Abstract
Purpose
The limitations imposed by human clinical studies and mammalian models of nephrolithiasis have hampered the development of effective medical treatments and preventative measures for decades. The simple but elegant Drosophila melanogaster is emerging as a powerful translational model of human disease, including nephrolithiasis and may provide important information essential to our understanding of stone formation. We present the current state of research using D. melanogaster as a model of human nephrolithiasis.
Materials and Methods
A comprehensive review of the English language literature was performed using PUBMED. When necessary, authoritative texts on relevant subtopics were consulted.
Results
The genetic composition, anatomic structure and physiologic function of Drosophila Malpighian tubules are remarkably similar to those of the human nephron. The direct effects of dietary manipulation, environmental alteration, and genetic variation on stone formation can be observed and quantified in a matter of days. Several Drosophila models of human nephrolithiasis, including genetically linked and environmentally induced stones, have been developed. A model of calcium oxalate stone formation is among the most recent fly models of human nephrolithiasis.
Conclusions
The ability to readily manipulate and quantify stone formation in D. melanogaster models of human nephrolithiasis presents the urologic community with a unique opportunity to increase our understanding of this enigmatic disease.
Keywords: Drosophila melanogaster, Malpighian tubule, disease model, nephrolithiasis
INTRODUCTION
The development of effective medical therapies for the prevention and treatment of nephrolithiasis has been hindered by a lack of understanding of the fundamental mechanisms of the disease. Human clinical studies and mammalian models of nephrolithiasis are constrained by financial costs, ethical standards and protracted biological cycles. Furthermore, the genetic and physiologic complexities of these models obscure the true impact that dietary manipulation, environmental alteration, and genetic variation have on the most basic processes underlying stone formation.
Drosophila melanogaster has been successfully employed in the study of a variety of human diseases spanning numerous organ systems. This versatile invertebrate is now emerging as a powerful translational model of human nephrolithiasis with an array of practical and functional advantages. Among these advantages are the low cost of acquisition and maintenance and a brief life cycle that facilitates rapid and economical modeling. The D. melanogaster genome is highly conserved, fully characterized, and easily exploited through a wide array of well-established and sophisticated genomic tools. Mutant stock lines are readily available and affordable. Most importantly, Drosophila Malpighian tubules are remarkably similar to human renal tubules in genetic activity, anatomic structure and physiologic function, yet their simplicity allows for direct observation and quantification of the effects of experimental conditions in ways other model systems cannot.
We present a review of the current state of research using D. melanogaster as a model of human nephrolithiasis. A detailed description of the structure, function and genetics of the Malpighian tubules is also included to highlight the current utility and prospective role of this novel invertebrate model.
STRUCTURE, FUNCTION AND GENETICS OF THE MALPIGHIAN TUBULES
The renal system of D. melanogaster is composed of two anatomically and functionally discrete organs, nephrocytes and Malpighian tubules. Nephrocytes are specialized groups of cells clustered near the heart and the esophagus that filter the fly’s haemolymph (circulatory fluid) and remove waste products in a manner analogous to the endocytic processes of podocytes in the human glomerulus.1 The Malpighian tubules are similar to the remainder of the human nephron and collecting duct. They generate urine via active transport of ions, water, and organic solutes from the haemolymph into the Malpighian tubule lumen.
D. melanogaster has four Malpighian tubules, one anterior and one posterior pair. Each pair of Malpighian tubules coalesces into a common ureter at the junction of the midgut and the hindgut (fig. 1). Like the human ureter, longitudinal and circular muscle layers surround the Drosophila ureter to facilitate the peristalsis of urine.2 A single Malpighian tubule is approximately 2 millimeters in length with an inner luminal diameter of 17 micrometers (fig. 2).3 The Malpighian tubules can be divided into three genetically and physiologically distinct domains; the initial, transitional, and main segments. The main segment contains about 75 of the 100–150 cells that make up each tubule.4 This segment is primarily responsible for Drosophila urine production, and is composed of two cell types, principal cells and stellate cells. These cells are comparable in structure and function to the principal cells and the intercalated cells of the human collecting duct tubules, and contain many homologous ion and organic solute transporters (fig. 3).
Figure 1.
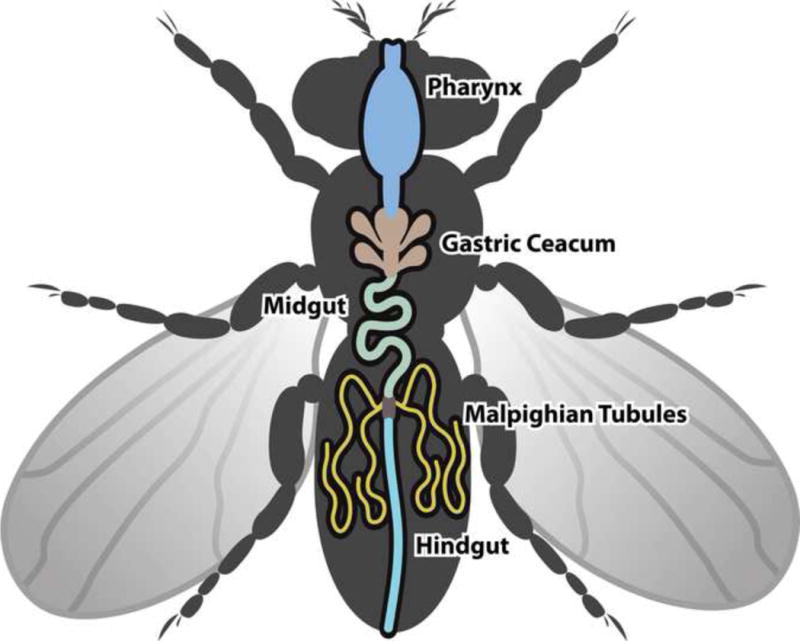
Cartoon of D. melanogaster excretory tract. Two pairs of Malpighian tubules, one anterior and one posterior, are each connected to the gut by a common ureter.
