Abstract
Both oxytocin (OT) and vasopressin (AVP) are known to modulate social behavior, and dysfunction in both systems has been postulated as a potential cause of certain psychiatric disorders that involve social behavioral deficits. In particular, there is growing interest in intranasal OT as a potential treatment for certain psychiatric disorders, and preliminary preclinical and clinical studies suggest efficacy in alleviating some of the associated symptoms. However, the vast majority of research participants in these studies have been male, and there is evidence for sexually differentiated effects of nonapeptides in both humans and non-human animals. To date, no study has investigated the effect of intranasal OT on brain function in human males and females within the same paradigm. Previously, in a randomized, placebocontrolled, double-blind fMRI study, we reported effects of intranasal OT and AVP on behavior and brain activity of human males as they played an interactive social game known as the Prisoner’s Dilemma Game. Here, we present findings from an identical study in human females, and compare these with our findings from males. Overall, we find that both behavioral and neural responses to intranasal OT and AVP are highly sexually differentiated. In women, AVP increased conciliatory behavior, and both OT and AVP caused women to treat computer partners more like humans. In men, AVP increased reciprocation of cooperation from both human and computer partners. However, no specific drug effects on behavior were shared between men and women. During cooperative interactions, both OT and AVP increased brain activity in men within areas rich in OT and AVP receptors and in areas playing a key role in reward, social bonding, arousal and memory (e.g., the striatum, basal forebrain, insula, amygdala and hippocampus), whereas OT and AVP either had no effect or in some cases actually decreased brain activity in these regions in women. OT treatment rendered neural responses of males more similar to responses of females in the placebo group and vice-versa, raising the prospect of an inverted u-shaped dose response to central OT levels. These findings emphasize the need to fully characterize the effects of intranasal OT and AVP in both males and females and at multiple doses before widespread clinical application will be warranted.
Keywords: oxytocin, vasopressin, fMRI, cooperation, sex differences
1. Introduction
Both oxytocin (OT) and vasopressin (AVP) are known to modulate social behavior, and dysfunction in both systems has been postulated as a potential cause of various psychiatric disorders involving social behavioral deficits (Goodson & Thompson, 2010; Meyer-Lindenberg, Domes, Kirsch, & Heinrichs, 2011; Yamasue, Kuwabara, Kawakubo, & Kasai, 2009). There is growing interest in the use of intranasal oxytocin (OT) as a potential treatment for a variety of psychiatric disorders (Heinrichs, von Dawans, & Domes, 2009; Meyer-Lindenberg, et al., 2011; Striepens, Kendrick, Maier, & Hurlemann, 2011), including autism spectrum disorders (ASD), a group of neurodevelopmental disorders with an estimated prevalence of 1 per 88 children (Wingate M, 2012). Oxytocin is implicated in a wide range of social functions, including functions specifically compromised in ASD. For example, among healthy male volunteers, intranasal OT administration increased gazing to the eye region of the face (Guastella, Mitchell, & Dadds, 2008). In another study of healthy male volunteers, intranasal OT improved accuracy in inferring mental states from the eye region of the face (Domes, Heinrichs, Michel, Berger, & Herpertz, 2007). Preliminary studies show that intranasal OT can indeed alleviate some social cognitive deficits found in ASD individuals, increasing gaze to the eye region of the face (Andari, et al., 2010), improving performance on the Reading the Mind in the Eyes Task (Guastella, et al., 2010), and biasing social interactions towards more cooperative social partners (Andari, et al., 2010). Although there has been less research on intranasal AVP as a treatment for ASD, specific alleles of AVPR1a gene have been linked with ASD (Kim, et al., 2002; Wassink, et al., 2004), emphasizing the importance of studying the AVP system and its potential role in ASD as well. In addition to ASD, studies have begun to investigate the effectiveness of intranasal OT as a treatment for schizophrenia, social anxiety disorder and depression (Striepens, et al., 2011).
Most research that has investigated the effects of intranasal OT on human social cognition and behavior has been conducted with male subjects. Studies with female subjects are complicated by the fact that estradiol, levels of which vary across the menstrual cycle, is known to induce OT receptors (Champagne, Diorio, Sharma, & Meaney, 2001; Pedersen, Caldwell, Walker, Ayers, & Mason, 1994) and presumably increase responsivity to OT. Given that ASD is more common in males (Wingate M, 2012), studies investigating the effect of OT on ASD symptoms are also strongly biased towards males. In fact, across five such recently published studies, of 71 of 78 participants were male (Anagnostou, et al., 2012; Andari, et al., 2010; Guastella, et al., 2010; Hollander, et al., 2007; Hollander, et al., 2003).
Previously, we investigated the effects of intranasal OT and AVP on behavior and brain activity in human males (J. K. Rilling, et al., 2012). Healthy normal men were randomized to treatment with intranasal OT, AVP or placebo (PL) and their brain activity was measured with fMRI as they played a social interactive task known as the Prisoners Dilemma (PD) game with assumed human and computer partners. The iterated Prisoner’s Dilemma Game is a model for relationships based on reciprocal altruism (Axelrod & Hamilton, 1981; Trivers, 1971), that is able to probe psychological processes that are compromised in ASD, including the motivation to participate in mutually cooperative social interactions. OT was found to augment the caudate nucleus response to reciprocated cooperation from assumed human partners, raising the prospect that intranasal OT renders cooperative interactions more rewarding, and that it could potentially increase motivation of ASD subjects to engage with others (Stavropoulos & Carver, 2013). If implemented at an early age, this might facilitate greater attention to the social environment, augment social learning and help normalize the development of social behavior. OT also augmented activation of the left amygdala in response to reciprocated cooperation, another dopamine (DA) rich region. AVP treatment made men more likely to reciprocate cooperation and enhanced the neural response to cooperative intercations in a region spanning known vasopressin circuitry, including the BNST, lateral septum and stria terminalis.
Here, we conduct a study of female subjects using the same paradigm. Among rodents, the social bonds supporting both maternal care and female adult partner preference are heavily dependent on interactions between OT and DA in the ventral striatum (Skuse & Gallagher, 2009; Young, Murphy Young, & Hammock, 2005). We therefore predicted that OT would augment the ventral striatum response to cooperative social interactions to an even greater extent in females than we previously found for males. On the other hand, given evidence for sex differences in the response to AVP in both humans and rodents (Albers, 2012; Thompson, George, Walton, Orr, & Benson, 2006), along with evidence that AVP is more influential in males compared with females (Young, et al., 2005), we did not expect AVP to augment activation in BNST, lateral septum and stria terminalis in women as it did in men. Using a sequential-choice version of the PD game, we focused on the neural correlates of 1) the realization that one’s cooperation had been reciprocated (as player 1), and 2) the choice to cooperate (as player 2). This allowed us to examine both the reaction to (as player 1) and the anticipation of (as player 2) a mutually cooperative outcome. We find pronounced sex differences in the effects of OT and AVP on brain function and behavior, suggesting that pharmacologic manipulation of the OT and AVP systems to treat psychiatric disorders should be investigated in a sex-specific manner.
2. Methods (see supplemental materials for additional details)
2.1 Subjects
After screening (see supplemental materials), 87 women between the ages of 18 and 22 years (mean = 20.4 years) from the Emory University campus were randomized to receive intranasal OT (n = 29), intranasal AVP (n = 29), or intranasal PL (n = 29). 4 subjects (AVP n = 1, OT n = 2, and PL n = 1) were excluded from the neuroimaging analysis due to excessive motion (>1.5 mm). Also, 10 subjects (AVP n = 0, OT n = 5, and PL n = 5) had only partial neuroimaging data due to excessive motion that occurred in a limited portion of the scan. Data were compared with data from a previously collected sample of male subjects, also between ages 18–22 who were similarly randomized to receive intranasal OT (n = 27), intranasal AVP (n = 27), or intranasal PL (n = 36). Five of these subjects (AVP n = 2, OT n = 1, and placebo n = 2) were excluded from the neuroimaging analysis due to excessive motion (>1.3 mm) (n = 3) or to missing data (n = 2).
