Abstract
Dengue virus belongs to the virus family Flaviviridae. Dengue hemorrhagic disease caused by dengue virus is a public health problem worldwide. The viral non structural 2B and 3 (NS2B-NS3) protease complex is crucial for virus replication and hence, it is considered to be a good anti-viral target. Leaf extracts from Carica papaya is generally prescribed for patients with dengue fever, but there are no scientific evidences for its anti-dengue activity; hence we intended to investigate the anti-viral activity of compounds present in the leaves of Carica papaya against dengue 2 virus (DENV-2). We analysed the anti-dengue activities of the extracts from Carica papaya by using bioinformatics tools. Interestingly, we find the flavonoid quercetin with highest binding energy against NS2B-NS3 protease which is evident by the formation of six hydrogen bonds with the amino acid residues at the binding site of the receptor. Our results suggest that the flavonoids from Carica papaya have significant anti-dengue activities.
Abbreviations
ADME - Absorption, distribution, metabolism and excretion, BBB - Blood brain barrier, CYP - Cytochrome P450, DENV - – Dengue virus, DHF - Dengue hemorrhagic fever, DSS - Dengue shock syndrome, GCMS - – Gas chromatography- Mass spectrometry, MOLCAD - Molecular Computer Aided Design, NS - Non structural, PDB - Protein data bank, PMF - Potential Mean Force.
Keywords: Dengue virus, Carica papaya, Quercetin, ADMET, NS2B-NS3 protease
Background
Dengue is a serious public health problem worldwide [1]. There are currently no vaccines available for the prevention and treatment of DENV infection [2]. DENV is a positive – stranded encapsulated RNA virus and is composed of three structural protein genes, which encode the nucleocapsid or core (C) protein, a membrane-associated (M) protein, an enveloped (E) glycoprotein and seven non-structural (NS) proteins [3]. DENV is a member of the genus Flavivirus and belongs to the Flaviviridae family [4]. DENV causes a wide spectrum of clinical illnesses ranging from relatively mild self limiting disease associated with fever, malaise and other non specific symptoms to the more severe, potential lethal disease manifestations that leads to dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) [5]. There are four antigenically distinct, but closely related serotypes of the dengue virus (DENV -1, DENV - 2, DENV -3 and DENV -4), that exhibits a 65 – 70% sequence homology [6]. Dengue is transmitted mainly by Aedes aegypti mosquito and to some extent by Aedes albopictus [7]. The DENV- 2 is the most widespread of the four serotypes [8]. In India, the earliest isolation of DENV-2 is from Vellore, South India [9]. The two component of viral serine protease, NS2B and NS3, play a crucial role in viral replication as it is required for the synthesis of the polyprotein precursor prior to the assembly of the viral complex [10]. Thus NS2B-NS3 is considered to be a significant target for development of anti-dengue drugs.
Plant derived compounds remain a significant source for the development of new antiviral agents. Studies reveal the importance of phytochemical agents against DENV [11–13]. Carica papaya, commonly known as papaya is a tree-like herbaceous plant. Carica papaya is a member of the plant family Caricaceae. Carica papaya leaf extracts are prescribed as a tonic for heart and also for the treatment of fever, pyrexia, diabetes, gonorrhea, syphilis, inflammation and for dressing foul wounds [14–16]. Previous phytochemical analysis [17] reveals the presence of flavonoids, alkaloids, carbohydrates, saponins, glycosides, phytosterols, phenolics, terpenoids and tannins in the leaves of Carica papaya. Previous report [18] states that the administration of aqueous leaf extracts of Carica papaya exhibits potential anti-dengue activity as indicated by the increased platelet count from 55×103/µL to 168×103/µL, White blood cells from 3.7×103/µL to 7.7×103/µL and neutrophils from 46% to78% in patients with dengue fever.
