Abstract
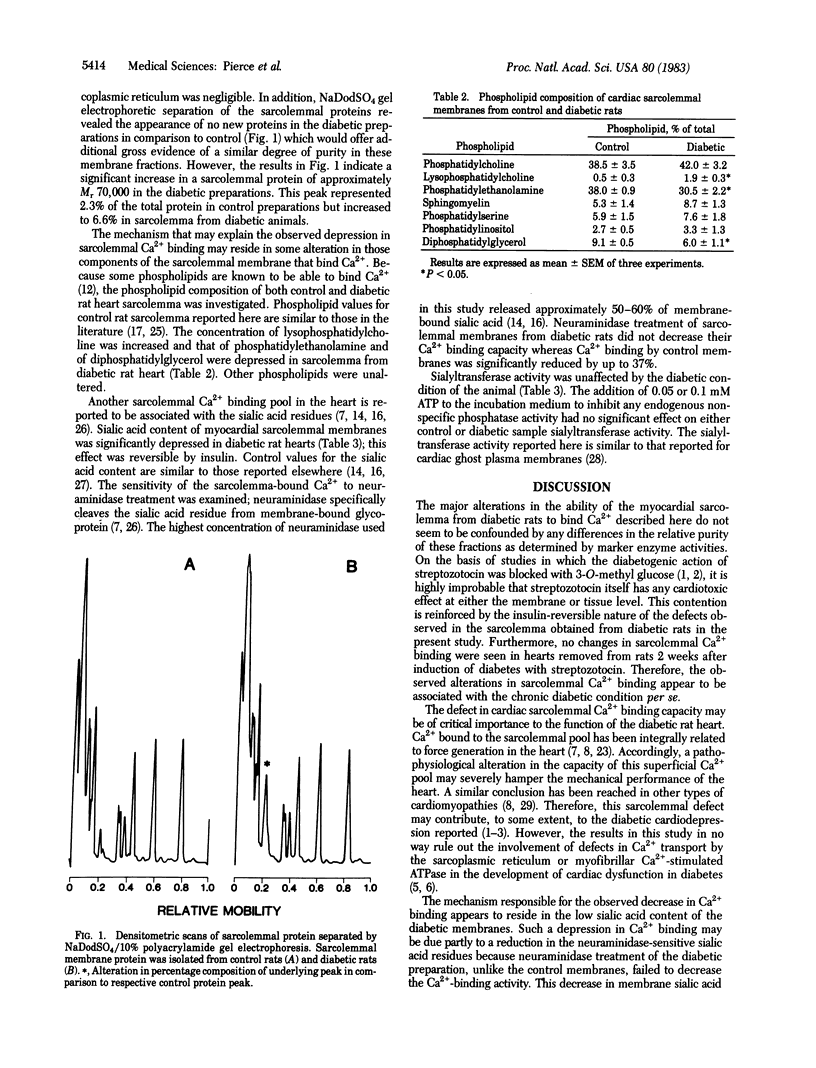
Chronic streptozotocin-induced diabetes in rats was associated with a significant loss in the ability of isolated cardiac sarcolemmal membranes to bind Ca2+. Administration of insulin to the diabetic rats normalized the sarcolemmal Ca2+ binding capacity. The content of sialic acid residues, which are considered to represent a superficial Ca2+ pool in sarcolemma, was decreased in preparations from diabetic rats, and this change also was reversible upon insulin treatment of the diabetic rats. Treatment of sarcolemma with neuraminidase decreased Ca2+ binding by 37% in control preparations but had no effect on diabetic preparations. Diphosphatidylglycerol content was decreased but other acidic phospholipids such as phosphatidylinositol and phosphatidylserine, which also bind Ca2+, were not altered during diabetes. An increase in lysophosphatidylcholine and a decrease in phosphatidylethanolamine contents were observed in membranes isolated from diabetic rats. These results suggest that some alterations occur in Ca2+ binding and composition of heart sarcolemma in chronically diabetic rats and may provide further insight into the pathogenesis of diabetic cardiomyopathy.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baba Y., Kai M., Setoyama S., Otsuji S. The lower levels of erythrocyte surface electric charge in diabetes mellitus. Clin Chim Acta. 1978 Mar 1;84(1-2):247–249. doi: 10.1016/0009-8981(78)90501-6. [DOI] [PubMed] [Google Scholar]
- Bailey L. E., Ma T. S. Functional deficiencies in interstitial glycoproteins in myopathic hamster hearts. Adv Myocardiol. 1980;1:95–112. [PubMed] [Google Scholar]
- Bardos P., Lacord-Bonneau M., Rakotoarivony J., Muh J. P., Weill J. Sialic acid transferase activities of normal and diabetic (db/db) mice kidney. Int J Biochem. 1980;12(3):505–507. doi: 10.1016/0020-711x(80)90138-x. [DOI] [PubMed] [Google Scholar]
- Baxter A., Durham J. P. A rapid, sensitive disk assay for the determination of glycoprotein glycosyltransferases. Anal Biochem. 1979 Sep 15;98(1):95–101. doi: 10.1016/0003-2697(79)90711-5. [DOI] [PubMed] [Google Scholar]
- Berenson G. S., Radhakrishnamurthy, Dalferes E. R., Jr, Ruiz H., Srinivasan S. R., Plavidal F., Brickman F. Connective tissue macromolecular changes in rats with experimentally induced diabetes and hyperinsulinism. Diabetes. 1972 Jun;21(6):733–743. doi: 10.2337/diab.21.6.733. [DOI] [PubMed] [Google Scholar]
- Bers D. M., Langer G. A. Uncoupling cation effects on cardiac contractility and sarcolemmal Ca2+ binding. Am J Physiol. 1979 Sep;237(3):H332–H341. doi: 10.1152/ajpheart.1979.237.3.H332. [DOI] [PubMed] [Google Scholar]
