Abstract
Although the plantar fascia (PF) has been studied quite well from a biomechanical viewpoint, its microscopic properties have been overlooked: nothing is known about its content of elastic fibers, the features of the extracellular matrix or the extent of innervation. From a functional and clinical standpoint, the PF is often correlated with the triceps surae muscle, but the anatomical grounds for this link are not clear. The aim of this work was to focus on the PF macroscopic and microscopic properties and study how Achilles tendon diseases might affect it. Twelve feet from unembalmed human cadavers were dissected to isolate the PF. Specimens from each PF were tested with various histological and immunohistochemical stains. In a second stage, 52 magnetic resonance images (MRI) obtained from patients complaining of aspecific ankle or foot pain were analyzed, dividing the cases into two groups based on the presence or absence of signs of degeneration and/or inflammation of the Achilles tendon. The thickness of PF and paratenon was assessed in the two groups and statistical analyses were conducted. The PF is a tissue firmly joined to plantar muscles and skin. Analyzing its possible connections to the sural structures showed that this fascia is more closely connected to the paratenon of Achilles tendon than to the Achilles tendon, through the periosteum of the heel. The PF extended medially and laterally, continuing into the deep fasciae enveloping the abductor hallucis and abductor digiti minimi muscles, respectively. The PF was rich in hyaluronan, probably produced by fibroblastic-like cells described as ‘fasciacytes’. Nerve endings and Pacini and Ruffini corpuscles were present, particularly in the medial and lateral portions, and on the surface of the muscles, suggesting a role for the PF in the proprioception of foot. In the radiological study, 27 of the 52 MRI showed signs of Achilles tendon inflammation and/or degeneration, and the PF was 3.43 ± 0.48 mm thick (99%CI and SD = 0.95), as opposed to 2.09 ± 0.24 mm (99%CI, SD = 0.47) in the patients in which the MRI revealed no Achilles tendon diseases; this difference in thickness of 1.29 ± 0.57 mm (99%CI) was statistically significant (P < 0.001). In the group of 27/52 patients with tendinopathies, the PF was more than 4.5 mm thick in 5, i.e. they exceeded the threshold for a diagnosis of plantar fasciitis. None of the other 25/52 paitents had a PF more than 4 mm thick. There was a statistically significant correlation between the thicknesses of the PF and the paratenon. These findings suggest that the plantar fascia has a role not only in supporting the longitudinal arch of the foot, but also in its proprioception and peripheral motor coordination. Its relationship with the paratenon of the Achilles tendon is consistent with the idea of triceps surae structures being involved in the PF pathology, so their rehabilitation can be considered appropriate. Finally, the high concentration of hyaluronan in the PF points to the feasibility of using hyaluronan injections in the fascia to treat plantar fasciitis.
Keywords: Achilles tendon, fasciacyte, hyaluronan, paratenon, plantar aponeurosis, plantar fascia, plantar fasciitis
Introduction
The plantar fascia (PF) can undergo a form of pathological degeneration called plantar fasciitis (Lemont et al. 2003; Benjamin, 2009) that is one of the most common causes of heel pain. It accounts for 1% of all orthopedic visits (Riddle & Schappert, 2004). It afflicts 10% of runners and is also common among workers and athletes whose activities have a high impact on the foot (Chandler & Kibler, 1993). There is no broad consensus among orthopedic surgeons, or any acknowledged guidelines on the management of plantar fasciitis (Di Giovanni et al. 2012). In 90–95% of patients, pain relief is achieved with conservative treatments that include rest, ice packs, specific exercises and stretching, anti-inflammatory medication, shoe inserts, and night splints, but they can often take from 6 months to a year to take effect (Cornwall & McPoil, 1999). Other long-lasting plantar fasciitis treatment options include extracorporeal shock wave therapy, which proved effective in 35% of patients involved in a randomized controlled multicenter trial, but placebo was also effective in 30% of patients (Haake et al. 2003). Another solution is corticosteroid injections, but this treatment has caused rupture of the fascia in 2–10% of patients (Acevedo & Beskin, 1998; Kim et al. 2010a; McMillan et al. 2012). Total or partial surgical release of the PF should only be an option when all else has failed (Tweed Barnes & Allen, 2009), the consequences of plantar fasciotomy being a 7.4 ± 4.1 mm reduction of the plantar arch (Kitaoka et al. 1997) and a 15% elongation of the sole (Arangio et al. 1997). In the long term, after fasciotomy, foot pain may also recur due to overpronation of the foot (Tweed Barnes & Allen, 2009).
Plantar fasciitis is diagnosed on clinical grounds, possibly with the support of imaging to rule out any other diseases and confirm a thickening of the PF. The issue of heel spurs in patients with plantar fasciitis is still debated: it is not clear whether they cause or co-exist with plantar fasciitis (Onwuanyi, 2000; Johal & Milner, 2012). Other PF disorders include: rupture, usually following corticosteroid injections to treat plantar fasciitis (Kim et al. 2010a), although this may also occur spontaneously (Louwers et al. 2010); and plantar fibromatosis, or Ledderhose disease (Bardelli et al. 1991; English et al. 2012), which resembles and is often associated with Dupuytren's contracture (Gudmundsson et al. 2013).
The PF has been studied quite well from a biomechanical point of view. It has a fundamental role in the biomechanics of the foot, in supporting its medial longitudinal arch and in the mechanisms of propulsion, dissipating the forces and stresses involving the foot during gait or in other loading conditions (Hicks, 1954; Bolgla & Malone, 2004). It was also demonstrated recently that the PF is capable of storing strain energy and converting it into propulsive force, behaving like a quasi-elastic tissue (Natali et al. 2010; Pavan et al. 2011).
In contrast, very little attention has been paid to the anatomical-histological aspects of the PF, which has been considered simply as an aponeurosis because almost all of its type 1 collagen fibers are arranged in the longitudinal direction. Nothing is known about its elastic fiber content, the features of its extracellular matrix or the extent of its innervation. The relationship between the PF and the Achilles tendon is another point of debate. According to some authors, the embryological origin of the PF sees it united with the Achilles tendon (Shaw et al. 2008), and this connection comprises a layer of periosteal fibers that declines in thickness and elastic properties with aging (Snow et al. 1995). In an anatomical study, Kim et al. (2010b) found that only 5% of 40 cadavers examined had a lower calcaneal insertion of the Achilles tendon and thus retained a connection between their Achilles tendon and their PF. In a later study, the same authors (2011) assessed the insertion of the Achilles tendon on magnetic resonance images (MRI) and reported that older people had a more proximally inserted Achilles tendon than younger people; they suggested that the proportion of people in the normal population with a PF–Achilles tendon connection would be higher (given the older age of the cadavers used in their previous study). These recent results indicate that, from a morphological point of view, a connection between the Achilles tendon and the PF is more likely in younger people. From a clinical point of view, on the other hand, many rehabilitation protocols recommend always considering the Achilles tendon and the triceps surae muscle in rehabilitation treatments for plantar fasciitis, although how these structures might influence the plantar fascia remains to be seen.
The aim of this study was to examine the macroscopic and microscopic characteristics of the PF to shed light on the elements and factors involved in diseases of this tissue. A secondary aim was to verify its possible correlation with surae structures in cadavers and the living.
Materials and methods
Macroscopic study
Anatomical studies (approved by the local ethical committee) were conducted on 12 unembalmed leg specimens (mean age: 71.9 years; range 67–92 years; seven males, five females; see Table 1) obtained from the ‘body donation program’ at the Anatomy Institute of the University of Padua (Porzionato et al. 2012). Eleven feet were dissected via a longitudinal cutaneous incision along the midline from the heel to the third toe. Initially, the skin alone was cut and raised medially and laterally to examine the subcutaneous tissue, which was then carefully removed to expose the PF. The fascia was isolated proximally to its calcaneal origin and distally up to the insertion in the metatarsophalangeal joints. The muscle insertions in the fascia and the connections with contiguous structures were removed to isolate the PF completely.