GCMS analysis of previous report [19] states that the leaves of Carica papaya mainly consists of seven phenolic compounds namely quercetin, protocatechuic acid, p-coumaric acid, caffeic acid, chlorogenic acid, kaempferol, and 5,7-dimethoxycoumarin. However, these compounds are not analyzed for their anti-dengue properties. Hence we attempted this research, and our results suggest that, the flavonoid quercetin has inhibitory activity on NS2B-NS3 protease which is very essential for the viral assembly. We believe our study might be a good starting point for the development of anti-dengue molecules from Carica papaya.
Methodology
Receptor 3D structure:
The NS2B cofactor was essential for the protease catalytic activity of NS3. The three dimensional structure of this protease associated with NS2B cofactor available in the protein data bank (PDB) [20]. The three dimensional structure of DENV-2 NS2B-NS3 was retrieved from the protein data bank, PDB ID: 2FOM [21]. All water molecules were removed and on the final stage hydrogen atoms were added to the target protein molecule.
Collection of active compounds:
A dataset of 7 compounds from Carica papaya were selected from the literature reported by Canini et al. (2007) [19]. The 2D structure of seven ligands were retrieved from the NCBI PubChem database ( http://www.ncbi.nlm.nih.gov/pccompound/), all the ligand molecules were converted into 3D structure using the 3D converter module of the SYBYL (Tripos international, USA) program and then energy minimized. The structures of the compounds were shown in (Figure 1).
Figure 1.
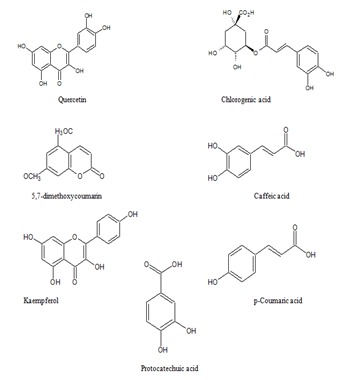
Compounds from Carica papaya leaf extract.
Molecular docking:
Molecular docking simulation was performed using Surflex-dock program incorporated in SYBYL 2.0 (Tripos international, USA) [22]. At the beginning of the docking, the protein receptor was optimized by removing the unrelated substructure. The side chains of the protein structure were fixed using default settings such as addition of water molecules, addition of hydrogen, unknown atom types were assigned and bumps were relaxed. The Kollman-all atom charges were assigned to protein atoms. Finally, the whole structure was subjected to a staged minimization using the default parameters. Protomol is a computational depiction of the intended binding site, built from the hydrogen containing protein mol2 file where the putative ligands were aligned. This was done by the protein residues which constitute the active site using criteria tuned to create a docking target. Surflex-Dock combines empirical scoring function with a molecular similarity-based search which considers four terms, including the hydrophobic complementarity, polar complementarity, entropic terms and salvation terms. Before docking, ligands were optimized using SYBYL 2.0 Molecular Modeling Suite of Tripos, hydrogen atoms were added and charges were loaded with using the Gasteiger and Marsili charge calculation method. Finally the ligands were minimized using Powell method. The Molecular Computer Aided Design (MOLCAD) program was used as a visualization tool to examine the interaction between the ligand and protein. MOLCAD afford several methods to create a molecular surface, in the present study the fast Connolly method which is based on marching cube algorithm were utilized to generate the surfaces. Consensus scoring (CScore) function were used to represent binding affinities of active compounds, which uses the multiple scoring function to evaluate the binding affinity. CScore which combines the use of Gold score (Gscore), Dock score (D_score), ChemScore, Potential Mean Force (PMF_score) along with polar and crash score. Gscore which focuses on hydrogen bonding interactions [23], D_score which utilizes the energetic contribution from hydrophobic and electrostatic interactions [24]. ChemScore function described by Eldridge et al. (1997) [25] estimates the contributions to binding energy from interactions include lipophilic atoms, lipophilic interactions, metal-ligand binding, hydrogen bonding. PMF_Score [26] whose function is based upon ligand-receptor atom-pair interaction potentials that are statistical in nature rather than empirical. Consensus score which combines the use of multiple scoring function produce more accurate results than single scoring approach.