- Chien K. R., Reeves J. P., Buja L. M., Bonte F., Parkey R. W., Willerson J. T. Phospholipid alterations in canine ischemic myocardium. Temporal and topographical correlations with Tc-99m-PPi accumulation and an in vitro sarcolemmal Ca2+ permeability defect. Circ Res. 1981 May;48(5):711–719. doi: 10.1161/01.res.48.5.711. [DOI] [PubMed] [Google Scholar]
- Dhalla N. S., Anand-Srivastava M. B., Tuana B. S., Khandelwal R. L. Solubilization of a calcium dependent adenosine triphosphatase from rat heart sarcolemma. J Mol Cell Cardiol. 1981 Apr;13(4):413–423. doi: 10.1016/0022-2828(81)90283-2. [DOI] [PubMed] [Google Scholar]
- Dhalla N. S., Das P. K., Sharma G. P. Subcellular basis of cardiac contractile failure. J Mol Cell Cardiol. 1978 Apr;10(4):363–385. doi: 10.1016/0022-2828(78)90384-x. [DOI] [PubMed] [Google Scholar]
- Dhalla N. S., Pierce G. N., Panagia V., Singal P. K., Beamish R. E. Calcium movements in relation to heart function. Basic Res Cardiol. 1982 Mar-Apr;77(2):117–139. doi: 10.1007/BF01908167. [DOI] [PubMed] [Google Scholar]
- Fein F. S., Strobeck J. E., Malhotra A., Scheuer J., Sonnenblick E. H. Reversibility of diabetic cardiomyopathy with insulin in rats. Circ Res. 1981 Dec;49(6):1251–1261. doi: 10.1161/01.res.49.6.1251. [DOI] [PubMed] [Google Scholar]
- Feuvray D., Idell-Wenger J. A., Neely J. R. Effects of ischemia on rat myocardial function and metabolism in diabetes. Circ Res. 1979 Mar;44(3):322–329. doi: 10.1161/01.res.44.3.322. [DOI] [PubMed] [Google Scholar]
- Frank J. S., Langer G. A., Nudd L. M., Seraydarian K. The myocardial cell surface, its histochemistry, and the effect of sialic acid and calcium removal on its stucture and cellular ionic exchange. Circ Res. 1977 Nov;41(5):702–714. doi: 10.1161/01.res.41.5.702. [DOI] [PubMed] [Google Scholar]
- Gertz B. J., Haugaard E. S. Effect of diabetes and fasting on the uridine triphosphate content and uridine kinase activity of rat cardiac and skeletal muscle. Metabolism. 1979 Apr;28(4):358–362. doi: 10.1016/0026-0495(79)90107-0. [DOI] [PubMed] [Google Scholar]
- Gordon L. M., Sauerheber R. D., Esgate J. A. Spin label studies on rat liver and heart plasma membranes: effects of temperature, calcium, and lanthanum on membrane fluidity. J Supramol Struct. 1978;9(3):299–326. doi: 10.1002/jss.400090303. [DOI] [PubMed] [Google Scholar]
- Harrow J. A., Das P. K., Dhalla N. S. Influence of some divalent cations on heart sarcolemmal bound enzymes and calcium binding. Biochem Pharmacol. 1978;27(22):2605–2609. doi: 10.1016/0006-2952(78)90334-9. [DOI] [PubMed] [Google Scholar]
- Joyner W. L., Mayhan W. G., Johnson R. L., Phares C. K. Microvascular alterations develop in Syrian hamsters after the induction of diabetes mellitus by streptozotocin. Diabetes. 1981 Feb;30(2):93–100. doi: 10.2337/diab.30.2.93. [DOI] [PubMed] [Google Scholar]