Figure 2.
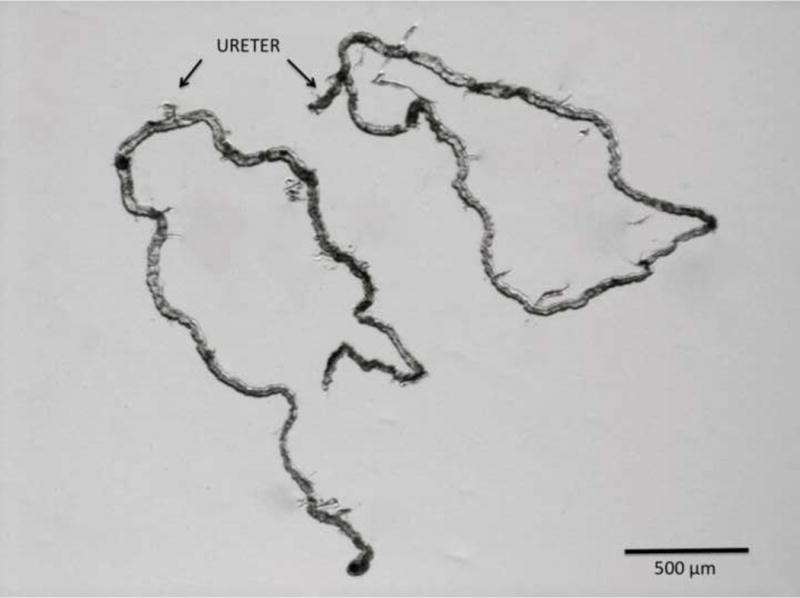
Photomicrograph of two pair of Malpighian tubules dissected free from an adult D. melanogaster. Each pair coalesces into a common ureter.
Figure 3.
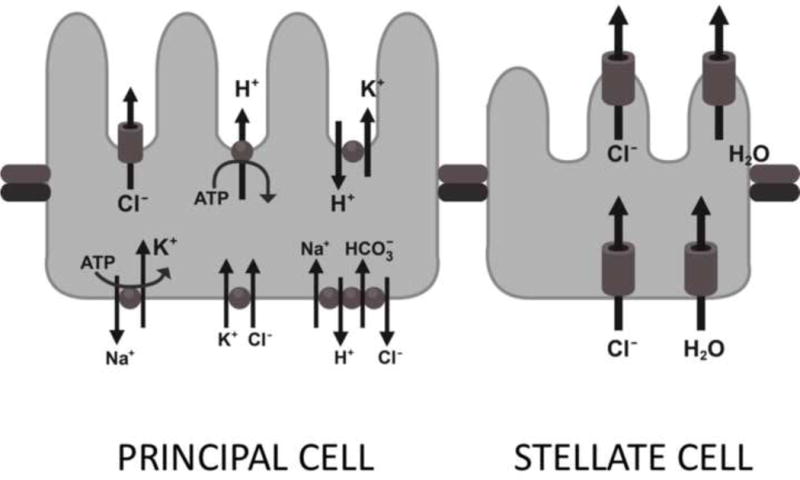
The principal and stellate cell of the D. melanogaster Malpighian tubules.
The larger principal cells are concentrated in the main segment of the tubule and contain basolateral ion cotransporters for Na+, K+ and Cl−, as well as a Na+-dependent solute transporter and a Na+-dependent Cl−/HCO3− exchanger.5 Ion transport at the apical cell membrane of the principal cell is accomplished via a Vacuolar-type H+-ATPase, which pumps protons from the cell into the Malpighian tubule lumen, providing the gradient necessary for secondary movement of Na+ and K+ into the lumen by the Na+/H+ and K+/H+ exchangers.6 The stellate cells, which are more evenly distributed throughout the initial, transitional, and main segments of the posterior Malpighian tubules, provide mainly chloride transport and water conductance.7
The Malpighian tubules regulate whole-body calcium levels through secretion and the subsequent formation of intraluminal “concretions” that are variably composed of concentric rings of calcium, magnesium, potassium, carbonate, phosphate, chloride and an organic matrix of glycosaminoglycans or proteoglycans.8 In flies fed a high-calcium diet, increased concretion formation causes obstruction and distention of the Malpighian tubules.9
In addition to the shared structural and physiological properties, there is excellent conservation of the gene function profile of the Malpighian tubules. The D. melanogaster genome is comprised of three pairs of autosomal chromosomes and one pair of sex chromosomes (fig. 4). The entire Drosophila genome is approximately the size of a single human chromosome, yet there is remarkable conservation across species. In cross genomic analysis, more than 70% of human disease loci have been found to have a homolog in the D. melanogaster genome, including 68% of genes linked to human cancers.10,11 The renal system is among the most highly conserved organ systems in the fly, with dozens of Drosophila genes that correspond to genetic diseases of the human kidney (Table 1).7,12,13 This conservation is dramatically illustrated by gene enrichment studies in which the expression levels of a gene within a specific tissue type is compared to gene expression levels in the entire organism. These data are used to infer tissue properties and physiological detail in experimental animals, and to identify phenotypes suitable for the study of human diseases. The Drosophila melanogaster genome contains more than 200 genes with expression levels that are more than 10-fold enriched in the Malpighian tubules compared to the entire fly. Among the Drosophila genes with a human disease homolog, 50 are enriched 3-fold or more within the Malpighian tubules, implying conservation of function of the excretory systems of two species that have been separated by millions of years of evolution (Table 1).12,14
Figure 4.