Drug Scan:
Drug scan was done using the online chemo informatics tool Molinspiration and admetSAR to check whether the compound has fulfilled the conditions as drug candidate.
Results
Molecular docking:
Docking of seven ligand molecules onto the catalytic site of the serine protease enzyme NS2B-NS3 is performed using SYBYL – Surflex docking [27]. After docking, the best pose for each ligand molecules are selected based on the CScore. Among the seven compounds screened, quercetin is found to have best binding affinity in terms of CScore. CScore offers multiple approaches for the evaluation of ligand-receptor interactions. CScore can be used to rank multiple configurations of the same ligand docked with a receptor, or to rank selected configurations of different ligands docked to the same receptor. To have a better understanding of the interaction of ligands with the complex, binding energies as well as the hydrogen bond interactions, are tabulated in Table 1 (see supplementary material). Evaluation of docking results depends upon the number of hydrogen bonds formed between the ligand and the protein. The molecular docking result of the best pose obtained is shown in (Figure 2), protein structure is shown in surface model and the ligand is shown in stick model. Figure 3 shows the hydrogen bond interactions between the quercetin and NS2B-NS3 serine protease. A total of six hydrogen bonds are formed and the key residues Asn 152, Ala 164, Lys 74, Asn 167, Leu 149 and Gly 87 are labeled.
Figure 2.
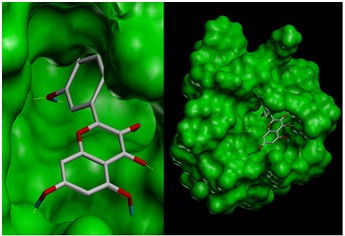
Docked structure of Dengue virus NS2B-NS3 protease with Quercetin
Figure 3.
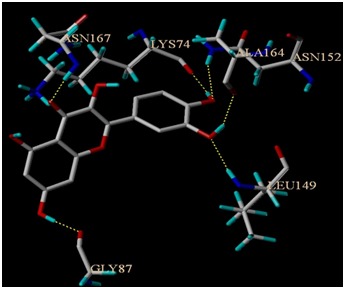
Hydrogen bonding interactions between the compound quercetin and NS2B-NS3 protease
ADME profiling:
Molecular properties and drug likeliness of the compounds are analysed on the basis of “Lipinski's Rule of Five” [28] using the tool Molinspiration server (http://www.molinspiration.com/). The rule describes molecular properties important for a drug's pharmacokinetics in the human body, including their absorption, distribution, metabolism and excretion. These compounds are checked for the drug-likeliness and the results are shown in Table 2 (see supplementary material). Our analysis showed that all the compounds passed through the filter except Chlorogenic acid which shows one violation against the Lipinski's rule of five. We also employed the use of admetSAR tool [29] for in silico screening ADMET profiles of the active compounds. The admetSAR server predicts the ADMET-associated properties of the active compounds for different types of models all of which showed positive results. These results are depicted in Table 3 (see supplementary material).