Table 1.
Cadaver parameters and plantar fascia size
| Size of the PF | ||||||||
|---|---|---|---|---|---|---|---|---|
| Donor id | Foot left/right | Age | Sex | Height (cm) | Weight (kg) | LG (cm) | WP (cm) | WD (cm) |
| 1 | L | 67 | M | 183 | 136 | 14 | 3 | 7.5 |
| R | 14 | 3 | 7.5 | |||||
| 2 | L | 76 | M | 172 | 41 | 13 | 2 | 5.5 |
| R | 13 | 2 | 5.5 | |||||
| 3 | R | 92 | F | 162 | 50 | 9.5 | 1 | 4.5 |
| 4 | L | 67 | F | 167 | 84 | 11 | 2 | 6.5 |
| R | 11 | 2 | 6.5 | |||||
| 5 | L | 67 | M | 165 | 79 | 10.5 | 1.5 | 5 |
| R | 10.5 | 1.5 | 5 | |||||
| 6 | R | 81 | F | 185 | 68 | 13.5 | 3 | 7 |
| 7 | R | 67 | F | 160 | 42 | 11.5 | 1 | 4.5 |
| 8 | R | 69 | M | 175 | 73 | 12.5 | 2 | 6 |
LG, length of the PF along the main longitudinal axis; WP, proximal width of the PF near the heel insertion; WD, distal width of the PF.
One foot was fixed in formalin and serial transversal sections were obtained every 2 cm from the cutis to the interosseous muscles for the purpose of microscopically examining the relationships between the PF and the skin and muscles. One fascia was isolated and fixed in formalin, then serial longitudinal sections were obtained and prepared for histological examination. Specimens from the proximal, central and distal portions were taken for histological studies on all the other fasciae.
Further anatomical evaluations were performed on specimens from the anatomical collection of the Anatomy Institute of Padova University, consisting of transverse, 2- to 5-cm-thick slices of three whole human bodies (two males and one female), preserved in Kaiserling's solution. In these specimens, the connection between the plantar fascia and the Achilles tendon and paratenon was analyzed.
Histological study
For each foot, eight specimens of the PF were taken: two samples were taken from the proximal portion of the plantar fascia, near the heel insertion, three samples from central portion (one from the medial part of the PF, one from the middle part and one from the lateral part) and three from distal portions, taking care to keep them anatomically intact. The specimens were mounted on cardboard to avoid deformations and artifacts, then fixed in 10% formalin solution and embedded in paraffin. Sections 10 μm thick were cut and stained with hematoxylin and eosin, van Gieson for elastic fibers, Azan–Mallory for collagen fibers, and Alcian blue for hyaluronan. Four immunohistochemical stains were also applied: anti-S100, based on a previously described method (Stecco et al. 2007) to identify any nerve structures, hyaluronan binding protein (HABP) as a hyaluronan marker, anti-collagen II and III antibodies (rabbit antibodies, Abcam) to identify the presence of collagen type II and III into the PF. The optimal dilution of the antibodies was evaluated in the datasheet, in a series of previous studies and various tests in our laboratory. Finally, the collagen III antibody was used with a dilution of 1 : 1000, the collagen II antibody with a dilution of 1 : 200 after proteinase K digestion. The sections were incubated using the DAKO Autostainer System. All preparations were observed under a DM4500-B light microscope (Leica Microsystems, Wetzlar, Germany) and recorded in full color mode (24-bit) with a digital camera (DFC 480, Leica Microsystems).
Radiological study
This analysis (approved by the regional ethical committee) was conducted on 52 MRI of the hindfoot of patients with symptoms of non-specific ankle and/or foot pain, randomly and anonymously drawn from a radiology database of the Euganea Medica center (37 males, 14 females; mean age 44.2 years). Each MRI was obtained on a 1.5 T MR system (Philips Medical Systems, Gyroscan Intera, Best, Netherlands), acquiring the images with a standard protocol as follows: high-resolution, T1-weighted spoiled gradient-echo (FLASH) images with a short TE (600/10), flip angle 90°, bandwidth 78 KHz, 113 × 180 mm rectangular field of view, and 211 × 152 matrix (pixel size, 0.54 × 0.35 mm). The sagittal sections obtained were 3 mm thick, with a gap of 0.3 mm between them. The images were analyzed in multi-planar reformatting reconstruction (MPR) mode on the axial, sagittal and coronal planes. MR scan analysis and post-processing were done on an Aquarius workstation Terarecon 3.6.2.3 (San Matteo, CA, USA). All images were reviewed by the same author (C.M.) and the parameters were analyzed (Fig. 1) using Sectra software (Sectra Imtec AB, Linköping, Sweden). First of all, the MRIs were divided into homogeneous and inhomogeneous Achilles tendon signals on axial and sagittal T1 images. If the intense signal was inhomogeneous, the signal appearance was classified according to Soila (Soila et al. 1999) as follows:
Fig. 1.
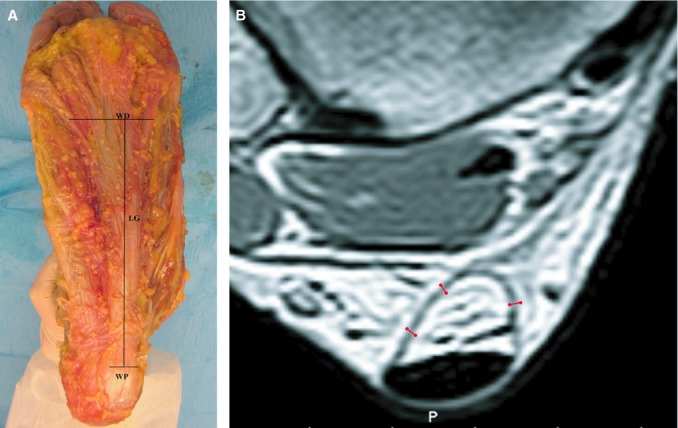
(A) Dissection of the foot, macroscopic view of the plantar fascia. The PF appeared as a pearly white, glistening layer of fiber bundles a few millimeters thick, tapering off in a proximal-distal direction, mainly arranged longitudinally. LG, length of the PF along the main longitudinal axis; WP, proximal width of the PF near the heel insertion; WD, distal width of the PF. (B) Axial MR image of the Achilles tendon. The thickness of the paratenon was measured a level with the passage between muscle and tendon, where muscle fibers were no longer visible. For each case, three measures were done, and the mean value calculated.
type 1: intratendinous intermediate signal intensity strands;
type 2: punctuate or patchy intermediate signal intensity in intratendineous foci;
type 3: diffuse ground glass-type intermediate signal intensity, not judged as punctuate.
For the Achilles tendinopathies, the presence of tendinosis, tendinitis, typical tear, atypical tear, Maglund's and bursitis retrocalcaneal according to Pierre-Jerome (Pierre-Jerome et al. 2010). were evaluated.
The Achilles tendinopathies were assessed using an anatomical classification of Achilles tendonitis recently proposed by Del Buono (Del Buono et al. 2013). i.e.
intramuscular
free tendon: (A) proximal, (B) middle, (C) distal
calcaneal.