2.2 Prisoner’s Dilemma task
The iterated Prisoner’s Dilemma game is a model for relationships based on reciprocal altruism (Trivers, 1971). In the game, two players choose to either cooperate or defect and receive a payoff that depends upon the interaction of their respective choices. The game version we use here is a sequential-choice PD game in which player 1 chooses and player 2 is then able to view player 1’s choice before making her own choice. Player 1 must decide whether or not to trust player 2 (i.e., cooperate), whereas player 2 must decide whether or not to reciprocate cooperation (or defection). Each of the four game outcomes is associated with a different payoff. Player cooperation followed by partner cooperation (CC) pays $2 to both player and partner, player cooperation followed by partner defection (CD) pays $0 to the player and $3 to the partner, player defection followed by partner defection (DD) pays $1 to both player and partner, and player defection followed by partner cooperation (DC) pays $3 to the player and $0 to the partner.
2.3. Preparation of OT, AVP and PL (see supplementary materials)
2.4. Behavioral procedures (See supplemental material for additional details)
Administration of OT, AVP or PL
Both experimenters and subjects were blind to the treatment subjects received. All solutions were administered intranasally. The OT group self-administered 24 IU oxytocin (Syntocinin-Spray, Novartis), and the AVP group self-administered 20 IU of AVP (American Reagent Laboratories, Shirley, NY, USA).
Blood sample
20 min after drug administration, a blood sample was drawn for measurement of plasma OT, AVP and estradiol levels. Due to difficult vascular access, samples were not obtained from 16 subjects. Samples were centrifuged at 4 °C within 20 min of blood draw. Plasma was collected and frozen at −80 °C until assay.
Confederate introductions
Prior to entering the scanner, subjects met two female confederates, one of whom partnered the subject when the subject played as player 1 and another who partnered the subject when the subject played as player 2. Subjects were told that they would play 30 rounds of an iterated PD game with each of the two partners.
Our goal was for subjects to be fully immersed in the task at 50 min post drug administration (see supplementary materials). We therefore aimed to start both the task and fMRI scan at 40 min after drug administration. In actuality, this time period averaged 43 min post nasal spray administration (SD = 3.09 min) across subjects.
Task
Prior to the start of each game, the visual display inside the scanner indicated with which partner the subject was about to play the game. While being imaged with fMRI, subjects played 30 rounds of a sequential-choice, iterated Prisoner’s Dilemma game in each of four sessions. For two sessions, subjects were told they were playing with the human partners they were introduced to. For the other two sessions, subjects were told they were playing with a computer partner. In actuality, subjects were always playing with a pre-programmed computer algorithm (see supplemtary materials) for all four sessions. Subjects were compensated with two-thirds of their total earnings across all four sessions.
For both human and computer partners, in one of the two sessions, subjects played in the role of first mover (player 1) and their partner played in the role of second mover (player 2). In the other session, roles were reversed. The order of human and computer sessions was counterbalanced across subjects so that half of the subjects were scanned in the order: player 1 with human partner (H1), player 2 with human partner (H2), player 1 with computer partner (C1), player 2 with computer partner (C2), and the other half were scanned in the order: C1, C2, H1, H2. Note that statistical comparisons were made between human and computer runs as player 1 or as player 2, but player 1 runs were not compared with player 2 runs so these need not be counterbalanced.
E-prime software (Psychology Software Tools, Pittsburgh, PA, USA) was used for stimulus presentation. Stimuli were projected onto a screen that subjects could view through a mirror mounted on the head coil. Subject responses were recorded using a response box. A timeline for a single PD trial is depicted in Figure 1. At the beginning of each round, the round number and partner’s photo were displayed for 2 s. Player 1 then had 4 s to choose to cooperate or defect. Players were informed that if they did not decide within this 4 s interval, their response would default to defection. Player 1’s choice was immediately revealed to player 2 and displayed for 1 s. A variable length fixation epoch of either 2, 4 or 6 s followed. Afterwards, player 2 had 4 s to cooperate or defect. Once player 2 decided, the outcome of the round was displayed for 4 s. Finally, the trial concluded with another variable length fixation epoch of either 2, 4 or 6 s. Trials were approximately 20 s long. Five null trials were interspersed among 30 PD trials in each session. Null trials consisted of 14 s of fixation. One session lasted approximately 12 min. All four sessions lasted about 48 min.
Figure 1.
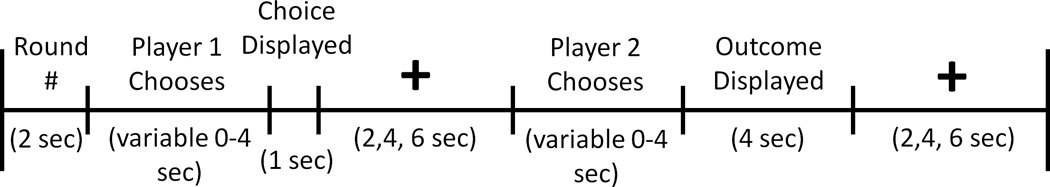
Timeline of PD Task
Behavioral analysis
For player 1 data, outcome frequencies and transition probabilities were compared across partner types (human, computer) using paired t-tests and across treatment groups (AVP, OT, PL) using two sample t-tests. T-tests were used rather than ANOVA because we were specifically interested in whether each drug condition differed from placebo, rather than whether there were differences among all three groups. Outcomes included CC (player cooperate followed by partner cooperate), CD (player cooperate followed by partner defect), DC (player defect followed by partner cooperate) and DD (player defect followed by partner defect), while transition probabilities were the probability of cooperating conditional on the outcome of the previous round. Thus, there were four different transition probabilities: the probability of cooperating after a CC outcome (pC/CC), after a CD outcome (pC/CD), after a DC outcome (pC/DC) and after a DD outcome (pC/DD). Sex differences in drug effects were evaluated using two way ANOVA. For player 2 data, outcomes included CC (partner cooperate followed by player cooperate), CD (partner defect followed by player cooperate), DC (partner cooperate followed by player defect), and DD (partner defect followed by player defect). Note that we always list the player’s choice first, irrespective of whether the player is adopting the role of player 1 or player 2. Outcome frequencies and probabilities of reciprocating partner cooperation and defection were compared across partner types and treatment groups, using paired and two sample t-tests, respectively. Again, sex differences in drug effects were evaluated using two way ANOVA.
To evaluate the potential modulation by estradiol levels of OT behavioral effects, we constructed a series of multiple linear regression models in which each behavioral measure served as a dependent variable, and plasma estradiol levels, drug treatment (0=PL, 1=OT), and their interaction term were entered as independent variables. A significant interaction term was evidence that estradiol levels had modulated the effect of OT on behavior.
2.5. Neuroimaging procedures
Imaging data were acquired, processed, modeled and statistically evaluated in the exact same way as for our previous study of male subjects (J. K. Rilling, et al., 2012).