- Karli J. N., Karikas G. A., Hatzipavlou P. K., Levis G. M., Moulopoulos S. N. The inhibition of Na+ and K+ stimulated ATPase activity of rabbit and dog heart sarcolemma by lysophosphatidyl choline. Life Sci. 1979 May 14;24(20):1869–1875. doi: 10.1016/0024-3205(79)90238-8. [DOI] [PubMed] [Google Scholar]
- Katz A. M., Messineo F. C. Lipid-membrane interactions and the pathogenesis of ischemic damage in the myocardium. Circ Res. 1981 Jan;48(1):1–16. doi: 10.1161/01.res.48.1.1. [DOI] [PubMed] [Google Scholar]
- Ku D. D., Sellers B. M. Effects of streptozotocin diabetes and insulin treatment on myocardial sodium pump and contractility of the rat heart. J Pharmacol Exp Ther. 1982 Aug;222(2):395–400. [PubMed] [Google Scholar]
- Langer G. A. The structure and function of the myocardial cell surface. Am J Physiol. 1978 Nov;235(5):H461–H468. doi: 10.1152/ajpheart.1978.235.5.H461. [DOI] [PubMed] [Google Scholar]
- Martin A. F., Pagani E. D., Solaro R. J. Thyroxine-induced redistribution of isoenzymes of rabbit ventricular myosin. Circ Res. 1982 Jan;50(1):117–124. doi: 10.1161/01.res.50.1.117. [DOI] [PubMed] [Google Scholar]
- Matsukubo M. P., Singal P. K., Dhalla N. S. Negatively charged sites and calcium binding in the isolated rat heart sarcolemma. Basic Res Cardiol. 1981 Jan-Feb;76(1):16–28. doi: 10.1007/BF01908160. [DOI] [PubMed] [Google Scholar]
- McConnaughey M. M., Jones L. R., Watanabe A. M., Besch H. R., Jr, Williams L. T., Lefkowitz R. J. Thyroxine and propylthiouracil effects on alpha- and beta-adrenergic receptor number, ATPase activities, and sialic acid content of rat cardiac membrane vesicles. J Cardiovasc Pharmacol. 1979 Nov-Dec;1(6):609–623. doi: 10.1097/00005344-197911000-00002. [DOI] [PubMed] [Google Scholar]
- Owens K., Pang D. C., Weglicki W. B. Production of lysophospholipids and free fatty acids by a sarcolemmal fraction from canine myocardium. Biochem Biophys Res Commun. 1979 Jul 27;89(2):368–373. doi: 10.1016/0006-291x(79)90639-9. [DOI] [PubMed] [Google Scholar]
- Panagia V., Lamers J. M., Singal P. K., Dhalla N. S. Ca2+- and Mg2+-dependent ATPase activities in the deoxycholate-treated rat heart sarcolemma. Int J Biochem. 1982;14(5):387–397. doi: 10.1016/0020-711x(82)90024-6. [DOI] [PubMed] [Google Scholar]
- Penpargkul S., Fein F., Sonnenblick E. H., Scheuer J. Depressed cardiac sarcoplasmic reticular function from diabetic rats. J Mol Cell Cardiol. 1981 Mar;13(3):303–309. doi: 10.1016/0022-2828(81)90318-7. [DOI] [PubMed] [Google Scholar]
- Penpargkul S., Schaible T., Yipintsoi T., Scheuer J. The effect of diabetes on performance and metabolism of rat hearts. Circ Res. 1980 Dec;47(6):911–921. doi: 10.1161/01.res.47.6.911. [DOI] [PubMed] [Google Scholar]