For all patients, the thickness of the PF 2 cm from its calcaneal insertion was measured on mid-sagittal T1-W images, and the thickness of the paratenon on axial T1 images on a level with the passage between muscle and tendon at which muscle fibers were no longer visible. For each patient, three measures were done, and the mean value calculated (Fig. 1B). Mean values and standard deviations of all the measurements obtained were calculated and Student's t-test was applied. The correlation between plantar fascia and paratenon thickness was performed with Graphpad software (GraphPad Software, La Jolla, CA, USA). To reveal differences between the thickness of the plantar fascia and Achilles paratenon, statistical analysis was performed by the Kruskal–Wallis test. P < 0.05 was considered to be statistically significant. Statistical calculations were carried out by Prism 3.0.3 (GraphPad Software, San Diego, CA, USA).
Results
Macroscopic study
After the skin dissection, the firm insertion of the plantar fascia (PF) in the cutis thanks to fibrous septae was evident in all cases, particularly in the proximal and distal portions of the PF. Subcutaneous fat was abundant, especially in the area of calcaneus, where it forms the fat pad of the heel. On removing this fat, the PF appeared as a pearly white, glistening layer of fiber bundles a few millimeters thick, tapering off in the proximal-distal direction, mainly arranged longitudinally and with a viscous-elastic consistency to the touch (Fig. 1). The PF were a mean 2 and 6 cm crosswise at the proximal and distal ends, respectively, and 12 cm lengthwise from the medial tuberculum to the metatarsophalangeal joint (Table 1).
On the inside, the PF was firmly attached to the superficial muscles of the sole, particularly in the proximal insertions. Distally, some perpendicular septae were detached from the PF and enveloped the tendons of the abductor hallucis and the four tendons of the flexor digitorum brevis, like the septa of Legueu and Juvara in the hand1. Various compartments for the tendons were thus formed, closely connected to the PF. Many inter- and intramuscular septae originated from the inner side of the PF, extending into the deep fascia of the foot, separating the three superficial muscles of the sole (abductor hallucis, flexor digitorum brevis, abductor digiti quinti), and being the origin of many muscle fibers at the same time.
All dissections revealed a PF divided into three parts (medial, central and lateral). The central portion was the thickest, arising on the medial tuberculum of the calcaneus and extending forward to cover the plantar surface of the flexor digitorum brevis muscle, before dividing unevenly into five digitations inserted each in a different metatarsophalangeal joint capsule. Most of the PF fibers were arranged longitudinally and obliquely, whereas there were some superficial fibers lying transversally, particularly on the proximal and distal portions. In all cases the PF continued over the calcaneal bone with a thin band (approximately 1–2 mm thick) corresponding to the periosteum of the calcaneal bone (Fig. 2). This layer surrounded the calcaneus and was in continuity with the paratenon of the Achilles tendon. The lateral portion covered the plantar surface of the abductor digiti quinti muscle, then continued with this muscle's fascia and laterally with the dorsal fascia. It was distally thin and proximally thick, where it formed a strong band in which some abductor digiti quinti muscle fibers were inserted, and it continued onto the inferior peroneal retinaculum. Distally, it was inserted in the fifth metatarsal joint capsule. The medial portion was less obvious and thinner than the others. It covered the plantar surface of the abductor hallucis muscle and included many insertions of this muscle's fibers. The medial portion of the PF extended to the flexor retinaculum of the foot and medially to the dorsal fascia of the foot. It was ultimately inserted in the first metatarsal joint.
Fig. 2.
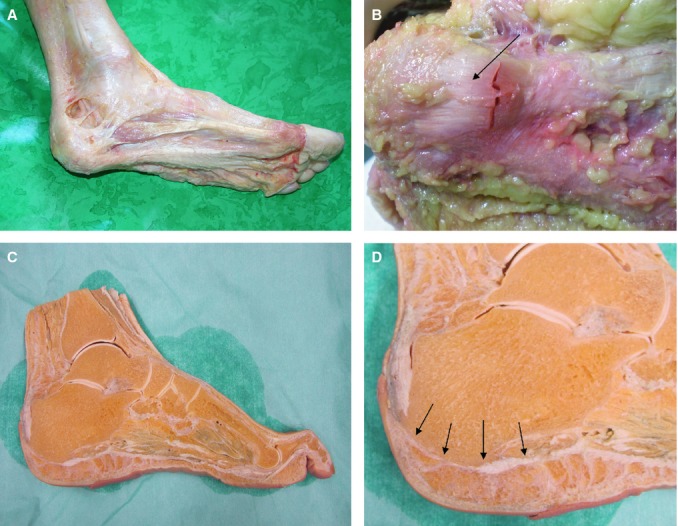
Continuity of the plantar fascia with the paratenon of the Achilles tendon. (A,B) Dissection of fresh cadaver. In (B) the fibrous tissue over the heel is evident. (C,D) Slice of foot preserved in Kaiserling's solution coming from the anatomical collection of Padova university. The arrows highlight the continuity between plantar fascia and Achilles paratenon.
Microscopic study
On full-thickness specimens obtained 2 cm from the calcaneal origin, the PF was 3.15 mm thick in the central portion and 1.56 mm laterally; 10 cm from the calcaneal origin, it was thinner, the central portion differing less in size from the lateral part, 1.41 mm and 0.66 mm, respectively (Fig. 3).
Fig. 3.
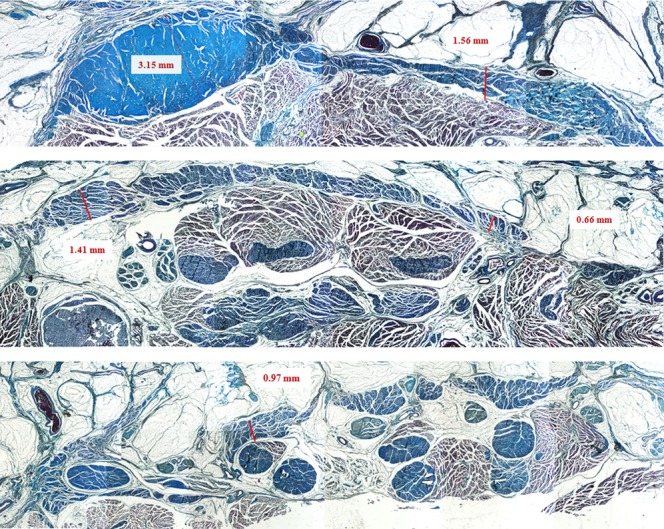
Transversal sections of the plantar fascia at the proximal, intermediate and distal level, Azan–Mallory stain.
The PF is composed mostly of type I collagen fibers arranged in a proximal-distal direction, with a few transversal and vertical collagen fibers. The anti-collagen III immunohistochemical study revealed the presence of type III collagen only in the loose connective tissue and, above all, where the large fibrous bundles are in different directions. This type of collagen is richly represented also in the perimysium of the plantar muscles (Fig. 4A,B). The anti-collagen II immunohistochemical study highlighted the type II collagen only in the samples near the heel, both in the extracellular matrix and around some cells with chondrocyte features (Fig. 4C,D).
Fig. 4.
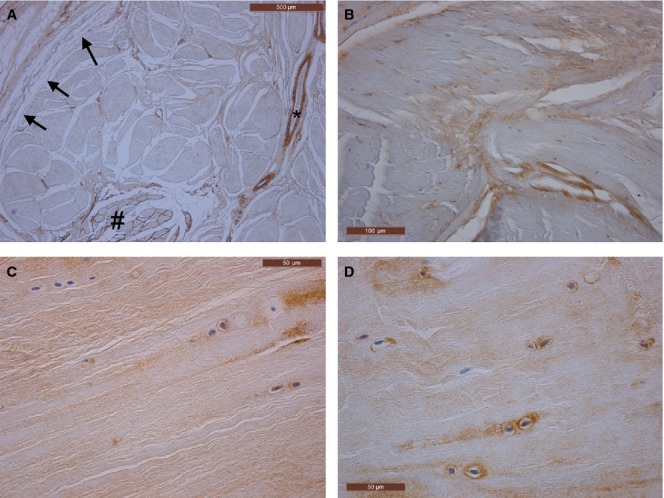
Immunohistochemical stains type III (A,B) and type II anti-collagen (C,D) of the plantar fascia. (A) The collagen type III is well represented in the vessel walls (*) and in the perimysium of the plantar muscles (#), whereas in the plantar fascia it is present only in the loose connective tissue (arrows) among the large collagen fibrous bundles. (B) Type III collagen is evident above all the point at which the large collagen fibrous bundles change direction. (C,D) Samples of the plantar fascia near the heel; type II collagen is evident in the extracellular matrix and around some large cells (chondrocytes).