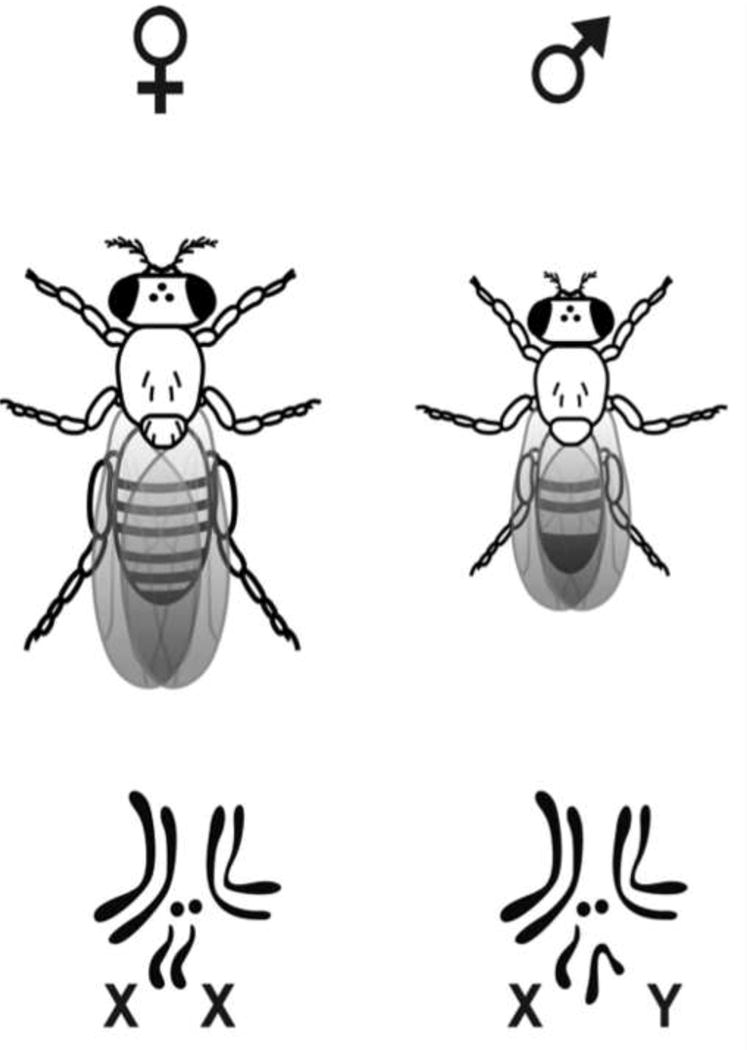
Visual segregation of Drosophila melanogaster by sex is easily accomplished due to the longer, pointed abdomen of the female. The genome is comprised of three autosomal pairs and one sex pair.
Table 1.
Drosophila genes corresponding to genetic diseases of the human kidney.
| Fly Gene | MT Enrichment | Corresponding Human Disease | Human Gene Locus | Gene Function |
|---|---|---|---|---|
| CG7642 | 18X | Xanthinuria, type 1 | 2p23-p22 | xanthine oxidase |
| CG12602 | 2.3X | Renal tubular acidosis, distal | 7q33-q34 | V-ATPase subunit |
| CG17369 | 3.7X | Renal tubular acidosis | 2cen-q13 | V-ATPase subunit |
| CG1709 | – | Renal tubular acidosis, distal | 7q33-q34 | V-ATPase subunit |
| CG4675 | 4.9X | Renal tubular acidosis, proximal | 4q21 | sodium-borate co-transporter |
| CG4357 | – | Gitelman syndrome | 16q13 | sodium-chloride co-transporter |
| CG5284 | 4.2X | Bartter syndrome, type 4; Dent disease | Xp11 | voltage-sensitive chloride channel |
| CG31547 | – | Bartter syndrome, type 1 | 15q15-q21 | potassium-chloride co-transporter |
| CG31116 | 11X | Bartter syndrome, type 2 and 4 | 3q27-q28 | voltage-sensitive chloride channel |
| CG3926 | 9.7X | Hyperoxaluria, primary type 1 | 27q36-37 | alanine-glyoxylate aminotransferase |
| CG6126 | 7.5X | Hypouricemia | 11q13 | urate transporter |
| CG9023 | 5.1X | Diabetes insipidus, nephrogenic | 9p13 | aquaporin water channel |
| CG17119 | 7X | Cystinosis, nephropathic | 17p13 | lysosomal cystine transporter |
DROSOPHILA MODELS OF HUMAN NEPHROLITHIAIS
The conservation of genetic composition and transporter protein structure, as well as the similarities of physiologic function of the Malpighian tubules has facilitated the development of several Drosophila stone models, including those of genetically linked and environmentally induced nephrolithiasis. Most recently, a D. melanogaster model of calcium oxalate nephrolithiasis has been described.
Genetically Linked Nephrolithiasis
Numerous inborn metabolic diseases such as hyperoxaluria, cystinuria, and hyperaminoaciduria (Fanconi syndrome) manifest with urinary stone disease in humans.15 Although they are among the most rare causes of human nephrolithiasis, exploring the genetic basis of these diseases in Drosophila may reveal mechanisms underlying stone formation and its regulation that are common to other stone types.