Anatomical image acquisition
Subjects were positioned head first in the supine position inside the scanner (Siemens Trio 3T), with padded head restraint to minimize head motion during scanning. Each scanning session began with a 15 s scout, followed by a 5 min T1-weighted MPRAGE 3d scan that was acquired in the sagittal plane and accelerated by generalized autocalibrating partially parallel acquisitions (GRAPPA) with a factor of 2 (TR = 2600 ms, TE = 3.02 ms, matrix = 256 × 256 × 176, FOV = 256 mm × 256 mm × 176 mm, slice thickness = 1.00 mm, gap = 0 mm).
fMRI image acquisition
Subjects were imaged while playing the PD game. Functional scans used an EPI sequence with the following parameters: TR = 2000 ms, TE = 28 ms, matrix = 64 × 64, FOV = 224 mm, slice thickness = 2.5 mm, 34 axial slices with a slice gap of 1.05 mm. TE was minimally decreased from the typical value (32 ms) in order to reduce magnetic susceptibility artifact in the orbitofrontal region. The duration of each EPI scan was about 12 min (30 PD rounds × ∼20 s per round, plus five null trials × 14 s per trial). After each of the four sessions, while still in the scanner, subjects rated their emotional reaction to the four PD game outcomes (CC, CD, DC, and DD). Seven-point Likert scales were used to rate the following emotions or feelings: afraid, angry, happy, guilty, disappointed, and relieved.
fMRI image analysis
Image preprocessing was conducted with Brain Voyager QX (version 2.0.8) (Brain Innovation, Maastricht, The Netherlands). Preprocessing involved image alignment by six-parameter 3-D motion correction, slice timing correction using cubic spline interpolation, spatial smoothing with a 8-mm full width at half maximum (FWHM) 3D Gaussian kernel, and temporal high-pass filtering with a frequency cutoff of three cycles per run to remove low frequency drifts. Images were subsequently normalized into Talairach space (Talairach & Tournoux, 1988). Subjects were scanned when playing both in the role of player 1 and in the role of player 2. For the player 1 runs, a separate general linear model (GLM) was defined for each subject that examined the neural response to both the epoch in which the choice to cooperate or defect was made, as well as to the epoch in which the game outcome was revealed. More specifically, the following regressors were defined for each subject in the role of player 1: (1) the beginning epoch when round number and the partner’s face or a picture of the computer were displayed, (2) the choice epoch when the subject chose to cooperate (Choice C), (3) the choice epoch when the subject chose to defect (Choice D), (4) CC outcomes, (5) CD outcomes, (6) DC outcomes, and (7) DD outcomes. These regressors were specified separately for runs with human and computer partners, resulting in a total of 14 distinct regressors per subject. For player 2 runs, a separate general linear model (GLM) was defined for each subject that examined the neural response to the epoch in which the partner’s choice was revealed, the epoch while the subject was choosing, and the epoch in which outcome was revealed. More specifically, the following regressors were defined for each subject in the role of player 2: (1) the beginning epoch when the subject saw her partner’s picture and the round number, (2) partner choice C, (3) partner choice D, (4) player choice C, (5) player choice D, (6) CC outcome, (7) CD outcome, (8) DC outcome, (9) DD outcome. These regressors were specified separately for runs with human and computer partners. Each regressor was convolved with a canonical model of the hemodynamic response.
We performed region of interest (ROI) analyses based on the a priori hypothesis that intranasal OT would enhance activation in both the caudate nucleus and the amygdala in response to reciprocated cooperation in women as it did previously in men. The caudate nucleus ROI was defined as a 9 mm-side cube (729 mm3) centered on the activation maximum for the contrast between mutual cooperation and the average of the other three PD game outcomes from our previous study (J. Rilling, et al., 2002), as well as its left hemisphere homologue in which we previously observed augmented activity following intranasal OT treatment in men. The cube volume was chosen to match that of the functional activation as closely as possible. The left amygdala ROI was defined as a 10 mm-side cube centered on the coordinates of the activation peak reported in Gamer et al. (Gamer et al., 2010), after converting from MNI to Talairach coordinates, and then manually adjusting the ROI location to more closely match the anatomical features of the MNI template. For each subject, contrasts of parameter estimates for predictors were averaged across all voxels within each ROI. We also computed the difference in these contrasts between human and computer partners, i.e., the interaction between the factor specified by the contrast and the factor of partner type. In a second level, random-effects analysis, individual subject contrast beta values were compared across treatment groups with two sample t-tests. A statistical threshold of p < 0.05 was adopted.
This ROI analysis was supplemented with a whole brain analysis in which contrasts of parameter estimates for various regressors were computed at every voxel within the brain. We also directly compared the values of these contrasts between human and computer partners. In a second level, random-effects analysis, individual subject contrast values were compared across drug treatment groups with two sample t-tests. To ensure that results were not driven by drug-mediated suppression of activation for computer partners, we further masked out activations where PL>drug for computer partners for all contrasts. The resulting map of the t statistic was thresholded at p < 0.05 corrected for multiple comparisons based on the volume of clusters of contiguous voxels, using a 3D extension (implemented in BrainVoyager) of a 2D Monte Carlo simulation procedure (Forman, et al., 1995)
To compare the current female data with previously collected data from males, we constructed a single model combining all male and female data, and then compared contrasts between the two sexes using a two sample t-test. The resulting map of the t statistic was thresholded at p < 0.05 corrected for multiple comparisons based on the volume of clusters of contiguous voxels, using a 3D extension (implemented in BrainVoyager) of a 2D Monte Carlo simulation procedure (Forman, et al., 1995).
Given that estrogen induces OT receptors (Champagne, et al., 2001; Pedersen, et al., 1994), we also constructed general linear models in which estradiol levels were included as covariates, and tested for differences between the coefficients relating estradiol to brain activity between the OT and PL groups. This analysis was done using the Oxford Center for Functional Magnetic Resonance Imaging of the Brain's software library (FSL, http://www.fmrib.ox.ac.uk/fsl/)(Smith, et al., 2004) (Supplementary Materials). Finally, to determine if individual variation in estradiol levels were obscuring main effects of OT compared with placebo, we constructed a model in which estradiol levels were treated as a covariate of no interest and compared results with those from the above models that excluded estradiol.
3. Results (see supplemental materials for additional details)
3.1 Behavior
3.1.1.Player 1 (see supplementary materials for additional information)
Drug treatment effects when playing with putative human partners
There were no effects of drug treatment on any outcome frequencies or any transition probabilities when playing with putative human partners (figure 2, supplementary figure 1).
Figure 2.
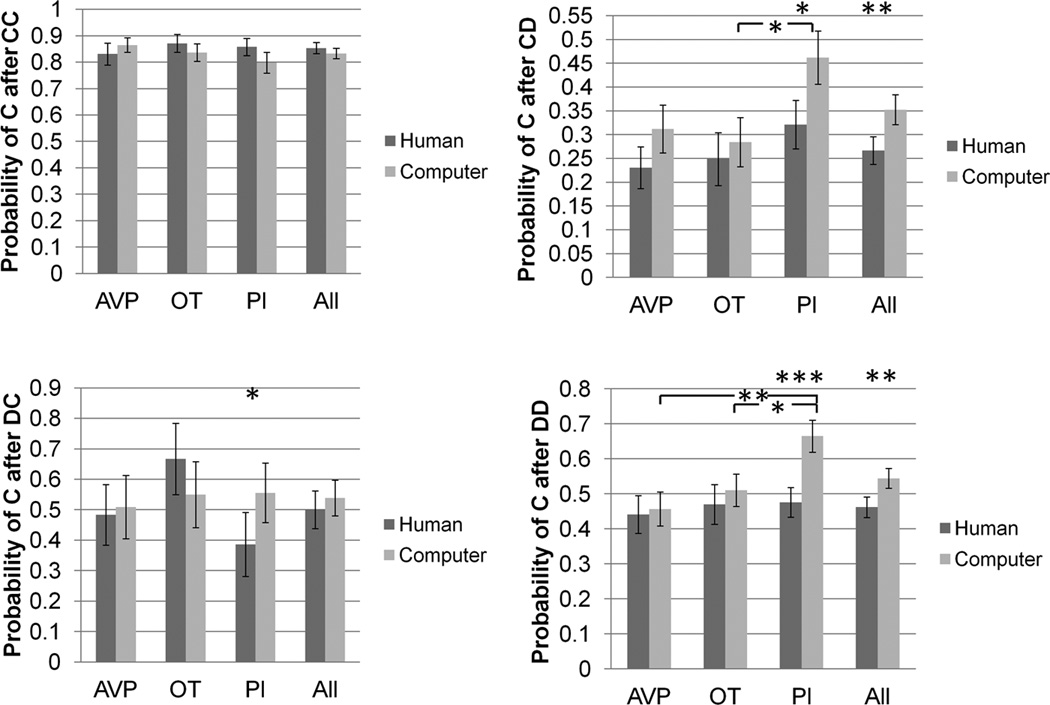
Transition probabilities as player 1, as a function of partner type and drug treatment group. * p<0.05, ** p<0.01, *** p<0.001.