Loose connective tissue visible among the various fibrous bundles revealed a few very thin elastic fibers on van Gieson staining (Fig. 5) and the presence of hyaluronan on Alcian blue staining (Fig. 6). The immunohistochemical anti-HABP stain specific for hyaluronan showed that this glycosaminoglycan is located among collagen fibers. Using the Alcian blue stain, many cells stained very intensely in the proximal part of the PF, near the heel insertion, and the cells were large with a basophilic cytoplasm (typical of chondrocytes), whereas the cells staining strongly with Alcian blue in other parts of the PF were completely different: they were tiny fusiform fibroblast-like cells arranged singly or in rows in the direction of the collagen fibers.
Fig. 5.
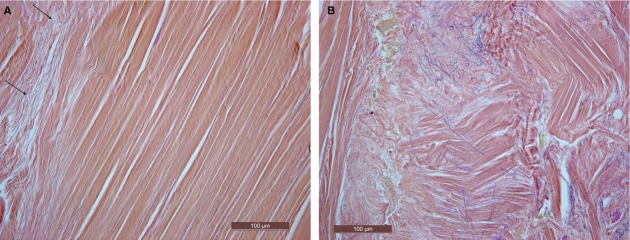
Samples of the plantar fascia, van Gieson stain. The elastic fibers are present only in the loose connective tissue among the various collagen fibrous bundles (arrows), not in the fibrous component of the plantar fascia (A). The elastic fibers form a thin, irregular mesh (B).
Fig. 6.
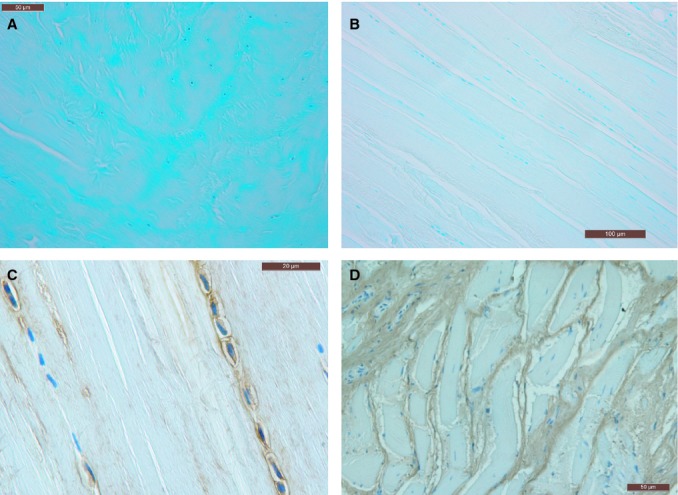
Alcian blue stain (A,B) in the proximal portion near the heel (A) and in the distal portion (B). In (A) the chondrocytes are evident and in (B) the fasciacytes. (C,D) Immunohistochemical anti-HABP stain specific for hyaluronan showed that this glycosaminoglycans is in the cytoplasm of the fasciocytes (C) and disposed among collagen fibers (D).
Using the anti-S100 immunohistochemical stain revealed numerous nerve endings and Ruffini and Pacini corpuscles inside the PF. They were particularly evident in the medial, lateral and distal parts of the PF, where the PF is thinner and joins the abductor hallucis and digiti minimi fasciae, or where it is connected to the metatarsophalangeal joints. The inner surface of the PF (in which many fibers of the muscles of the sole of the foot are inserted) is innervated more than the outer surface (Fig. 7).
Fig. 7.
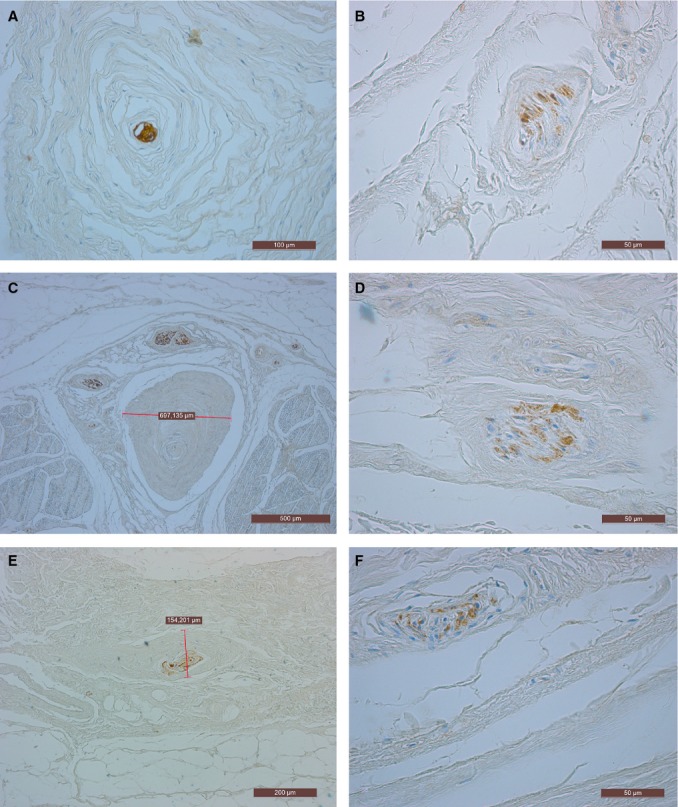
Innervation of the plantar fascia, anti-S100 immunohistochemical stain. (A,C,E) Pacini corpuscles. (B,D,F) Ruffini corpuscles.
Radiological study
The Achilles tendon showed signs of tendinitis in 27 of the 52 MRIs considered. In the other 25/52 patients, the Achilles tendon appeared on MRI as a homogeneous structure of low signal intensity and rounded shape, characterized by a hyperintense signal representing small vessels, and with no signs of inflammation or degeneration (Tables 2 and 3). This latter group showed signs of other pathological conditions, such as cysts, foot ligament disorders or osteoarthritis.
Table 2.
Parameters of MRI without signs of Achilles tendinosis
| MRI | Age | Sex | PF thickness (mm) | Paratenon thickness (mm) |
|---|---|---|---|---|
| 1 | 30 | F | 1.39 | 0.91 |
| 2 | 41 | F | 1.76 | 1.12 |
| 3 | 65 | F | 2.1 | 0.88 |
| 4 | 18 | M | 2.11 | 1.49 |
| 5 | 39 | F | 1.74 | 0.91 |
| 6 | 40 | F | 1.39 | 0.92 |
| 7 | 34 | M | 2.4 | 0.87 |
| 8 | 47 | F | 2.08 | 1.34 |
| 9 | 48 | M | 3.1 | 0.9 |
| 10 | 25 | M | 2.8 | 1.12 |
| 11 | 23 | F | 1.66 | 0.62 |
| 12 | 37 | M | 2.8 | 1 |
| 13 | 48 | M | 2.8 | 0.71 |
| 14 | 18 | F | 2.08 | 0.63 |
| 15 | 23 | M | 2.43 | 0.63 |
| 16 | 19 | M | 2.1 | 0.54 |
| 17 | 25 | M | 1.74 | 0.59 |
| 18 | 27 | M | 2 | 0.55 |
| 19 | 26 | M | 1.7 | 0.78 |
| 20 | 76 | F | 2.46 | 0.75 |
| 21 | 76 | F | 1.78 | 0.7 |
| 22 | 28 | M | 1.74 | 0.56 |
| 23 | 53 | M | 2.43 | 0.62 |
| 24 | 31 | M | 1.4 | 0.66 |
| 25 | 23 | M | 2.2 | 0.74 |
| Mean value (± SD) | 36.8 (± 16.79) | 2.09 (± 0.47) | 0.82 (± 0.25) |
Table 3.