Xanthinuria Type I and II are human inherited autosomal recessive disorders caused by defective purine metabolism. Enzyme deficiencies result in total-body accumulation and increased urinary excretion of xanthine, and the disease commonly manifests as xanthine nephrolithiasis in children and adolescents.16 Xanthinuria Type I is caused by a deficiency in XDH, the enzyme responsible for converting hypoxanthine to xanthine, and xanthine to uric acid. Cloning of the human genes for XDH (2p22/23) from two affected siblings led to identification of the mutations responsible for the classical form of human Xanthinuria Type I.17
Xanthinuria Type II also results from XDH deficiency, but involves deficiencies of additional enzymatic co-factors. The rosy and maroon-like mutant strains of D. melanogaster possess genetic homologs of the altered human XDH genes, and similarly these flies suffer from whole-body accumulation of xanthine and the formation of xanthine concretions within their Malpighian tubules.18 The pathophysiology of Xanthinuria Type II was unknown until cloning of the maroon-like gene in Drosophila led to identification of the human homolog of this gene (human molybdenum cofactor sulfurase gene).19 In a manner similar to human Xanthinuria patients, the accumulation of Malpighian tubule concretions in the rosy and maroon-like models can be manipulated with dietary modification and alteration of transporter protein expression, but unlike humans the effects these changes in Drosophila can be readily observed and quantified in a matter of days (fig. 5).20
Figure 5.
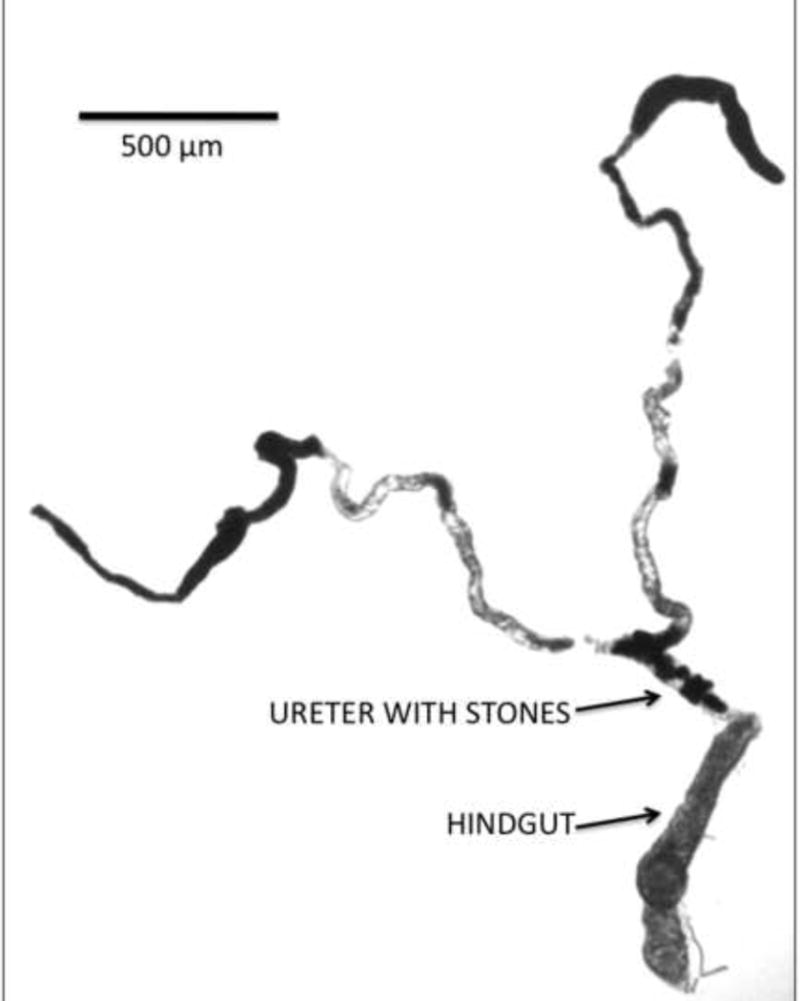
Xanthine stones in the Malpighian tubules lumens of rosy mutants deficient in XDH.
Environmentally Induced Nephrolithiasis
In 2007 a large-scale recall of pet food was initiated after several Chinese brands were found to be contaminated with the industrial chemical melamine. Outbreaks of renal failure and the subsequent deaths of thousands of canines in Asia and North America led to the discovery of melamine-induced nephrolithiasis and nephrotoxicosis as the cause.21 Contamination of infant formula with melamine led to the death of four children and the hospitalization of over 6000 babies in China the following year. As in the canine poisonings, the majority of the children presented with renal failure and melamine-induced nephrolithiasis.22
The human kidney excretes melamine intact, and even low urinary concentrations of melamine can result in the formation of calcium oxalate, calcium phosphate, or uric acid nephrolithiasis.23 In the Drosophila model of melamine-induced nephrolithiasis, flies fed food stock containing increasing levels of melamine formed crystals in their Malpighian tubules in a dose-dependent manner, over a period of just 21 days. Simple light microscopy was used to qualify the degree of crystal formation in the translucent Malpighian tubules, and to assess the effects of dose variation and the administration of stone-inhibiting substances. Scanning electron microscopy revealed the crystals were variably composed of calcium, oxygen, phosphate and carbon. A dose-dependent decrease in lifespan, from a mean of approximately 40 days to less than 10 days, was also noted in flies fed increasing concentrations of melamine. Attempted mitigation of the effects of melamine with potassium-citrate resulted in a reversal of the effects on D. melanogaster lifespan, but no significant effect on Malpighian tubule crystal formation.24 As with other Drosophila models of environmentally induced nephrolithiasis, the melamine-induced nephrolithiasis Drosophila model has limited applicability to the formation of idiopathic calcium oxalate nephrolithiasis in humans, as the crystals are neither calcium oxalate nor uric acid.