- Pettit G. W., Vick R. L. Contribution of pancreatic insulin to extrarenal potassium homeostasis: a tow-compartment model. Am J Physiol. 1974 Feb;226(2):319–324. doi: 10.1152/ajplegacy.1974.226.2.319. [DOI] [PubMed] [Google Scholar]
- Philipson K. D., Bers D. M., Nishimoto A. Y. The role of phospholipids in the Ca2+ binding of isolated cardiac sarcolemma. J Mol Cell Cardiol. 1980 Nov;12(11):1159–1173. doi: 10.1016/0022-2828(80)90063-2. [DOI] [PubMed] [Google Scholar]
- Pierce G. N., Dhalla N. S. Cardiac myofibrillar ATPase activity in diabetic rats. J Mol Cell Cardiol. 1981 Dec;13(12):1063–1069. doi: 10.1016/0022-2828(81)90296-0. [DOI] [PubMed] [Google Scholar]
- Potter J. D. The content of troponin, tropomyosin, actin, and myosin in rabbit skeletal muscle myofibrils. Arch Biochem Biophys. 1974 Jun;162(2):436–441. doi: 10.1016/0003-9861(74)90202-1. [DOI] [PubMed] [Google Scholar]
- Regan T. J., Wu C. F., Yeh C. K., Oldewurtel H. A., Haider B. Myocardial composition and function in diabetes. The effects of chronic insulin use. Circ Res. 1981 Dec;49(6):1268–1277. doi: 10.1161/01.res.49.6.1268. [DOI] [PubMed] [Google Scholar]
- Sedlis S. P., Corr P. B., Sobel B. E., Ahumada G. G. Lysophosphatidyl choline potentiates Ca2+ accumulation in rat cardiac myocytes. Am J Physiol. 1983 Jan;244(1):H32–H38. doi: 10.1152/ajpheart.1983.244.1.H32. [DOI] [PubMed] [Google Scholar]
- Senges J., Brachmann J., Pelzer D., Hasslacher C., Weihe E., Kübler W. Altered cardiac automaticity and conduction in experimental diabetes mellitus. J Mol Cell Cardiol. 1980 Dec;12(12):1341–1351. doi: 10.1016/0022-2828(80)90120-0. [DOI] [PubMed] [Google Scholar]
- Spiro R. G. Glycoproteins: their biochemistry, biology and role in human disease. N Engl J Med. 1969 Nov 6;281(19):1043–concl. doi: 10.1056/NEJM196911062811905. [DOI] [PubMed] [Google Scholar]
- Taeko S., Daly M. J., Anand-Srivastava M. B., Dhalla N. S. Influence of neuraminidase treatment on rat heart sarcolemma. J Mol Cell Cardiol. 1980 Feb;12(2):211–217. doi: 10.1016/0022-2828(80)90090-5. [DOI] [PubMed] [Google Scholar]
- Takeo S., Duke P., Taam G. M., Singal P. K., Dhalla N. S. Effects of lanthanum on the heart sarcolemmal ATPase and calcium binding activities. Can J Physiol Pharmacol. 1979 May;57(5):496–503. doi: 10.1139/y79-075. [DOI] [PubMed] [Google Scholar]
- Tuana B. S., Dhalla N. S. Purification and characterization of a Ca2+-dependent ATPase from rat heart sarcolemma. J Biol Chem. 1982 Dec 10;257(23):14440–14445. [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Westberg N. G., Michael A. F. Human glomerular basement membrane: chemical composition in diabetes mellitus. Acta Med Scand. 1973 Jul-Aug;1-2(1):39–47. doi: 10.1111/j.0954-6820.1973.tb19411.x. [DOI] [PubMed] [Google Scholar]