Discussion
Dengue infection has been re-emerging as a serious life threat with increase in the infection rates every year. The number of cases of severe dengue disease continues to grow in endemic areas of Southeast Asia, Central and South America, and other subtropical regions [30]. Therefore, there is an urgent need to develop effective anti-dengue compounds to combat this epidemic infection. In the present study we have analysed the activity of compounds in Carica papaya leaf extracts against NS2B-NS3 serine protease of DENV2 virus. DENV2 is a single stranded RNA with type I cap structure at the 5' end and codes for single polyprotein precursor arranged in order NH2-C-prM-E-NS1-NS2A-NS2B-NS3-NS4A-NS4B-NS5-COOH [31]. It is well known that DENV NS3 is a multifunctional protein with an N-terminal protease domain (NS3pro) [32]. The viral protease is comprised of two viral proteins NS2B and NS3 that are associated with each other to form a heterocomplex. The N-terminal region of the non-structural 3 (NS3) protease forms complex with NS2B cofactor which is essential for viral replication [33]. Prohibiting the processing and release of the viral proteins from the polyprotein precursor would inhibit viral genome replication, thus reducing the number of virion progency produced. Since NS2B-NS3 protease plays a central role in the viral life cycle, it acts as an attractive therapeutic target for antiviral compound. Several studies provide considerable evidence for the importance of NS2B-NS3 protease as potential drug target [34, 35]. Receptor-ligand binding interaction studies carried out by performing docking of the ligands that are found to be competitively inhibiting the activities of the DENV-2 NS2B-NS3 serine protease. From our analysis we found quercetin as best inhibitors as they show better ligand-enzyme interactions and stability. Our results correlate with the experimental work done by Zandi et al. (2011) [36], which show significant antiviral activity against DENV-2 in Vero cells. Quercetin has also been reported to have inhibitory effects on several viruses [37]. Even though p-Coumaric acid shows best binding affinity in terms of hydrogen bond it shows poor values in case of ligand-receptor atom pair interaction. Considering protocatechuic acid the D score which reflects electrostatic and hydrophobic energy showed the best score whereas all the other parameters gave poor results. Chlorogenic acid, 5, 7-dimethoxycoumarin and kaempferol forms five hydrogen bonds with the active site residues of NS2B-NS3 protease but shows low binding affinity comparing to quercetin. Caffeic acid shows only four hydrogen bond interactions which are the least among the seven compounds analysed. Evaluation of Absorption, Distribution, Metabolism, and Excretion (ADME) properties of lead compounds is a major challenge in the process of drug development [38]. Most drug fails in the drug development process are due to poor pharmacokinetic properties and toxicity [39]. Development of high throughput and fast ADMET profiling assays facilitate the identification of active lead compounds at early drug discovery. ADME profiling into the earliest phase of the discovery process, leads to the development of effective lead compounds in the process of drug design [40]. ADME profiling of active compounds proves that all the compounds except chlorogenic acid have no side effects on absorption. Presence of high-resistance tight junctions between endothelial cells of brain capillaries forms the barrier and prevents the brain uptake of most pharmaceuticals [41]. Experimentally, Blood brain barrier (BBB) is measured as the ratio of the compound concentration in the brain to that in the blood. Knowledge of the penetration of drugs through BBB is one of the key parameters to be optimized in drug discovery [42]. Oral bioavailability often considered as an important parameter to determine the drug likeness of active compounds as therapeutic agents [43]. Oral drug bioavailability can also be markedly influenced by physiological, physiochemical and certain biopharmaceutical factors [44]. High penetration of BBB is required for central nervous system (CNS)-active drugs, whereas for non- CNS low penetration is desirable to minimize CNS-related side effects. Incidence of CNS involvement in patients with dengue infection has been reported in the many studies [45]. Recently two case of meningitis caused by oligosymptomatic dengue has been reported in the city of Kolkata, West Bengal, India [46]. The BBB permeability of compound depends on several factors such as lipophilicity, hydrogen-bond desolvation potential, molecular size and pKa/charge [47, 47]. ADMET-associated properties of the active compounds for different types of models such as BBB penetration, P-glycoprotein substrate, renal organic cation transporter, human intestinal absorption and Caco2 permeability showed positive results which strongly supports the ability of compounds to act as drug. Cytochrome P450 (CYP) is group of isozymes involves in the metabolism of drugs, fatty acids, steroids, bile acids and carcinogens. Human genome encodes nearly fifty-seven CYP of which fifteen are involved in the metabolism of drugs and other xenobiotic chemicals [49]. Nearly 75% of phase I drug metabolism depends on the involvement of CYP enzymes [50]. In the case of metabolism, various CYP substrate and inhibitor models are calculated and the result shows that these active compounds are Non-substrate and Non-inhibitor of CYP enzymes. In terms of toxicity, all the compounds are found to be non toxic. Our findings reveal that among the seven compounds screened, quercetin has potential inhibitory activity against NS2B-NS3 serine protease. Further ADME and toxicity risk assessment strongly suggest that quercetin have marked antiviral activity against DENV2 virus. Based on our observations, we suggest that, the flavonoid quercetin in Carica papaya might exert its antiviral activity by blocking the viral assembly mechanism of DENV2 virus. Our results strongly suggest that quercetin is a good candidate for the development of effective anti-dengue compounds. On the whole, we conclude that the flavonoid quercetin could be further investigated and can possibly be developed as an effective anti-dengue compound.