Several other lithogenic substances have been used to develop environmentally induced models of human nephrolithiasis in rats, including ethylene glycol, hydroxyl-L-proline, vitamin D, and various oxalate-containing compounds.25,26 In rat models of environmentally induced nephrolithiasis, lithogenic substances must be administered daily via gastric tube after dissolution in salad oil, via intraperitoneal injection, through subcutaneous injection or by a combination of these methods, for periods of up to 28 days. Direct observation of the effects of the various experimental conditions on the formation of crystals in the murine renal tubules requires the use of polarized light microscopy after excision, sectioning, and complex staining of the rat kidneys.25,26
Similar models of environmentally induced nephrolithiasis in D. melanogaster are considerably less cumbersome. Dose-dependent calcium oxalate crystal formation is readily visible as soon as 14 days in the Malpighian tubules of flies fed diets containing increasing concentrations of ethylene glycol, hydroxyl-L-proline, or sodium oxalate. A dose-dependent decrease in lifespan, from a mean 40 days in controls to less than 10 days in flies fed 1% ethylene glycol, can also be easily demonstrated in Drosophila. The addition of potassium citrate to the lithogenic diet of these experimental flies resulted in an obvious decrease in the formation of calcium oxalate crystal in the Malpighian tubules in all of the experimental models, and resulted in an increased lifespan in the ethylene glycol model.27
Several of these Drosophila models of environmentally induced nephrolithiasis exhibit the formation of calcium oxalate crystals in the Malpighian tubules. As with genetically linked nephrolithiasis, these Drosophila models are not entirely representative of idiopathic calcium stone formation in humans, but common mechanisms of crystal formation and inhibition may be defined. Moreover, the practical advantages of D. melanogaster allow rapid evaluation of scores of target genes and stone-related metabolic pathways, and facilitate efficient large-scale screening of contributory or preventative compounds.
Calcium Oxalate Nephrolithiasis
The most common type of human kidney stones are largely composed of calcium oxalate, and likely result from a combination of genetic and environmental factors, including diet. Calcium oxalate stones also form in humans due to well-defined monogenic mutations involving cell membrane transporters, ion receptors and transmembrane ion channels. These diseases include hypercalciuric metabolic diseases such as Dent’s disease and Bartter syndrome, and hyperoxaluric metabolic disease including the various forms of primary hyperoxaluria.28,29 Lastly, calcium oxalate stones can form secondarily in patients with other metabolic disease such as those linked to hyperuricosuria.30 The recently developed fruit fly model of calcium oxalate nephrolithiasis, Drosophila Prestin, represents a major step toward describing the basic mechanisms underlying the formation of the most common type of human nephrolithiasis.
The Drosophila Prestin gene model of human calcium oxalate nephrolithiasis was developed by selective knockdown of Slc26a6, a luminal oxalate transporter found in the Malpighian tubules.31 In humans, Slc26 anion transporters are distributed in tissues throughout the body and perform a variety of physiologic functions.32 The D. melanogaster genome contains 9 homologs of the 11 human Slc26 transporters. The Drosophila Prestin gene (CG5485) codes for proteins homologous to the human Slc26a5 and Slc26a6 transporters.31 The functions of Slc26a5/6 in humans include Cl−/HCO3− exchange and oxalate secretion in the kidney, as well as regulation of oxalate secretion in the small intestine (Cl−/oxalate2− exchange).32,33 As in humans, the Slc26a5/a6 transporter homologs in Drosophila are found in various tissues, including the Malpighian tubules, and also mediate Cl−/oxalate2− exchange.31 To accurately model the excretion of oxalate in human renal tubules, selective knockdown of Slc26a5/a6 in the D. melanogaster Malpighian tubules was performed using the GAL4/UAS system. The GAL4/UAS system accomplishes tissue-specific knockdown of genes by inserting the transcription activating driver (the GAL4-driver) into one line of flies and then crossing these flies with another line that contains both the corresponding promoter region (the UAS-responder) and the downstream desired gene sequence. This selective knockdown results in a loss of function in the desired tissue (the Malpighian tubule), while maintaining normal function of the gene throughout the rest of the organism.34
The fidelity of the Drosophila Prestin model of human calcium oxalate nephrolithiasis is demonstrated through the directly observable effects of dietary manipulation on fly stone formation, and the resultant mitigation of these effects brought about by the selective knockdown of Slc26a5/a6. For example, calcium oxalate crystals are not normally visible in the Malpighian tubules of wild-type D. melanogaster fed a standard diet, but the addition of sodium-oxalate to the diet of wild-type Drosophila larvae results in the formation of calcium oxalate crystals in the Malpighian tubules within 2 days. Adult flies fed an oxalate-rich diet produce calcium oxalate crystals in 6–12 hours (fig. 6). This calcium oxalate crystal formation can be quantified with birefringence microscopy of dissected tubules or with micro-CT scanning of whole flies (fig. 7). Dynamic in vitro formation of calcium oxalate crystals can also be observed in real time in the dissected wild-type adult Malpighian tubules submerged in a sodium-oxalate bath.33 On the other hand, selective knockdown of Slc26a5/a6 in the Malpighian tubules of the Drosophila Prestin model results in a statistically significant decrease in the intraluminal formation of calcium oxalate crystals.35
Figure 6.