Parameters of MRI with signs of Achilles tendinosis
| MRI | Age | Sex | PF thickness (mm) | Paratenon thickness (mm) |
|---|---|---|---|---|
| 1 | 75 | F | 2.2 | 1.1 |
| 2 | 40 | F | 1.7 | 1.2 |
| 3 | 31 | M | 2.8 | 1.3 |
| 4 | 30 | M | 3.1 | 1.4 |
| 5 | 44 | M | 3.6 | 1.3 |
| 6 | 40 | M | 4.6 | 1.3 |
| 7 | 40 | M | 5.6 | 1.3 |
| 8 | 40 | M | 4.3 | 1.4 |
| 9 | 40 | M | 3.4 | 1.4 |
| 10 | 53 | M | 3.5 | 1.4 |
| 11 | 53 | M | 3.5 | 2 |
| 12 | 46 | M | 2.7 | 1.1 |
| 13 | 41 | M | 3.5 | 1.3 |
| 14 | 41 | M | 4 | 1.1 |
| 15 | 46 | M | 4.6 | 1.6 |
| 16 | 40 | M | 2.4 | 1.4 |
| 17 | 60 | M | 5.3 | 1.1 |
| 18 | 44 | F | 4.6 | 1.4 |
| 19 | 48 | M | 3.2 | 1.4 |
| 20 | 67 | M | 2.2 | 1.5 |
| 21 | 88 | M | 3.5 | 1.1 |
| 22 | 71 | M | 2.8 | 1.1 |
| 23 | 81 | M | 2.4 | 1.2 |
| 24 | 73 | M | 3.5 | 1.6 |
| 25 | 59 | M | 3.7 | 1.4 |
| 26 | 81 | F | 2.7 | 1.4 |
| 27 | 57 | M | 3.2 | 1.1 |
| Mean value (± SD) | 52.9 (± 16.3) | 3.42 (± 0.95) | 1.34 (± 0.20) |
Based on the Del Buono classification (Del Buono et al. 2013), the tendinopathies in 9/27 patients were located in the calcaneal region, four on the distal, eight on the medial, and five on the proximal free tendon, and one was intramuscular.
The thickness of the PF was compared between the two groups: in the group of patients with evidence of tendinopathy, the PF was 3.43 ± 0.48 mm thick (99%CI and SD: 0.95; range 1.71–5.6 mm), whereas in patients with no signs of Achilles tendon inflammation or degeneration, the PF was 2.09 ± 0.24 mm thick (99%CI and SD: 0.47). The difference of 1.29 ± 0.57 mm (99%CI) was statistically significant (P < 0.001) (Fig. 8).
Fig. 8.
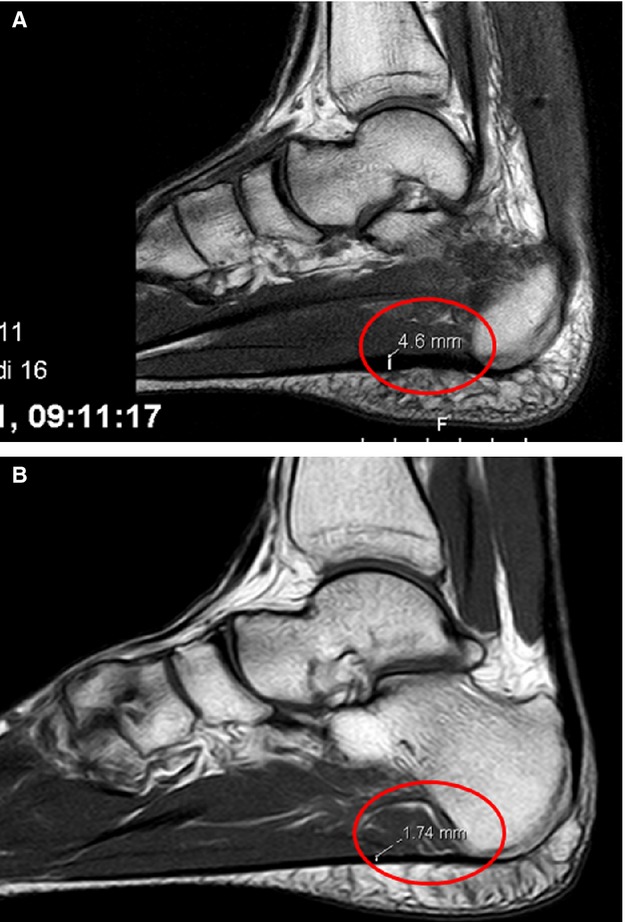
MRI of healthy patients (B) and patients with Achilles tendon pathology (A). The thickness of plantar fascia is highlighted.
In patients with tendinopathies, 14/27 had a PF more than 3.5 mm thick, and in five of these it was more than 4.5 mm (considered the threshold for a diagnosis of PF). In these last five cases, the tendinopathy involved the calcaneal region in three, the distal free tendon in one, and the medial free tendon in one. In patients with no signs of Achilles tendon disease, none had a PF more than 4 mm thick.
Discussion
In the past, the plantar fascia has received more attention from the biomechanical engineers and clinicians than from anatomists. This is evident also analyzing the anatomical terminology. In the Nomina Anatomica (1998) only the term plantar aponeurosis is used to indicate this structure, but it is inserted in the chapter ‘fasciae’. In the various anatomical textbooks, the terms ‘plantar fascia’ and ‘plantar aponeurosis’ are used interchangeably. In fact, the term ‘aponeurosis’ is generally used to indicate a tissue with a unidirectional arrangement of collagen fibers, whereas a fascia is a structure with a multidirectional arrangement of the fibers (Langevin & Huijing, 2009). No published works have discussed which term is more appropriate for this tissue and it is not clear whether the PF contains only longitudinal or also multidirectional fibers. In our microscopic study, different stains were used to reveal the arrangement and composition of the plantar fascia: the collagen fibers were found arranged mainly in a proximal-to-distal longitudinal direction, but there were also various fibers lying in vertical, transverse and oblique directions. This multilayer configuration of the collagen fibers is a typical feature of fasciae rather than aponeurosis, so we suggest that the term ‘plantar fascia’ would be a more appropriate name for this tissue. With the immunohistochemical stains it was possible to affirm that almost all the tissue is formed of type I collagen; only in the loose connective tissue where the large fibrous bundles change directions is there also type III collagen. Few elastic fibers could be revealed in the loose connective tissue, but generally we can affirm that the plantar fascia is not an elastic tissue.