Drug treatment effects when playing with computer partners
There were no effects of drug treatment on the frequency of CD or DC outcomes when playing with computer partners. However, subjects in the OT group (mean=11.8) experienced 2.6 fewer CC outcomes than subjects in the PL group (mean = 14.4) (p = 0.04). Also, there were 2.9 fewer DD outcomes in the PL group (mean = 8.4) compared with either the OT (mean = 11.3) (p = 0.03) or AVP (mean = 11.4) group (p = 0.03). There were no effects of drug treatment on pC/CC or pC/DC. However, pC/CD in the PL group (46.2%) was 17.8% higher than the OT group (28.4%) (p = 0.02). Also, pC/DD in the PL group (66.5%) was 15.4% higher than the OT group (51.1%) (p = 0.02) and 20.8% higher than the AVP group (45.7%) (p = 0.003) (figure 2, supplementary figure 1).
Interaction between partner type and drug treatment effect
For pC/DD, the difference between human and computer partners was larger for the PL than the OT group by 17% (p = 0.03). The difference between human and computer partners was also larger for the PL than the AVP group by 19% (p = 0.007) (figure 2).
Comparison with Males
Among men, there were no significant behavioral differences between drug and placebo groups for either human or computer partners. Thus, none of the player 1 effects observed in women were also found in men. Despite this, formal statistical tests for differences between males and females on these behavioral measures did not reach significance.
3.1.2 Player 2 (see supplementary materials for additional information)
Drug treatment effects when playing with human partners
There were no effects of drug treatment on the frequency of CC, DC or DD outcomes when playing with human partners. However, the OT group (mean = 0.14) had 0.79 fewer CD outcomes than the AVP group (mean = 0.93) (p = 0.003). There were no effects of drug treatment on the probability of reciprocating cooperation with human partners. However, the AVP group (72%) was 20% less likely than the OT group (92%) to reciprocate defection (p = 0.03). The AVP group was also 18% less likely than the PL group (90%) to reciprocate defection (p = 0.03) (figure 3, supplementary figure 2).
Figure 3.
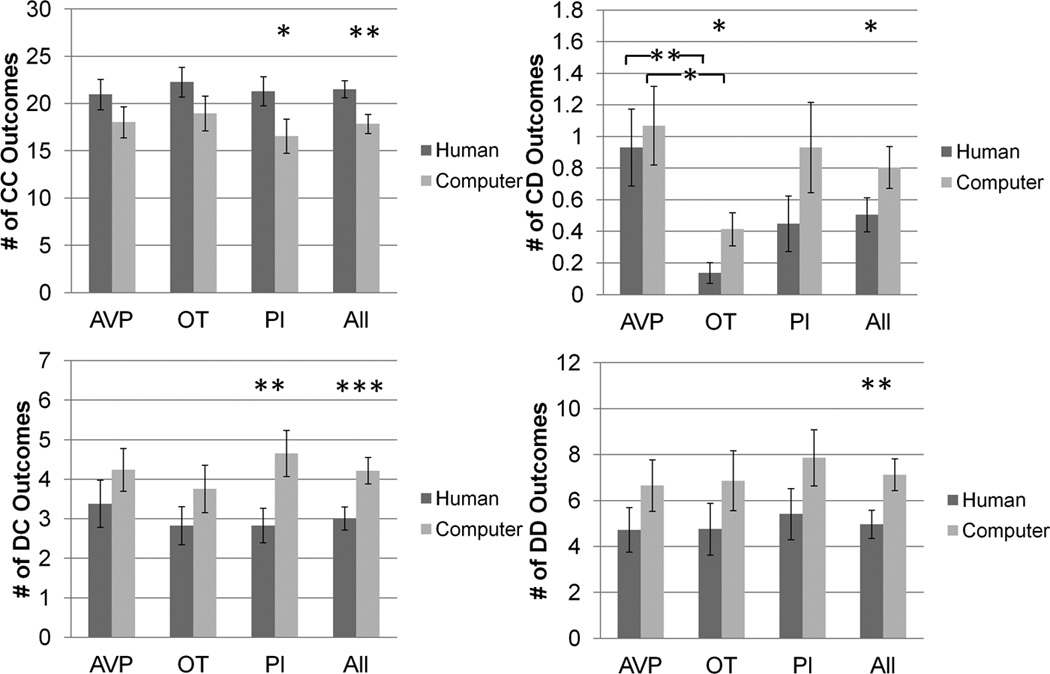
CC, CD, DC and DD outcome frequency as player 2, as a function of partner type and drug treatment groups. * p<0.05, ** p<0.01, *** p<0.001.
Drug treatment effects when playing with computer partners
There were no effects of drug treatment on the frequency of CC, DC or DD outcomes when playing with computer partners. However, the OT group (mean = 0.4) had 0.7 fewer CD outcomes than the AVP group (mean = 1.1) (p = 0.02). There were no effects of drug treatment on the probability of reciprocating cooperation or on the probability of reciprocating defection with computer partners (figure 3, supplementary figure 2).
Interaction between partner type and drug treatment effect
No effects (figure 3).
Comparison with Males
AVP-treated males reciprocated cooperation from both human and computer partners at higher rates than PL-treated males (J. K. Rilling, et al., 2012). Neither of these effects were present in females, and a formal statistical comparisons between men and women was marginally significant only for reciprocated cooperation from human partners (p=0.06). OTtreated males were also more likely to reciprocate defection from computer partners compared with PL-treated males. This effect was not found in females, but there was no significant difference between men and women. Finally, AVP-treated females were less likely to reciprocate defection from human partners than PL-treated females. This effect was not observed in males, and this sex difference was statistically significant (p=0.04).
Formal statistical comparisons between men and women also revealed additional sex differences not evident from comparing the main effects of each sex independently. 1) While AVP treatment tended to (non-significantly) decrease the number of CD outcomes with human partners for males, it had the opposite tendency in females (males vs. females, p=0.004). 2) AVP treatment also tended to decrease the number of CD outcomes with computer partners for males, while having little effect in females (males vs. females, p=0.05). 3) AVP treatment tended to decrease the number of DC outcomes with human partners for males, but not females (males vs females, p=0.02). There was a trend in the same direction for DC outcomes with computer partners (males vs. females, p=0.06). 4) AVP increased the number of CC outcomes with human partners to a greater extent in men compared with women (males vs. females, p=0.05). 5) Finally, AVP treatment tended to decrease reciprocation of defection from computer partners in females but not males (males vs. females, p=0.05).
3.1.3 Estradiol modulation of OT effects in women
Estradiol did not modulate the effect of OT on any behavioral measures with either human or computer partners, in the role of either player 1 or player 2. Nor did controlling for estradiol levels as a nuisance variable reveal any additional effects of OT on behavior.