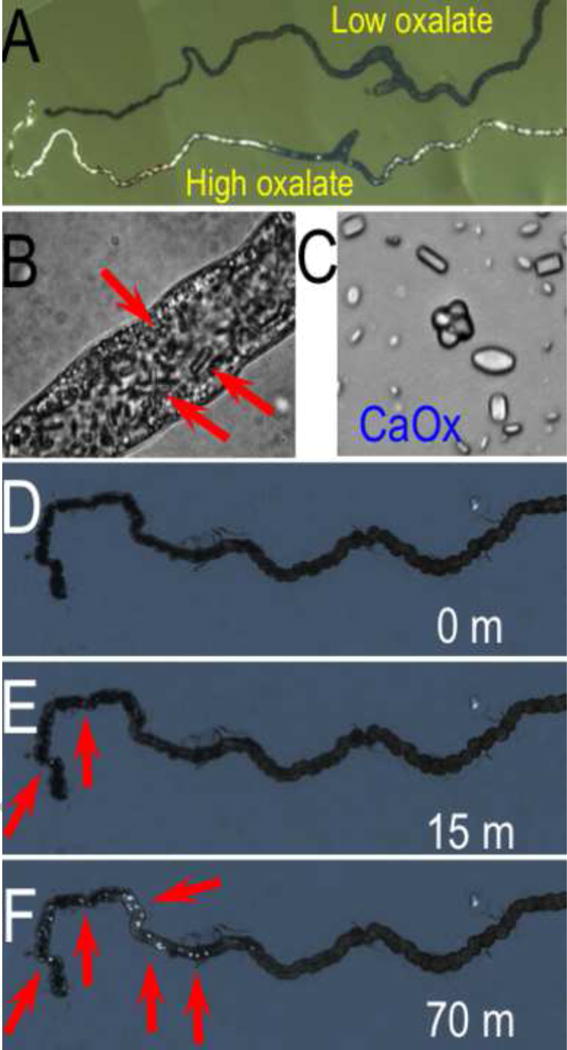
Photomicrographs of (A) calcium oxalate crystal accumulation D. melanogaster fed diet supplemented with sodium oxalate (bottom), compared to control (top), (B) magnification of intraluminal calcium oxalate crystals, (C) magnified view of crystals, and (D–F) crystal formation in dissected Malpighian tubules in sodium oxalate bath. (Hirata, et al., 2012).
Figure 7.
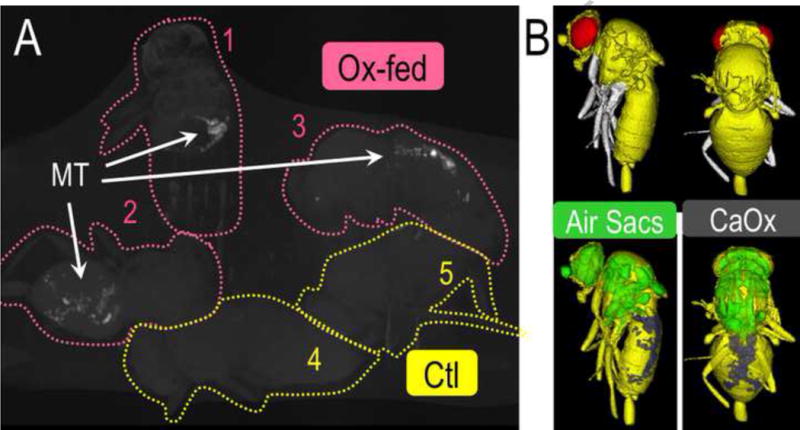
(A) MicroCT scan images of calcium oxalate crystal accumulation in three adult D. melanogaster fed a diet supplemented with sodium oxalate as compared to images of two controls, and (B) Surface renderings constructed from Micro-CT scan images (Hirata, et al., 2012). (Ox-Fed=oxalate fed flies, Ctl=control flies, MT=Malpighian tubules)
LIMITATIONS DROSOPHILA MODELING OF HUMAN NEPHROLITHIASIS
Foremost among the perceived limitations of Drosophila as a model for the study of human nephrolithiasis are the differences in anatomic arrangement of the renal and excretory systems. The renal system of D. melanogaster is aglomerular, and the filtration products are not directly delivered to the tubules from an intimately associated glomerulus. In Drosophila, two anatomically separate and functionally distinct cell populations are responsible for haemolymph filtration and ion transport. As described in detail above, ion transport is accomplished in Drosophila by the principal and stellate cells of the Malpighian tubules. The nephrocytes perform many of the functions of the vertebrate glomerulus, including filtration and detoxification of the haemolymph. Nephrocytes have a complex folded plasma membrane that forms multiple channels bordered by narrow slits that serve as filtration junctions. In a manner similar to the podocytes of the glomerulus, these small slits allow the nephrocytes to filter materials from the haemolymph based upon particle size and charge, and then sequester the filtered materials via endocytosis. These mechanisms can be readily visualized in a nephrocyte cell culture using variable sizes of fluorescently labeled dextrans, and with the feeding of Drosophila larvae with toxins such as silver nitrate.1 As with the Malpighian tubules, numerous genetic homologs of the human glomerulus have been identified within the Drosophila nephrocyte, and diseases such as human congenital nephrotic syndrome can be recapitulated in fly models.1 Thus, rather than imposing limitations, the anatomical separation of filtration and ion transport functions in D. melanogaster presents opportunities for detailed, single-tissue modeling of diseases affecting the analogous vertebrate renal structures. This single-tissue modeling of stone formation in Drosophila may provide the basis for subsequent experiments in more complex and expensive mammalian models, including humans.