Supplementary material
Acknowledgments
Dr. Anand Anbarasu gratefully acknowledges the Indian Council of Medical Research (ICMR), Government of India Agency for the research grant [IRIS ID: 2011-03260] to carry out this research. P. Lavanya thanks ICMR for the research fellowship through the ICMR grant IRIS ID: 2011-03260. We would like to thank the management of VIT University for providing us the necessary facilities to carry out this research project.
Footnotes
Citation:Senthilvel et al, Bioinformation 9(18): 889-895 (2013)
References
- 1.Bhatt S, et al. Nature. 2013;496:504. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Idrees S, Ashfaq UA. Genet Vaccines Ther. 2012;10:1. doi: 10.1186/1479-0556-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta N, et al. Indian J Med Res. 2012;136:373. [PMC free article] [PubMed] [Google Scholar]
- 4.Kuno G, et al. J Virology. 1998;72:73. doi: 10.1128/jvi.72.1.73-83.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shresta S, et al. J Virology. 2006;80:10208. doi: 10.1128/JVI.00062-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rico-Hesse R. Virology. 1990;174:479. doi: 10.1016/0042-6822(90)90102-w. [DOI] [PubMed] [Google Scholar]
- 7.Rosen L, et al. Am J Trop Med Hyg. 1983;32:1108. doi: 10.4269/ajtmh.1983.32.1108. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi H, et al. Antiviral Res. 2012;96:305. doi: 10.1016/j.antiviral.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 9.Kumar SRP, et al. J Gen Virol. 2010;91:707. doi: 10.1099/vir.0.017954-0. [DOI] [PubMed] [Google Scholar]
- 10.Mueller NH, et al. Antimicrob Agents Chemother. 2008;52:3385. doi: 10.1128/AAC.01508-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johari J, et al. Int J Mol Sci. 2012;13:16785. doi: 10.3390/ijms131216785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chattopadhyay D, et al. N Biotechnol. 2009;25:347. doi: 10.1016/j.nbt.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang LI, et al. BMC Complement Altern Med. 2012;12:3. doi: 10.1186/1472-6882-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aruoma OI, et al. Biofactors. 2006;26:147. doi: 10.1002/biof.5520260205. [DOI] [PubMed] [Google Scholar]
- 15.Mehdipour S, et al. Phytother Res. 2006;20:591. doi: 10.1002/ptr.1932. [DOI] [PubMed] [Google Scholar]
- 16.Sadek KM, et al. Acta Inform med. 2012;20:180. doi: 10.5455/aim.2012.20.180-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baskaran C, et al. Asian Pac J Trop Dis. 2012;2:658. [Google Scholar]
- 18.Ahmad N, et al. Asian Pac J Trop Biomed. 2011;1:330. doi: 10.1016/S2221-1691(11)60055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canini A, et al. J Food Compos Anal. 2007;20:584. [Google Scholar]
- 20.Berman HM, et al. Nucleic Acid Res. 2000;28:235. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erbel P, et al. Nat Struct Mol Biol. 2006;13:372. doi: 10.1038/nsmb1073. [DOI] [PubMed] [Google Scholar]
- 22.South Hanley Rd., St. Louis, Missouri, 63144, USA.: Tripos International; 1699. SYBYL-X 2.0. [Google Scholar]
