsumi Y., Anraku Y. Properties of H+-translocating adenosine triphosphatase in vacuolar membranes of SAccharomyces cerevisiae. J Biol Chem. 1981 Nov 10;256(21):10859–10863. [PubMed] [Google Scholar]
- Navon G., Shulman R. G., Yamane T., Eccleshall T. R., Lam K. B., Baronofsky J. J., Marmur J. Phosphorus-31 nuclear magnetic resonance studies of wild-type and glycolytic pathway mutants of Saccharomyces cerevisiae. Biochemistry. 1979 Oct 16;18(21):4487–4499. doi: 10.1021/bi00588a006. [DOI] [PubMed] [Google Scholar]
- Okorokov L. A., Lichko L. P., Kulaev I. S. Vacuoles: main compartments of potassium, magnesium, and phosphate ions in Saccharomyces carlsbergenis cells. J Bacteriol. 1980 Nov;144(2):661–665. doi: 10.1128/jb.144.2.661-665.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Navarro A., Ortega M. D. The mechanism of sodium efflux in yeast. FEBS Lett. 1982 Feb 22;138(2):205–208. doi: 10.1016/0014-5793(82)80442-0. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Navarro A., Sancho E. D. Cation exchanges of yeast in the absence of magnesium. Biochim Biophys Acta. 1979 Apr 4;552(2):322–330. doi: 10.1016/0005-2736(79)90286-4. [DOI] [PubMed] [Google Scholar]
- Rothstein A. Relationship of cation influxes and effluxes in yeast. J Gen Physiol. 1974 Nov;64(5):608–621. doi: 10.1085/jgp.64.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J. P., Ryan H. The role of intracellular pH in the regulation of cation exchanges in yeast. Biochem J. 1972 Jun;128(1):139–146. doi: 10.1042/bj1280139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D., Slayman C. L. Control of intracellular pH. Predominant role of oxidative metabolism, not proton transport, in the eukaryotic microorganism Neurospora. J Gen Physiol. 1982 Sep;80(3):377–402. doi: 10.1085/jgp.80.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman R. G., Brown T. R., Ugurbil K., Ogawa S., Cohen S. M., den Hollander J. A. Cellular applications of 31P and 13C nuclear magnetic resonance. Science. 1979 Jul 13;205(4402):160–166. doi: 10.1126/science.36664. [DOI] [PubMed] [Google Scholar]
- den Hollander J. A., Behar K. L., Shulman R. G. 13C NMR study of transamination during acetate utilization by Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 May;78(5):2693–2697. doi: 10.1073/pnas.78.5.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander J. A., Ugurbil K., Brown T. R., Shulman R. G. Phosphorus-31 nuclear magnetic resonance studies of the effect of oxygen upon glycolysis in yeast. Biochemistry. 1981 Sep 29;20(20):5871–5880. doi: 10.1021/bi00523a034. [DOI] [PubMed] [Google Scholar]