The co-mingling of waste products from the Malpighian tubules and the gut within the common cloaca of D. melanogaster has been cited as a drawback of the use of invertebrates for modeling of human nephrolithiasis.36 This perceived limitation is readily overcome by a combination of the simplicity of the D. melanogaster morphology and several well-developed experimental techniques. The two pairs of Malpighian tubules are easily dissected free from their attachments to the gut by severing the common ureter. These isolated tubules can then be anchored with a thin steel pin and submerged in media containing experimental compounds or haemolymph harvested from relevant model adult flies or larvae. The dissected tubules will continue to secrete fluid for more than 5 hours, and the resultant secretory products can be collected for subsequent analysis in a manner analogous to a human 24-hour urine collection.3
Among other challenges inherent to the D. melanogaster models of human renal function and nephrolithiasis formation are the differences in mechanisms of calcium homeostasis. For example, as an invertebrate, Drosophila melanogaster lacks the skeletal calcium stores that are an integral part of maintaining calcium balance in humans. Calcium metabolism in D. melanogaster has been well characterized, and although some of the mechanisms have been studied, the full potential of the model has not been realized. For example, 25–30% of the calcium content of the whole adult Drosophila is stored as calcium-containing concretions in the distal segment of the anterior pair of Malpighian tubules. Variations in dietary calcium are mitigated in D. melanogaster by combined tubular secretion of free calcium in the urine, and sequestration of insoluble calcium in the luminal concretions.9 As is the case with human nephrolithiasis, the pH-dependent formation of these luminal concretions can be inhibited with common medications such as acetazolamide and hydrochlorothiazide.37
Current Drosophila models of human nephrolithiasis are limited to genetically linked and environmentally induced stone formation and a new model of calcium oxalate stone formation. There has not been work performed in the areas of uric acid or magnesium ammonium phosphate stone formation. This may be due to the focus on idiopathic calcium stones, particularly calcium oxalate stones, or because the etiology of these two stone types (uric acid and infection stones) have been better studied in humans. Bacterial infection models in Drosophila may not replicate the changes in the urinary milieu caused by the urea-splitting organisms that culminate in human infection stones. Intestinal bacterial infections in Drosophila models do not cause immediate death however, but rather an indolent process similar to that seen in humans, so this may be another area of stone research that could be exploited using the fly model in the future. Uric acid stones would ostensibly be simpler to model than infections stones, and may represent, along with infection stones, an avenue of additional research.
CONCLUSIONS
The technologies and techniques for the surgical treatment of nephrolithiasis have evolved greatly in the last 20 years but little progress has been made in the development of effective medications for prevention or treatment.
Conventional medical therapy for nephrolithiasis only alters the gross urinary constituents to decrease the risk of stone formation, but stone formation is actually the endpoint of a complex and still poorly understood pathophysiologic process. Contemporary medications do not directly target these fundamental mechanisms, and thus many drugs have undesirable side effects or limited short-and long-term efficacy. New cost-effective, efficacious and readily deployable therapeutic strategies are needed. The disease models and genomic tools of D. melanogaster present the urologic community with a unique opportunity to develop novel therapies for nephrolithiasis through an increased understanding of the pathophysiology of this disease in its various manifestations.
Acknowledgments
Mr. Richmond Lee created Figures 1, 4, and 6.
This work was supported in part by a grant from the AUA Foundation Research Scholars Program and Boston Scientific Corporation, The Endourological Society, The “Friends of Joe”, the NIH K12-DK-07-006: Multidisciplinar K12 Urologic Research Career Development Program, and NIH grants EY017732, P50-DK083007, DK092408.
Key of Definitions for Abbreviations and Acronyms
- XDH
Xanthine dehydrogenase
- UAS
Upstream activation sequence
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weavers H, Prieto-Sánchez S, Grawe F, et al. The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature. 2009;457:322. doi: 10.1038/nature07526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wessing A, Eichelberg D. Malpighian tubules, rectal papillae and excretion. In: Ashburner M, Wright TRF, editors. The genetics and biology of Drosophila. 2c. New York: Academic Press Inc; 1978. pp. 1–42. [Google Scholar]
- 3.Dow JAT, Maddrell SHP, Görtz A, et al. The Malpighian tubules of Drosophila melanogaster: a novel phenotype for studies of fluid secretion and its control. J Exp Biol. 1994;197:421. doi: 10.1242/jeb.197.1.421. [DOI] [PubMed] [Google Scholar]
- 4.Sözen M, Armstrong JD, Yang M, et al. Functional domains are specified to single-cell resolution in a Drosophila epithelium. Proc Natl Acad Sci U S A. 1997;94:5207. doi: 10.1073/pnas.94.10.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romero MF, Henry D, Nelson S, et al. Cloning and characterization of a Na+-driven anion exchanger (NDAE1). A new bicarbonate transporter. J Biol Chem. 2000;275:24552. doi: 10.1074/jbc.M003476200. [DOI] [PubMed] [Google Scholar]
- 6.O’Donnell MJ, Ianowski PJ, Linton SM, et al. Inorganic and organic anion transport by insect renal epithelia. Biochim et Biophys Acta. 2003;1618:194. doi: 10.1016/j.bbamem.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Dow JAT, Romero MF. Drosophila provides rapid modeling of renal development, function, and disease. Am J Physiol Renal Physiol. 2010;299:F1237. doi: 10.1152/ajprenal.00521.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wessing A, Zierold K, Hevert F. Two types of concretions in Drosophila Malpighian tubules as revealed by X-ray microanalysis: a study on urine formation. J Insect Physiol. 1992;38:543. [Google Scholar]