- 23.Jones G, et al. J Mol Biol. 1995;245:43. doi: 10.1016/s0022-2836(95)80037-9. [DOI] [PubMed] [Google Scholar]
- 24.Meng EC, et al. J Comp Chem. 1992;13:505. [Google Scholar]
- 25.Eldridge MD, et al. J Comput Aided Mol Des. 1997;11:425. doi: 10.1023/a:1007996124545. [DOI] [PubMed] [Google Scholar]
- 26.Muegge I, Martin YC. J Med Chem. 1999;42:791. doi: 10.1021/jm980536j. [DOI] [PubMed] [Google Scholar]
- 27. Jain AN, et al. J Med Chem. 2003;46:499. doi: 10.1021/jm020406h. [DOI] [PubMed] [Google Scholar]
- 28.Lipinski CA, et al. Adv Drug Deliv Rev. 1997;46:3. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 29.Cheng F, et al. J Chem Inf Model. 2012;52:3099. doi: 10.1021/ci300367a. [DOI] [PubMed] [Google Scholar]
- 30.Whitehead SS, et al. Nat Rev Microbiol. 2007;5:518. doi: 10.1038/nrmicro1690. [DOI] [PubMed] [Google Scholar]
- 31.Frimayanti N, et al. Int J Mol Sci. 2011;12:1089. doi: 10.3390/ijms12021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qi RF, et al. Acta Biochim Biophys Sin. 2008;40:91. doi: 10.1111/j.1745-7270.2008.00382.x. [DOI] [PubMed] [Google Scholar]
- 33.Falgout B, et al. J Virol. 1991;65:2467. doi: 10.1128/jvi.65.5.2467-2475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chappell KJ, et al. Curr Med Chem. 2008;15:2771. doi: 10.2174/092986708786242804. [DOI] [PubMed] [Google Scholar]
- 35.Kim YM, et al. J Biol Chem. 2013;288:12891. doi: 10.1074/jbc.M112.442723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zandi K, et al. Virol J. 2011;8:560. doi: 10.1186/1743-422X-8-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mucsi I, Pragai BM. Experientia. 1985;41:930. doi: 10.1007/BF01970018. [DOI] [PubMed] [Google Scholar]
- 38.Brito MA. Braz J Pharm Sci. 2011;47:797. [Google Scholar]
- 39.Lin JH, Lum AYH. Pharmacol Rev. 1997;49:403. [PubMed] [Google Scholar]
- 40.Tsaioun K, et al. BMC Neurol. 2009;9:S1. doi: 10.1186/1471-2377-9-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stamatovic SM, et al. Curr Neuropharmacol. 2008;6:179. doi: 10.2174/157015908785777210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alavijeh MS, et al. NeuroRx. 2005;2:554. doi: 10.1602/neurorx.2.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas VH, et al. Expert Opin Drug Metab Toxicol. 2006;2:591. doi: 10.1517/17425255.2.4.591. [DOI] [PubMed] [Google Scholar]
- 44.Hurst S, et al. Expert Opin Drug Metab Toxicol. 2007;3:469. doi: 10.1517/17425225.3.4.469. [DOI] [PubMed] [Google Scholar]
- 45.Araujo F, et al. Emerg Infect Dis. 2012;18:677. doi: 10.3201/eid1804.111552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goswami RP, et al. J Infect Dev Ctries. 2012;6:208. doi: 10.3855/jidc.2241. [DOI] [PubMed] [Google Scholar]
- 47.Abraham MH, et al. Eur J Med Chem. 2004;39:235. doi: 10.1016/j.ejmech.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 48.Atkinson F, et al. Curr Med Chem-CNS Agents. 2002;2:229. [Google Scholar]
- 49.Guengerich FP. Mol Interv. 2003;3:194. doi: 10.1124/mi.3.4.194. [DOI] [PubMed] [Google Scholar]
- 50.Bibi Z, et al. Nutr Metab. 2008;5:27. doi: 10.1186/1743-7075-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