The PF was found well innervated, especially where it joins with the fasciae of the abductor hallucis and abductor digiti minimi muscles, and where the sole muscles are inserted. The presence of Pacini and Ruffini corpuscles – usually considered responsible for mechanoreception (Moraes et al. 2008) – suggests that plantar fascia innervations have a role in proprioception and in the stability and control of foot movements. In the last years there has been increasing interest within therapeutic communities concerning the role that fascia plays in musculoskeletal strain disorders such myofascial pain, postural strain patterns and proprioception (Findley, 2009) but nothing has been published about the possible proprioceptive role of the plantar fascia. However, our findings reinforce previous studies about the antebrachial fascia (Stecco et al. 2007), the thoracolumbar fascia (Tesarz et al. 2011), crural fascia and ankle retinacula (Benjamin, 2009). Thanks to the many muscle insertions, the plantar fascia is capable of perceiving both the foot's position and the state of contraction of the various intrinsic muscles of the foot. If these muscles contract excessively, the PF (and the nerve endings it contains) might be overstretched. These properties of the plantar fascia shed new light on this complex tissue. The fascia could be seen as a coachman guiding the muscles in the sole of the foot and helping to coordinate all these structures during movement. Besides, the presence of septa similar to those of Legueu and Juvara in the hand also suggest a strong relation of the PF with the flexor tendons and lumbrical muscles of the foot.
The ubiquitous presence of hyaluronan (HA) inside the plantar fascia is a novel, intriguing finding. It enables the different fibrous bundles to glide, as well as being an efficient shock absorber and possibly also serving an anti-inflammatory purpose (Neuman et al. 2011). HA is known to be particularly prominent during embryogenesis, in tissues undergoing rapid growth, and whenever repair and regeneration occur (Lee & Spicer, 2000; Spicer & Tien, 2004). Fragmented HA seems to have highly angiogenic, inflammatory and immunostimulatory (Noble, 2002; Walton, 2008) effects, and reflects tissue stress. Based on these anatomical findings, it would seem pertinent to consider the medical use of hyaluronan injection as an alternative to corticosteroid injections for plantar fasciitis, since HA is physiologically better suited to the composition of the normal PF. Such injections could increase the visco-supplementation of the PF, reduce stress and friction between collagen fibers, and contain PF inflammation and degeneration. There are reports in the literature of a positive effect of hyaluronan injection in Achilles, rotator cuff and patellar tendonitis (Coombes et al. 2010). In animal studies, hyaluronan has revealed the capacity to reduce nociception (Gomis et al. 2009). Further research will also focus on the potential role of the tiny fusiform fibroblast-like cells staining strongly for Alcian blue: similar cells – named as ‘fasciacytes’ – have recently been found in the deep fasciae of the limbs (Stecco et al. 2011). These cells are probably responsible for the production and secretion of hyaluronan as synoviocytes on the internal surface of joint capsules (Chang et al. 2010) or as hyalocytes in the vitreous chamber of the eye (Sakamoto & Ishibashi, 2011). The chondrocytes found near the heel insertion would have a totally different meaning: they are the expression of a cartilagineous metaplasia that gradually reduces the connection between the Achilles tendon and the plantar fascia (Kim et al. 2011), reducing PF elasticity as a consequence. Mechanical stress is considered one of the important factors in cartilagineous metaplasia, and in ligament ossification, too (Tsukamoto et al. 2006). The accumulation of tensile stress in the PF is probably responsible for the progressive chondroplasia of the calcaneal entheses of the PF, and it could be seen as an adaptive mechanism to ensure the integrity of the interface in response to greater mechanical loads (Benjamin et al. 2000). Our findings thus confirm the gradual shrinking of the anatomical connection between the PF and the Achilles tendon, but this connection seems to persist with the paratenon of the Achilles tendon, thanks to a thin layer of periosteal fibers in the heel. The relationship between the PF and the Achilles paratenon was also confirmed by our MRI data showing a statistically significant correlation between their thicknesses. Various authors (Kvist et al. 1987; Graf et al. 1990; Sharma & Maffulli, 2005; Van Sterkenburg & van Dijk, 2011) have recently emphasized the role of the paratenon in the etiology of tendinopathy. In our setting, five of 27 patients with Achilles tendinitis had a PF more than 4.5 mm thick, whereas none of the patients whose MRIs showed no signs of tendinitis had a PF more than 4 mm thick (Fig. 9). The thickness of more than 4.5 mm using MR and ultrasound represent fasciitis (McNally & Shetty, 2010) and in a systemic review it was found that patients with chronic plantar heel pain are likely to have a thickened plantar fascia (McMillan et al. 2009). MR is also very sensitive for highlighting pathological changes around the plantar fascia (Pierre-Jerome et al. 2010), as perifascial edema, bone marrow edema of the calcaneus at the insertion of the plantar fascia, and high signal intensity within the plantar fascia. Moreover, in the case of aponeurotic thickening on T1 sequences the presence of intermediate signal intensity within the plantar fascia has been suggested (Healey & Chen, 2010). On the other hand, MRI is not usually used to diagnose plantar fasciitis (Healey & Chen, 2010) because of its high costs, and ultrasound (US) is often preferred. US is highly operator-dependent in that it requires a long learning curve and there is also a lack of standardization (Kane et al. 2004). Nevertheless, US evaluation of the thickness of the plantar fascia showed high reliability for both intra- and inter-rater evaluations (Cheng et al. 2012), and fascial thickening and hypoechoic echotexture were correlated with heel pain score at real-time sonoelastography of the plantar fascia (Sconfienza et al. 2013). The strong correlation between the thickness of PF and Achilles paratenon suggest in the case of plantar fascia thickening the characteristics of Achilles tendon and paratenon should also be evaluate, but it is probable that the reverse could happen, that is, that in the case of chronic Achilles tendinitis, the plantar fascia could be damaged. Indeed Warren (1990) reported a positive correlation between Achilles tendon loading and plantar fascia tension, and Carlson et al. (2000) demonstrated that excessive stretching and tightness of the Achilles tendon are risk factors of plantar fasciitis. Finally, these findings support the results reported by Bolívar et al. (2013), who identified a greater tightness of the posterior leg muscles in individuals with plantar fasciitis, and suggested that therapists intending to use a stretching protocol to treat plantar fasciitis should look for both hamstring and triceps surae tightness.
Fig. 9.
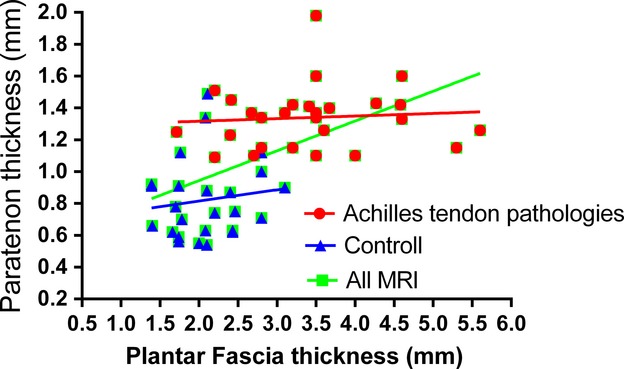
Correlation between plantar fascia thickness and thickness of Achilles tendon paratenon in patients with Achilles tendonitis and healthy people.
Acknowledgments
Our special thanks to Dr. Anna Rambaldo and Gloria Sarasin for technical assistance. This work was supported by MIUR Ex 60% 2011.
Footnotes
In 1892, two French anatomists, Legueu and Juvara, published an article which described multiple septa originating from the palmar aponeurosis and attached deeply into the anterior interosseous muscle fascia and metacarpal bone. Eight well-defined septa could be encountered in each hand. A septum is located on either side of each flexor digitorum profundus and superficialis tendons of the index through the small fingers. These septa formed seven compartments or septal canals, four containing the flexor tendons and three containing the common digital nerves and arteries and the lumbrical muscles (Bilderback & Rayan, 2004).
Author contributions
Carla Stecco: dissections and histology, data interpretation, writing the paper. Marco Corradin: dissection and imaging study. Veronica Macchi: imaging study. Andrea Porzionato: data analysis. Aldo Morra: imaging study. Carlo Biz: data interpretation. Raffaele De Caro: critical revision and approval of manuscript.