3.2 Neuroimaging
3.2.1 Player 1
To evaluate the overall degree of similarity in brain activity between our current female sample and our previously collected male sample, we compared the contrast between reciprocated (CC) and unreciprocated (CD) cooperation from putative human partners across all three drug treatment groups combined (supplementary figure 3). This analysis revealed widespread and highly specific overlap between males and females in terms of both activations and deactivations. Among other areas, both sexes showed activation in the head of the caudate nucleus, as well as prominent deactivation (i.e., greater activation to CD) in the dorsal anterior cingulate cortex (dACC) and bilateral anterior insula.
Neuropeptide modulation of the response to reciprocated cooperation
Previously, we reported that intranasal OT treatment augmented the left caudate nucleus response to reciprocated cooperation among men (J. K. Rilling, et al., 2012). Within the same region of interest, defined independently of both samples using an ROI from Rilling et al (2002), we tested for drug and partner effects on the response to reciprocated cooperation among women. In the PL group, the left caudate nucleus activated more strongly to reciprocated compared with unreciprocated cooperation from a human partner (t(25) = 3.02, p < 0.006). However, OT did not significantly modulate the response to reciprocated cooperation in women (supplementary figure 4). In addition to the left caudate nucleus, we also previously found intranasal OT to augment activation within the left amygdala in response to reciprocated cooperation among men. Within this ROI, OT had the opposite effect in women, decreasing activation relative to the placebo group in response to reciprocated cooperation from human partners (t(47) = −2.38, p = 0.02). The sex difference was statistically significant (sex x dug treatment ANOVA interaction effect p=0.005). The interaction effect was such that OT rendered the male left amygdala response similar to the female left amygdala response in the placebo condition, and vice-versa (supplementary figure 5).
We also examined neuropeptide effects on the response to reciprocated cooperation in whole brain analyses of the contrasts [(CC human-CC computer) OT- (CC human – CC computer)PL)] and [(CC human-CC computer)AVP-(CC human-CC computer)PL)]. To ensure that results were not driven by neuropeptide-mediated suppression of activation with computer partners, we further masked out activations from the contrasts (CC computer)PL-(CC computer)OT and (CC computer)PL-(CC computer)AVP.
[(CC human-CC computer) OT- (CC human – CC computer)PL)]
In females, this contrast yielded no significant activation, and significant deactivation focused on the posterior cingulate cortex (supplementary table 1).
Overlap with males
There was no overlap with activation maps for males for this contrast.
Differences with males
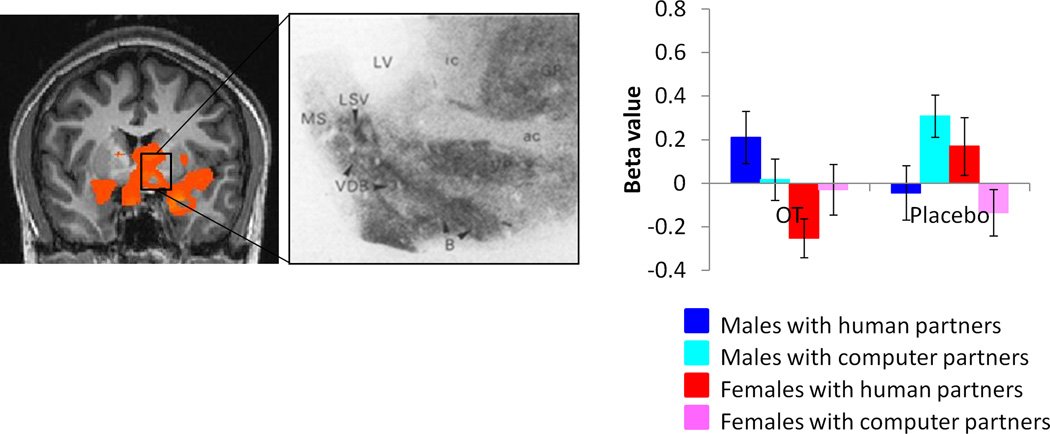
Males showed higher activation than females for this contrast across widespread regions, including but not limited to the left amygdala, left nucleus accumbens, bilateral insula and bilateral hippocampus. This activation also encompassed basal forebrain regions rich in OT receptors (Loup, Tribollet, Dubois-Dauphin, & Dreifuss, 1991), including the preoptic area, basal nucleus of Meynert, diagonal band of Broca, and lateral septum (supplementary table 1, figure 4, supplementary figure 6).
Figure 4.
Regions showing higher activation in males vs. females for the contrast [(CC human-CC computer) OT- (CC human – CC computer)PL)], exclusively masked by the contrast [(CC computer)PL-(CC computer)OT] male - [(CC computer)PL-(CC computer)OT] female (p<0.05 corrected). Inset shows human OT receptor density from Loup et al, 1991. Beta value plot is for the entire functional activation. ac = anterior commissure; B = basal nucleus of Meynert; ic = internal capsule; LSV = lateral septal nucleus; LV = lateral ventricle; MS = medial septal nucleus; VDB = nucleus of the vertical limb of the diagonal band. Images are thresholded at p<0.05 corrected for multiple comparisons at the cluster level.
[(CC human-CC computer) AVP- (CC human – CC computer)PL)]
In females, this contrast yielded no significant activation, and significant deactivation across widespread subcortical regions that overlapped extensively with areas activated by OT in males (supplementary table 2).
Overlap with males
There was no overlap with males for this contrast.
Differences with males
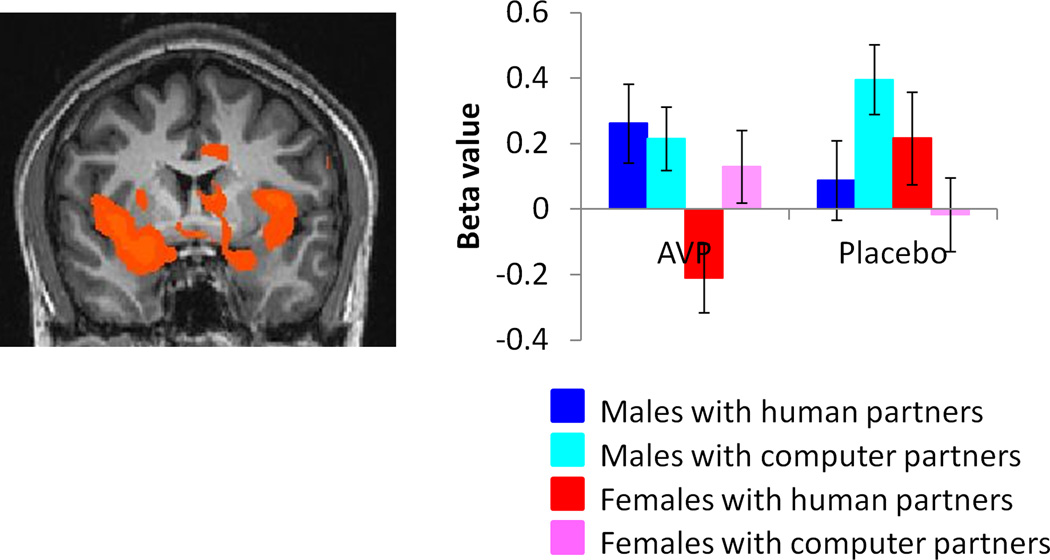
As for OT, males showed higher activation than females for this contrast within the amygdala, nucleus accumbens, hippocampus, insula and throughout the basal forebrain (supplementary table 2, figure 5, supplementary figure 7). In particular, females did not show activation in the the BNST, lateral septum and stria terminalis as observed previously in our male sample (J. K. Rilling, et al., 2012).
Figure 5.
Regions showing higher activation in males vs. females for the contrast [(CC human-CC computer) AVP- (CC human – CC computer)PL)], exclusively masked by the contrast [(CC computer)PL-(CC computer)AVP] male - [(CC computer)PL-(CC computer)AVP] female (p<0.05 corrected). Plotted beta values are averages over the entire extent of functional activation. Images are thresholded at p<0.05 corrected for multiple comparisons at the cluster level.