- 9.Dube KA, McDonald DG, O’Donnell MJ. Calcium homeostasis in larval and adult Drosophila melanogaster. Arch Insect Biochem Physiol. 2000;44:27. doi: 10.1002/(SICI)1520-6327(200005)44:1<27::AID-ARCH4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 10.Chien S, Reiter LT, Bier E, et al. Homophila: human disease gene cognates in Drosophila. Nucleic Acids Res. 2002;30:149. doi: 10.1093/nar/30.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubin GM, Yandell MD, Wortman JR, et al. Comparative genomics of the eurkaryotes. Science. 2000;287:2204. doi: 10.1126/science.287.5461.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chintapalli VR, Wang J, Dow JAT. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007;39:715. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- 13.Donorkin S, Reiter LT. Drosophila orthologues to human disease genes: an update on progress. Prog Nucleic Acid Res Mol Biol. 2008;82:1. doi: 10.1016/S0079-6603(08)00001-9. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Kean L, Yang J, et al. Function-informed transcriptome analysis of Drosophila renal tubule. Genome Biol. 2004;5:R69. doi: 10.1186/gb-2004-5-9-r69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cochat P, Pichault V, Bacchetta J, et al. Nephrolithiasis related to inborn metabolic diseases. Pediatr Nephrol. 2010;25:415. doi: 10.1007/s00467-008-1085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pais VM, Jr, Lowe G, Lallas CD, et al. Xanthine urolithiais. Urology. 2006;67:1084. doi: 10.1016/j.urology.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 17.Ichida K, Amaya Y, Kamatani N, et al. Identification of two mutations in human xanthine dehydrogenase gene responsible for classical type I xanthinuria. J Clin Invest. 1997;99:2391. doi: 10.1172/JCI119421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell HK, Glassman E. Hypoxanthine in Rosy and Maroon-like mutants of Drosophila melanogaster. Science. 1959;129:268. doi: 10.1126/science.129.3344.268. [DOI] [PubMed] [Google Scholar]
- 19.Ichida K, Matsumara T, Sakuma R, et al. Mutation of human molybdenum cofactor sulfurase gene is responsible for classical xanthinuria type II. Biochem Biophys Res Commun. 2001;282:1194. doi: 10.1006/bbrc.2001.4719. [DOI] [PubMed] [Google Scholar]
- 20.Chi T, Kolipinski M, Kahn A, et al. A novel urinary stone animal model using Drosophila melanogaster. J Urol. 2010;183:e765. abstract 1970. [Google Scholar]
- 21.Thompson ME, Lewin-Smith MR, Kalasinsky VF, et al. Characterization of melamine-containing and calcium oxalate crystals in three dogs with suspected pet food-induced nephrotoxicosis. Vet Pathol. 2008;45:417. doi: 10.1354/vp.45-3-417. [DOI] [PubMed] [Google Scholar]
- 22.Parry J. Contaminated infant formula sickens 6200 babies in China. BMJ. 2008;337:a1738. doi: 10.1136/bmj.a1738. [DOI] [PubMed] [Google Scholar]
- 23.Wu CF, Liu CC, Chen BH, et al. Urinary melamine and adult urolithiasis in Taiwan. Clin Chima Acta. 2010;411:184. doi: 10.1016/j.cca.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Chen WC, Lin WY, Chen HY, et al. Melamine-induced urolithiasis in a Drosophila model. J Agric Food Chem. 2012;60:2753. doi: 10.1021/jf204647p. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Cao Z, Zhang Z, et al. A comparative study on several models of experimental renal calcium oxalate stones formation in rats. J Huazhong Univ Sci Technolog Med Sci. 2007;27:83. doi: 10.1007/s11596-007-0124-z. [DOI] [PubMed] [Google Scholar]
- 26.Oh SY, Kwon JK, Lee SY, et al. A comparative study of experimental rat models of renal calcium oxalate stone formation. J Endourol. 2011;25:1057. doi: 10.1089/end.2010.0386. [DOI] [PubMed] [Google Scholar]
- 27.Chen YH, Liu HP, Chen HY, et al. Ethylene glycol induces calcium oxalate crystal deposition in Malpighian tubules: a Drosophila model for nephrolithiasis/urolithiasis. Kidney International. 2011;80:369. doi: 10.1038/ki.2011.80. [DOI] [PubMed] [Google Scholar]
- 28.Stechman MJ, Loh NY, Thakker RV. Genetic causes of hypercalciuric nephrolithiasis. Pediatr Nephrol. 2009;24:2321. doi: 10.1007/s00467-008-0807-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoppe B. An update on primary hyperoxaluria. Nat Rev Nephrol. 2012;8:467. doi: 10.1038/nrneph.2012.113. [DOI] [PubMed] [Google Scholar]
- 30.Sorensen CM, Chandhoke PS. Hyperuricosuric calcium nephrolithiasis. Endocrinol Metab Clin North Am. 2002;31:915. doi: 10.1016/s0889-8529(02)00038-5. [DOI] [PubMed] [Google Scholar]
- 31.Hirata T, Czapar A, Brin L, et al. Ion and solute transport by Prestin in Drosophila and Anopheles. J Insect Physiol. 2012;58:563. doi: 10.1016/j.jinsphys.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dorwart MR, Shcheynikov N, Yang D, et al. The solute carrier 26 family of proteins in epithelial ion transport. Physiology. 2008;23:104. doi: 10.1152/physiol.00037.2007. [DOI] [PubMed] [Google Scholar]
- 33.Sindić A, Chang MH, Mount DB. Renal physiology of SLC26 anion exchangers. Curr Opin Nephrol Hypertens. 2007;16:484. doi: 10.1097/MNH.0b013e3282e7d7d0. [DOI] [PubMed] [Google Scholar]
- 34.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 35.Hirata T, Cabrero P, Berkholz DS, et al. In vivo Drosophila genetic model for calcium oxalate nephrolithiasis. Am J Physiol Renal Physiol. 2012;303:F1555. doi: 10.1152/ajprenal.00074.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Assimos D. Re: Ethylene glycol induces calcium oxalate crystal deposition in Malpighian tubules: a Drosophila model for nephrolithiasis/urolithiasis. J Urol. 2012;187:1299. doi: 10.1016/j.juro.2011.12.125. [DOI] [PubMed] [Google Scholar]
- 37.Wessing A, Zierold K. The formation of type-I concretions in Drosophila Malpighian tubules studied by electron microscopy and X-ray microanalysis. J Insect Physiol. 1999;45:39. doi: 10.1016/s0022-1910(98)00097-3. [DOI] [PubMed] [Google Scholar]