References
- Acevedo JI, Beskin JL. Complications of plantar fascia rupture associated with corticosteroid injection. Foot Ankle Int. 1998;19:91–97. doi: 10.1177/107110079801900207. [DOI] [PubMed] [Google Scholar]
- Arangio GA, Chen C, Kim W. Effect of cutting the plantar fascia on mechanical properties of the foot. Clin Orthop Relat Res. 1997;339:227–231. doi: 10.1097/00003086-199706000-00031. [DOI] [PubMed] [Google Scholar]
- Bardelli M, D'Arienzo M, Veneziani C. Ledderhose's disease. Arch Putti Chir Organi Mov. 1991;39:335–339. [PubMed] [Google Scholar]
- Benjamin M. The fascia of the limbs and back – a review. J Anat. 2009;214:1–18. doi: 10.1111/j.1469-7580.2008.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Rufai A, Ralphs JR. The mechanism of formation of bony spurs (enthesophytes) in the achilles tendon. Arthritis Rheum. 2000;43:576–583. doi: 10.1002/1529-0131(200003)43:3<576::AID-ANR14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Bilderback KK, Rayan GM. The septa of Legueu and Juvara: an anatomic study. J Hand Surg Am. 2004;29:494–499. doi: 10.1016/j.jhsa.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Bolgla LA, Malone TR. Plantar fasciitis and the windlass mechanism: a biomechanical link to clinical practice. J Athl Train. 2004;39:77–82. [PMC free article] [PubMed] [Google Scholar]
- Bolívar YA, Munuera PV, Padillo JP. Relationship between tightness of the posterior muscles of the lower limb and plantar fasciitis. Foot Ankle Int. 2013;34:42–48. doi: 10.1177/1071100712459173. [DOI] [PubMed] [Google Scholar]
- Carlson RE, Fleming LL, Hutton WC. The biomechanical relationship between the tendoachilles, plantar fascia and metatarsophalangeal joint dorsiflexion angle. Foot Ankle Int. 2000;21:18–25. doi: 10.1177/107110070002100104. [DOI] [PubMed] [Google Scholar]
- Chandler TJ, Kibler WB. A biomechanical approach to the prevention, treatment and rehabilitation of plantar fasciitis. Sports Med. 1993;15:344–352. doi: 10.2165/00007256-199315050-00006. [DOI] [PubMed] [Google Scholar]
- Chang SK, Gu Z, Brenner MB. Fibroblast-like synoviocytes in inflammatory arthritis pathology: the emerging role of cadherin-11. Immunol Rev. 2010;233:256–266. doi: 10.1111/j.0105-2896.2009.00854.x. [DOI] [PubMed] [Google Scholar]
- Cheng JW, Tsai WC, Yu TY, et al. Reproducibility of sonographic measurement of thickness and echogenicity of the plantar fascia. J Clin Ultrasound. 2012;40:14–19. doi: 10.1002/jcu.20903. [DOI] [PubMed] [Google Scholar]
- Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet. 2010;376:1751–1767. doi: 10.1016/S0140-6736(10)61160-9. [DOI] [PubMed] [Google Scholar]
- Cornwall MW, McPoil TG. Plantar fasciitis: etiology and treatment. J Orthop Sports Phys Ther. 1999;29:756–760. doi: 10.2519/jospt.1999.29.12.756. [DOI] [PubMed] [Google Scholar]
- Del Buono A, Chan O, Maffulli N. Achilles tendon: functional anatomy and novel emerging models of imaging classification. Int Orthop. 2013;37:715–721. doi: 10.1007/s00264-012-1743-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giovanni BF, Moore AM, Zlotnicki JP, et al. Preferred management of recalcitrant plantar fasciitis among orthopaedic foot and ankle surgeons. Foot Ankle Int. 2012;33:507–512. doi: 10.3113/FAI.2012.0507. [DOI] [PubMed] [Google Scholar]
- English C, Coughlan R, Carey J, et al. Plantar and palmar fibromatosis: characteristic imaging features and role of MRI in clinical management. Rheumatology. 2012;51:1134–1136. doi: 10.1093/rheumatology/ker522. [DOI] [PubMed] [Google Scholar]
- FCAT. Terminologia Anatomica: International Anatomical Terminology. New York: Thieme Medical Publishers; 1998. [Google Scholar]
- Findley TW. Second international fascia research congress. Int J Ther Massage Bodywork. 2009;2:1–6. doi: 10.3822/ijtmb.v2i2.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomis A, Miralles A, Schmidt RF, et al. Intra-articular injections of hyaluronan solutions of different elastoviscosity reduce nociceptive nerve activity in a model of osteoarthritic knee joint of the guinea pig. Osteoarthritis Cartilage. 2009;17:798–804. doi: 10.1016/j.joca.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Graf J, Schneider U, Niethard FU. Microcirculation of the Achilles tendon and significance of the paratenon. A study with the plastination method. Handchir Mikrochir Plast Chir. 1990;22:163–166. [in German] [PubMed] [Google Scholar]
- Gudmundsson KG, Jónsson T, Arngrímsson R. Association of morbus Ledderhose with Dupuytren's contracture. Foot Ankle Int. 2013;34:841–845. doi: 10.1177/1071100713475352. [DOI] [PubMed] [Google Scholar]
- Haake M, Buch M, Schoellner C, et al. Extracorporeal shock wave therapy for plantar fasciitis: randomised controlled multicentre trial. Br Med J. 2003;327:75. doi: 10.1136/bmj.327.7406.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healey K, Chen K. Plantar fasciitis: current diagnostic modalities and treatments. Clin Podiatr Med Surg. 2010;27:369–380. doi: 10.1016/j.cpm.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Hicks J. The mechanics of the foot. II. The plantar aponeurosis and the arch. J Anat. 1954;88:25–30. [PMC free article] [PubMed] [Google Scholar]
- Johal KS, Milner SA. Plantar fasciitis and the calcaneal spur: fact or fiction? Foot Ankle Surg. 2012;18:39–41. doi: 10.1016/j.fas.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Kane D, Balint PV, Sturrock R, et al. Musculoskeletal ultrasound – a state of the art review in rheumatology. Part 1: current controversies and issues in the development of musculoskeletal ultrasound in rheumatology. Rheumatology. 2004;43:823. doi: 10.1093/rheumatology/keh214. [DOI] [PubMed] [Google Scholar]
- Kim C, Cashdollar MR, Mendicino RW, et al. Incidence of plantar fascia ruptures following corticosteroid injection. Foot Ankle Spec. 2010a;3:335–337. doi: 10.1177/1938640010378530. [DOI] [PubMed] [Google Scholar]
- Kim PJ, Richey JM, Wissman LR, et al. The variability of the Achilles tendon insertion: a cadaveric examination. J Foot Ankle Surg. 2010b;49:417–420. doi: 10.1053/j.jfas.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Kim PJ, Martin E, Ballehr L, et al. Variability of insertion of the Achilles tendon on the calcaneus: an MRI study of younger subjects. J Foot Ankle Surg. 2011;50:41–43. doi: 10.1053/j.jfas.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Kitaoka HB, Luo ZP, An KN. Effect of plantar fasciotomy on stability of arch of foot. Clin Orthop Relat Res. 1997;344:307–312. [PubMed] [Google Scholar]
- Kvist M, Jozsa L, Jarvinen MJ, et al. Chronic Achilles paratenonitis in athletes: a histological and histochemical study. Pathology. 1987;19:1–11. doi: 10.3109/00313028709065127. [DOI] [PubMed] [Google Scholar]