3.2.2 Player 2
Neuropeptide modulation of the choice to cooperate
We examined neuropeptide effects on the choice to cooperate in a whole brain analysis with the contrasts [(Choice C human- Choice C computer) OT- (Choice C human – Choice C computer)PL)] and [(Choice C human – Choice C computer)AVP-(Choice C human - Choice C computer)PL)]. To ensure that results were not driven by neuropeptide-mediated suppression of activation with computer partners, we further masked out activations from the contrasts (Choice C computer)PL-(Choice C computer)OT and (Choice C computer)PL-(Choice C computer)AVP.
[(Choice C human - Choice C computer) OT- (Choice C human - Choice C computer)PL)]
In females, this contrast yielded no significant activations or deactivations.
Differences with males
Males showed stronger activation than females in VLPFC, VMPFC, DLPFC, subgenual ACC, ventral and dorsal striatum, basal forebrain, thalamus, ventral temporal cortex, posterior cingulate, occipital cortex and cerebellum (supplementary table 3, supplementary figure 8).
[(Choice C human – Choice C computer)AVP-(Choice C human - Choice C computer)PL)]
In females, this contrast yielded significant deactivation in occipital cortex (supplementary table 4).
Overlap with males
There was no significant overlap between males and females for this contrast.
Differences with males
Males showed stronger activation than females in VLPFC, DLPFC, caudate tail, posterior cingulate, ventral temporal cortex, cerebellum and across widespread regions in parietal and occipital cortex (supplementary table 4, supplementary figure 9).
Overlap between OT and AVP effects
For each of the above contrasts, we compared activation maps for OT-PL and AVP-PL. Of the four contrasts (male CC, female CC, male choice C, female Choice C), only one showed substantial overlap between OT and AVP effects. In males, both OT and AVP increased activity in prefrontal cortex, dorsal and ventral striatum, anterior insula and ventral temporal cortex during the choice to cooperate.
3.2.3 Estradiol modulation of OT effects in women
Estradiol levels did not significantly modulate the effect of OT on brain activity for any of the four contrasts. Nor did including estradiol levels as a nuisance variable alter the results of the above contrasts comparing OT and PL groups among women (see supplementary materials).
4. Discussion (see supplemental materials for additional details)
4.1 Behavior
4.1.2 Drug effects
There were two important sex differences in drug effects on behavior. First, AVP rendered men but not women more likely to reciprocate cooperation from human partners. In contrast, AVP did not increase cooperation in men following partner defection. This suggests that AVP may have an important role in inter-male cooperation, and that its effects may be contextdependent. These findings are consistent with a recent hypothesis that AVP-related peptides may function to offset male aggression in specifically affiliative contexts (Goodson, 2013). The second sex difference was that, among women but not men, AVP increased the probability that subjects would cooperate as player 2, after their partner had defected. Since the subject willingly takes a financial loss by choosing to cooperate in this context, this likely represents a conciliatory gesture aimed at reestablishing cooperation. Our data suggest that AVP facilitates these conciliatory gestures in women, a finding consistent with an earlier study showing that AVP increases affiliative facial motor patterns in women in response to unfamiliar female faces (Thompson, et al., 2006). In addition to these two findings, we also found that among women, both OT and AVP lowered rates of cooperation with computer partners following an outcome of mutual defection in the previous round so that rates more closely matched those found with human partners in this context. Thus, OT and AVP may support anthropomorphism of computer partners and the associated betrayal aversion in women. OT had the same effect following a CD outcome in the previous round. Although these effects were not observed in men, the direct comparison between the two sexes did not yield a statistically significant result, whereas the above two effects had significant or marginally significant sex differences.
Overall, there were no significant effects of intranasal OT on cooperative behavior with assumed human partners. Recent studies show that the effects of intranasal OT on cooperative behavior depend on the participant’s attachment style (Bartz, Zaki, Bolger, & Ochsner, 2011). For example, intranasal OT increases cooperation among men who are high rather than low in attachment avoidance (De Dreu, 2012b). Another study found that whereas OT increased cooperative behavior in participants high in attachment anxiety and low in attachment avoidance, OT actually decreased cooperative behavior in participants high in both attachment anxiety and avoidance (J. Bartz, et al., 2011). Such effects may be attributable to developmental influences on OT receptor expression (Bales & Perkeybile, 2012), which in turn should affect responsiveness to intranasal OT. We did not characterize the attachment style of the participants in our study, so we are unable to evaluate the potential influence of attachment style on neural and behavioral responsiveness to OT.
Nevertheless, two previous studies have shown that OT increases cooperative behavior in men during a trust game, even without accounting for individual variation in attachment style (Baumgartner, Heinrichs, Vonlanthen, Fischbacher, & Fehr, 2008; Kosfeld, Heinrichs, Zak, Fischbacher, & Fehr, 2005). In these studies, participants interacted with anonymous partners in single-shot games. In our own study, participants instead believe they are interacting with human partners that they have met previously, and the interactions are iterative (30 rounds per partner). We suspect that the more limited behavioral effects of OT in our study can be attributed to a more powerful influence of the history of the interaction on the participant’s decision making. In other words, OT effects may be exacerbated when participants interact with a “blank slate”, in which circumstance characteristics of the partner (e.g., their prior behavior) do not influence decision making.
4.1.3 Estradiol effects
Estradiol is known to modulate OT release and OT receptor expression (Champagne, et al., 2001; Pedersen, et al., 1994). However, plasma estradiol levels did not modulate the effect of OT on any behavioral measures. It is possible that more widespread modulatory effects of estradiol would emerge in other behavioral tasks that are more sensitive to OT.
4.2 Imaging
4.2.1 Player 1
All male scans were acquired prior to all female scans, raising the possibility that sex differences in activation maps could be attribus to changes in scanner performance and data quality over this time interval. However, the main effect of reciprocated vs. unreciprocated cooperation (CC-CD) yielded very similar activation maps for males and females (supplementary figure 3), suggesting that data were of similar quality in the two groups and that any sex differences in drug effects are unlikely attributable to potential differences in scan quality.
We previously found that intranasal OT increased the response to reciprocated cooperation among men within both the left caudate nucleus and the left amygdala. Contrary to our expectations, ROI analyses showed that these effects were not present in women, and the left amygdala effect was in fact in the opposite direction. More generally, whole brain analyses showed that among men, both OT and AVP increased activation in response to CC outcomes in subcortical regions extending from nucleus accumbens through the basal forebrain to the amygdala and hippocampus, as well as the insula, many of which are rich in OT and AVP receptors. Among the functions of the activated regions are reward, social bonding, arousal and memory. In contrast to men, OT and AVP administration to women either decreased or had no effect on activation in these regions in response to CC outcomes. In general, OT treatment rendered the neural response of males more similar to that of females in the PL group, and vice-versa (figure 4). These results suggest that intranasal OT may enhance social reward and facilitate social bonding to a greater degree in men than in women.
4.2.2 Player 2
Similar to player 1 results and in contrast to men, neuropeptide administration never increased activity in women during the choice to cooperate. Whereas both OT and AVP had minimal impact on brain activity during the choice to cooperate in women, both strongly increased activation in both cortical and subcortical regions during the choice to cooperate in men. Player 1 and player 2 results were consistent in showing that both OT and AVP increase activity in men during cooperation. Moreover, for both player 1 and player 2 results, OT specifically increased activation in the dorsal and ventral striatum and basal forebrain during cooperation. Given the role of the ventral striatum in reward processing (Knutson, Taylor, Kaufman, Peterson, & Glover, 2005; O"Doherty, 2004; Schultz, Tremblay, & Hollerman, 2000), these results suggest that intranasal OT may augment the experienced (player 1) or anticipated (player 2) reward from mutual cooperation between men but not between women. This is not to say that endogenous OT does not normally support these functions among women.
Despite these effects on brain activation in men, intranasal OT did not increase cooperative behavior among men in our study. It has been shown to do so in other experimental contexts (Baumgartner, et al., 2008; De Dreu, 2012a, 2012b; Israel, Weisel, Ebstein, & Bornstein, 2012; Kosfeld, et al., 2005). Thus, it is possible that OT facilitates men’s cooperation with ingroup members, as in (De Dreu, 2012a), by augmenting striatal activity during cooperation. If it does the same for males with ASD, it could potentially help to normalize their social behavior by increasing their motivation to participate in and therefore learn from social interactions (Stavropoulos & Carver, 2013). One possible explanation for the lack of a behavioral effect of OT on cooperation in our study despite its augmentation of striatal activity is that the social reward facilitated by OT is outweighed by competing, perhaps more strategic, motivations. Our results also show that AVP can contribute to cooperation among men, and call for greater investigation of its role as a potential treatment for males with ASD, although potential pleitropic effects on stress and anxiety should be carefully investigated (Goodson, 2013; Shalev, et al., 2011).
Sex differences in the effects of OT and AVP have been previously reported in both non-human animals and in humans. For example, although AVP injections in the anterior hypothalamus of male hamsters stimulate aggression in the resident-intruder test, the same injections decrease aggression in females (Albers, 2012). In humans, intranasal AVP enhanced men’s agonistic facial motor patterns in response to unfamiliar male faces and decreased the perception of friendliness in those faces. However, in women, AVP enhanced affiliative facial motor patterns in response to unfamiliar female faces and increased perception of friendliness in those faces (Thompson, et al., 2006). In the current study, we likewise observed sex differences in the neural and behavioral effects of intranasal AVP. Regarding OT, intranasal OT has been found to decrease the amygdala response to fearful, angry and happy faces in men. On the other hand, intranasal OT increased the amygdala response to fearful faces in women, but had no effect on the amygdala response to angry or happy faces (Domes, Heinrichs, Glascher, et al., 2007; Domes, et al., 2010). In the current study, intranasal OT modulated amygdala function in a different manner. Specifically, OT treatment increased the left amygdala response to mutual cooperation with human partners in men, and decreased that response in women. However, the current study and the study of Domes et al examined the amygdala response to very different stimuli, cooperative social exchanges on the one hand and emotional faces on the other. Laterality effects may also help to explain the discrepancy since Domes et al found OT to decrease right amygdala activation in males, whereas we find it to increase left amygdala activity. Consistent with our finding, Gamer et al (Gamer, Zurowski, & Buchel, 2010) also found that OT augmented the left amygdala response to happy faces in male subjects.
What is the explanation for the sex differences in neural responsiveness to OT and AVP in our study? One possibility is that the effects of OT and AVP are influenced by gonadal steroid hormone environments (e.g., T, E), which markedly differ between men and women. For example, given that steroid hormones modulate OT and AVP receptor expression (Insel, 2010), men and women may differ in the density and distribution of OT and AVP receptors. Sex differences in human OT and AVP receptor distributions have not yet been systematically investigated. Another possibility is that there are sex differences in the ability of intranasally administered neuropeptides to cross the blood brain barrier. While these explanations might account for cases in which neuropeptide affects brain activity in one sex but not the other, it is a less compelling explanation for opposing effects (upregulation in males and downregulation in females) such as those we observed here. Women have higher CSF oxytocin levels than men do (Altemus et al 1999). Perhaps brain OT levels have an inverted-u shaped dose response relationship with neural activity, in which men have baseline levels to the left of the maximum, whereas women are closer to the maximum. In this case, raising brain OT levels in men would move them closer to the maximum level of activity, whereas raising levels in women would displace them to the right of the maximum, decreasing brain activity. This explanation is consistent with the left amygdala ROI results, and with the beta value plot in figure 4, both of which show that OT renders the response of males more similar to that of females in the PL group and the response of females more similar to that of males in the placebo group. This would not be without precedent, since the effects of OT on social recognition are also postulated to follow an inverted U-shaped dose response curve (Bielsky & Young, 2004). One final possibility relates to cross-reactivity in binding of OT and AVP to their receptors. For example, if central AVP levels are higher in men, additional exogenous AVP could saturate AVP receptors and cross-react with OT receptors (Manning, et al., 2012; Young & Flanagan-Cato, 2012) and this, in turn, could drive increases in activity in regions involved in reward and bonding.
4.2.3 OT vs. AVP effects
Given significant cross-reactivity between OT and AVP systems (Audigier & Barberis, 1985), coupled with evidence that OT and AVP can have similar effects on behavior (Bielsky & Young, 2004), it is important to determine whether intranasal OT and AVP activate similar or different brain regions. Our results show that there was incomplete overlap between the two, suggesting that OT and AVP are working through at least partially distinct receptor populations.
4.2.4 Estradiol effects
OT effects on brain activity were not modulated by plasma estrogen levels. Perhaps modulatory effects of estrogen will only emerge when levels extend beyond the range observed in this study. It is also possible that local synthesis of estradiol in the brain could modulate responsiveness to OT (Remage-Healey, Saldanha, & Schlinger, 2011).
5. Conclusion
Overall, OT and AVP had sexually differentiated effects on both behavior and brain activity. In women, AVP increased conciliatory behavior, and both OT and AVP caused women to treat computer partners more like humans. In men, AVP selectively increased reciprocation of cooperation from both human and computer partners. No common drug effects on behavior were found in both men and women. Although the main effect of reciprocated vs. unreciprocated cooperation (CC-CD) yielded very similar activation maps men and women, OT and AVP modulated brain activity very differently in the two sexes. During cooperative interactions, both OT and AVP frequently increased activity in men within areas rich in OT and AVP receptors and in areas playing a key role in reward, social bonding, arousal and memory, such as the striatum, basal forebrain, insula, amygdala and hippocampus. Importantly, both player 1 and player 2 results were consistent in showing that both OT and AVP increase activity in men during cooperation. In contrast, OT and AVP either decreased activity or had no effect in these and other regions in women. Proper interpretation of these results may require consideration of possible non-linear dose-response curves and acknowledgement that each peptide can bind to both OT and AVP receptors. There is considerable interest in the possibility of using neuropeptides to treat a variety of psychiatric disorders, principal among them being ASD. However, our data suggest that it is inappropriate to generalize findings from studies done with male subjects and patients to female subjects and patients. It will be important to fully characterize the dose-response functions of both OT and AVP in both men and women before adopting these drugs for widespread clinical use.
Supplementary Material
Acknowledgements
We thank Susan Rogers, Jianguo Xu, Elissar Andari and Elliott Albers for assistance with various aspects of this manuscript.
Funding
Supported by NIMH Grant R01 MH084068-01A1 and PHS Grant UL1 RR025008 from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources. Assay services were provided by the Biomarkers Core Laboratory at the Yerkes National Primate Research Center. This facility is supported by the Yerkes National Primate Research Center Base Grant 2P51RR000165-51.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflicts of interest.
Contributors
James K. Rilling designed the study, supervised data acquisition and analysis, and wrote the paper. Ashley DeMarco and Patrick Hackett collected and analyzed the data, and assisted with writing the paper. Sabrina Stair collected and analyzed the data. Xu Chen and Pritam Gautam assisted with data analysis. Richmond Thompson, Beate Ditzen and Giuseppe Pagnoni advised with respect to study design and interpretation of results, and assisted with writing the paper. Rajan Patel provided statistical advice and assisted with analyses. Ebrahim Haroon served as study physician.