- Langevin HM, Huijing PA. Communicating about fascia: history, pitfalls, and recommendations. Int J Ther Massage Bodywork. 2009;2:3–8. doi: 10.3822/ijtmb.v2i4.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Spicer AP. Hyaluronan: a multifunctional, megaDalton, stealth molecule. Curr Opin Cell Biol. 2000;12:581–586. doi: 10.1016/s0955-0674(00)00135-6. [DOI] [PubMed] [Google Scholar]
- Lemont H, Ammirati KM, Usen N. Plantar fasciitis: a degenerative process (fasciosis) without inflammation. J Am Podiatr Med Assoc. 2003;93:234–237. doi: 10.7547/87507315-93-3-234. [DOI] [PubMed] [Google Scholar]
- Louwers MJ, Sabb B, Pangilinan PH. Ultrasound evaluation of a spontaneous plantar fascia rupture. Am J Phys Med Rehabil. 2010;89:941–944. doi: 10.1097/PHM.0b013e3181f711e2. [DOI] [PubMed] [Google Scholar]
- McMillan AM, Landorf KB, Barrett JT, et al. Diagnostic imaging for chronic plantar heel pain: a systematic review and meta-analysis. J Foot Ankle Res. 2009;2:32. doi: 10.1186/1757-1146-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan AM, Landorf KB, Gilheany MF, et al. Ultrasound guided corticosteroid injection for plantar fasciitis: randomised controlled trial. Br Med J. 2012;344:e3260. doi: 10.1136/bmj.e3260. [DOI] [PubMed] [Google Scholar]
- McNally EG, Shetty S. Plantar fascia: imaging diagnosis and guided treatment. Semin Musculoskelet Radiol. 2010;14:334–343. doi: 10.1055/s-0030-1254522. [DOI] [PubMed] [Google Scholar]
- Moraes MR, Cavalcante ML, Leite JA, et al. Histomorphometric evaluation of mechanoreceptors and free nerve endings in human lateral ankle ligaments. Foot Ankle Int. 2008;29:87–90. doi: 10.3113/FAI.2008.0087. [DOI] [PubMed] [Google Scholar]
- Natali AN, Pavan PG, Stecco C. A constitutive model for the mechanical characterization of the plantar fascia. Connect Tissue Res. 2010;51:337–346. doi: 10.3109/03008200903389127. [DOI] [PubMed] [Google Scholar]
- Neuman MG, Nanau RM, Oruña L, et al. In vitro anti-inflammatory effects of hyaluronic acid in ethanol-induced damage in skin cells. J Pharm Pharm Sci. 2011;14:425–437. doi: 10.18433/j3qs3j. [DOI] [PubMed] [Google Scholar]
- Noble PW. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol. 2002;21:25–29. doi: 10.1016/s0945-053x(01)00184-6. [DOI] [PubMed] [Google Scholar]
- Onwuanyi ON. Calcaneal spurs and plantar heel pad pain. The Foot. 2000;4:182–185. [Google Scholar]
- Pavan P, Stecco C, Darwish S, et al. Investigation of the mechanical properties of the plantar aponeurosis. Surg Radiol Anat. 2011;33:905–911. doi: 10.1007/s00276-011-0873-z. [DOI] [PubMed] [Google Scholar]
- Pierre-Jerome C, Moncayo V, Terk MR. MRI of the Achilles tendon: a comprehensive review of the anatomy, biomechanics, and imaging of overuse tendinopathies. Acta Radiol. 2010;51:438–454. doi: 10.3109/02841851003627809. [DOI] [PubMed] [Google Scholar]
- Porzionato A, Macchi V, Stecco C, et al. Quality management of body donation program at the University of Padova. Anat Sci Educ. 2012;5:264–272. doi: 10.1002/ase.1285. [DOI] [PubMed] [Google Scholar]
- Riddle DL, Schappert SM. Volume of ambulatory care visits and patterns of care for patients diagnosed with plantar fasciitis: a national study of medical doctors. Foot Ankle Int. 2004;25:303–310. doi: 10.1177/107110070402500505. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Ishibashi T. Hyalocytes: essential cells of the vitreous cavity in vitreoretinal pathophysiology? Retina. 2011;31:222–228. doi: 10.1097/IAE.0b013e3181facfa9. [DOI] [PubMed] [Google Scholar]
- Sconfienza LM, Silvestri E, Orlandi D, et al. Real-time sonoelastography of the plantar fascia: comparison between patients with plantar fasciitis and healthy control subjects. Radiology. 2013;267:195–200. doi: 10.1148/radiol.12120969. [DOI] [PubMed] [Google Scholar]
- Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005;87:187–202. doi: 10.2106/JBJS.D.01850. [DOI] [PubMed] [Google Scholar]
- Shaw HM, Vázquez OT, McGonagle D, et al. Development of the human Achilles tendon enthesis organ. J Anat. 2008;213:718–724. doi: 10.1111/j.1469-7580.2008.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow SW, Bohne WH, DiCarlo E, et al. Anatomy of the Achilles tendon and plantar fascia in relation to the calcaneus in various age groups. Foot Ankle Int. 1995;16:418–421. doi: 10.1177/107110079501600707. [DOI] [PubMed] [Google Scholar]
- Soila K, Karjalainen PT, Aronen HJ, et al. High-resolution MR imaging of the asymptomatic Achilles tendon: new observations. AJR Am J Roentgenol. 1999;173:323–328. doi: 10.2214/ajr.173.2.10430128. [DOI] [PubMed] [Google Scholar]
- Spicer AP, Tien JY. Hyaluronan and morphogenesis. Birth Defects Res C Embryo Today. 2004;72:89–108. doi: 10.1002/bdrc.20006. [DOI] [PubMed] [Google Scholar]
- Stecco C, Gagey O, Belloni A, et al. Anatomy of the deep fascia of the upper limb. Second part: study of innervation. Morphologie. 2007;91:38–43. doi: 10.1016/j.morpho.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Stecco C, Stern R, Porzionato A, et al. Hyaluronan within fascia in the etiology of myofascial pain. Surg Radiol Anat. 2011;33:891–896. doi: 10.1007/s00276-011-0876-9. [DOI] [PubMed] [Google Scholar]
- Tesarz J, Hoheisel U, Wiedenhöfer B, et al. Sensory innervation of the thoracolumbar fascia in rats and humans. Neuroscience. 2011;194:302–308. doi: 10.1016/j.neuroscience.2011.07.066. [DOI] [PubMed] [Google Scholar]
- Tsukamoto N, Maeda T, Miura H, et al. Repetitive tensile stress to rat caudal vertebrae inducing cartilage formation in the spinal ligaments: a possible role of mechanical stress in the development of ossification of the spinal ligaments. Spine. 2006;5:234–242. doi: 10.3171/spi.2006.5.3.234. [DOI] [PubMed] [Google Scholar]
- Tweed Barnes MR, Allen MJ. An evaluation of the long-term effects of total plantar fasciotomy – a preliminary study. Foot. 2009;19:75–79. doi: 10.1016/j.foot.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Van Sterkenburg NM, van Dijk CN. Mid-portion Achilles tendinopathy: why painful? An evidence-based philosophy. Knee Surg Sports Traumatol Arthosc. 2011;19:1367–1375. doi: 10.1007/s00167-011-1535-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton A. Efficacy of myofascial release techniques in the treatment of primary Raynaud's phenomenon. J Bodyw Mov Ther. 2008;12:274–280. doi: 10.1016/j.jbmt.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Warren BL. Plantar Fasciitis in Runners. Sports Med. 1990;10:338–345. doi: 10.2165/00007256-199010050-00004. [DOI] [PubMed] [Google Scholar]