References
- Albers HE. The regulation of social recognition, social communication and aggression: vasopressin in the social behavior neural network. Hormones and Behavior. 2012;61:283–292. doi: 10.1016/j.yhbeh.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Anagnostou E, Soorya L, Chaplin W, Bartz J, Halpern D, Wasserman S, Wang AT, Pepa L, Tanel N, Kushki A, Hollander E. Intranasal oxytocin versus placebo in the treatment of adults with autism spectrum disorders: a randomized controlled trial. Mol Autism. 2012;3:16. doi: 10.1186/2040-2392-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci U S A. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audigier S, Barberis C. Pharmacological characterization of two specific binding sites for neurohypophyseal hormones in hippocampal synaptic plasma membranes of the rat. EMBO J. 1985;4:1407–1412. doi: 10.1002/j.1460-2075.1985.tb03794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod R, Hamilton WD. The Evolution of Cooperation. Science. 1981;211:1390–1396. doi: 10.1126/science.7466396. [DOI] [PubMed] [Google Scholar]
- Bales KL, Perkeybile AM. Developmental experiences and the oxytocin receptor system. Hormones and Behavior. 2012;61:313–319. doi: 10.1016/j.yhbeh.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Bartz J, Simeon D, Hamilton H, Kim S, Crystal S, Braun A, Vicens V, Hollander E. Oxytocin can hinder trust and cooperation in borderline personality disorder. Soc Cogn Affect Neurosci. 2011;6:556–563. doi: 10.1093/scan/nsq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci. 2011;15:301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25:1565–1574. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dreu CK. Oxytocin modulates cooperation within and competition between groups: an integrative review and research agenda. Hormones and Behavior. 2012a;61:419–428. doi: 10.1016/j.yhbeh.2011.12.009. [DOI] [PubMed] [Google Scholar]
- De Dreu CK. Oxytocin modulates the link between adult attachment and cooperation through reduced betrayal aversion. Psychoneuroendocrinology. 2012b;37:871–880. doi: 10.1016/j.psyneuen.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007;62:1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves "mindreading" in humans. Biol Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35:83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Gamer M, Zurowski B, Buchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proc Natl Acad Sci U S A. 2010;107:9400–9405. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL. Deconstructing sociality, social evolution and relevant nonapeptide functions. Psychoneuroendocrinology. 2013;38:465–478. doi: 10.1016/j.psyneuen.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Thompson RR. Nonapeptide mechanisms of social cognition, behavior and species-specific social systems. Curr Opin Neurobiol. 2010 doi: 10.1016/j.conb.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, Hickie IB. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67:692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biol Psychiatry. 2008;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30:548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L, Anagnostou E, Wasserman S. Oxytocin increases retention of social cognition in autism. Biol Psychiatry. 2007;61:498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Hollander E, Novotny S, Hanratty M, Yaffe R, DeCaria CM, Aronowitz BR, Mosovich S. Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger's disorders. Neuropsychopharmacology. 2003;28:193–198. doi: 10.1038/sj.npp.1300021. [DOI] [PubMed] [Google Scholar]
- Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65:768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel S, Weisel O, Ebstein RP, Bornstein G. Oxytocin, but not vasopressin, increases both parochial and universal altruism. Psychoneuroendocrinology. 2012;37:1341–1344. doi: 10.1016/j.psyneuen.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Young LJ, Gonen D, Veenstra-VanderWeele J, Courchesne R, Courchesne E, Lord C, Leventhal BL, Cook EH, Insel TR. Transmission disequilibrium testing of arginine vasopressin receptor 1A (AVPR1A) polymorphisms in autism. Molecular Psychiatry. 2002;7:503–507. doi: 10.1038/sj.mp.4001125. [DOI] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. J Neurosci. 2005;25:4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Res. 1991;555:220–232. doi: 10.1016/0006-8993(91)90345-v. [DOI] [PubMed] [Google Scholar]
- Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, Durroux T, Mouillac B, Corbani M, Guillon G. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J Neuroendocrinol. 2012;24:609–628. doi: 10.1111/j.1365-2826.2012.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behavioral and Brain Sciences. 1994;108:1163–1171. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Saldanha CJ, Schlinger BA. Estradiol synthesis and action at the synapse: evidence for "synaptocrine" signaling. Front Endocrinol (Lausanne) 2011;2:28. doi: 10.3389/fendo.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling J, Gutman D, Zeh T, Pagnoni G, Berns G, Kilts C. A neural basis for social cooperation. Neuron. 2002;35:395–405. doi: 10.1016/s0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD, Thompson R, Ditzen B, Patel R, Pagnoni G. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology. 2012;37:447–461. doi: 10.1016/j.psyneuen.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cerebral Cortex. 2000;10:272–283. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Shalev I, Israel S, Uzefovsky F, Gritsenko I, Kaitz M, Ebstein RP. Vasopressin needs an audience: Neuropeptide elicited stress responses are contingent upon perceived social evaluative threats. Hormones and Behavior. 2011 doi: 10.1016/j.yhbeh.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Skuse DH, Gallagher L. Dopaminergic-neuropeptide interactions in the social brain. Trends Cogn Sci. 2009;13:27–35. doi: 10.1016/j.tics.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stavropoulos KK, Carver LJ. Research Review: Social motivation and oxytocin in autism -implications for joint attention development and intervention. J Child Psychol Psychiatry. 2013 doi: 10.1111/jcpp.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striepens N, Kendrick KM, Maier W, Hurlemann R. Prosocial effects of oxytocin and clinical evidence for its therapeutic potential. Front Neuroendocrinol. 2011;32:426–450. doi: 10.1016/j.yfrne.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers; 1988. (Chapter Chapter) [Google Scholar]
- Thompson RR, George K, Walton JC, Orr SP, Benson J. Sex-specific influences of vasopressin on human social communication. Proc Natl Acad Sci U S A. 2006;103:7889–7894. doi: 10.1073/pnas.0600406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers RL. The Evolution of Reciprocal Altruism. Quarterly Review of Biology. 1971;46:35–57. [Google Scholar]
- Wassink TH, Piven J, Vieland VJ, Pietila J, Goedken RJ, Folstein SE, Sheffield VC. Examination of AVPR1a as an autism susceptibility gene. Mol Psychiatry. 2004;9:968–972. doi: 10.1038/sj.mp.4001503. [DOI] [PubMed] [Google Scholar]
- Wingate MMB, Kirby RS, Pettygrove S, Cunniff C, Meaney F, Schulz E, Miller L, Robinson C, Quintana G, Kaiser MY, Lee LC, Landa R, Newschaffer C, Constantino J, Fitzgerald R, Zahorodny W, Daniels J, Giarelli E, Pinto-Martin J, Levy SE, Nicholas J, Charles J, Zimmerman J, Maenner MJ, Durkin M, Rice C, Baio J, Van Naarden Braun K, Phillips K, Doernberg N, Yeargin-Allsopp M. Prevalence of autism spectrum disorders--Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012;61:1–19. [PubMed] [Google Scholar]
- Yamasue H, Kuwabara H, Kawakubo Y, Kasai K. Oxytocin, sexually dimorphic features of the social brain, and autism. Psychiatry Clin Neurosci. 2009;63:129–140. doi: 10.1111/j.1440-1819.2009.01944.x. [DOI] [PubMed] [Google Scholar]
- Young LJ, Flanagan-Cato LM. Editorial comment: oxytocin, vasopressin and social behavior. Hormones and Behavior. 2012;61:227–229. doi: 10.1016/j.yhbeh.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Murphy Young AZ, Hammock EA. Anatomy and neurochemistry of the pair bond. J Comp Neurol. 2005;493:51–57. doi: 10.1002/cne.20771. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.