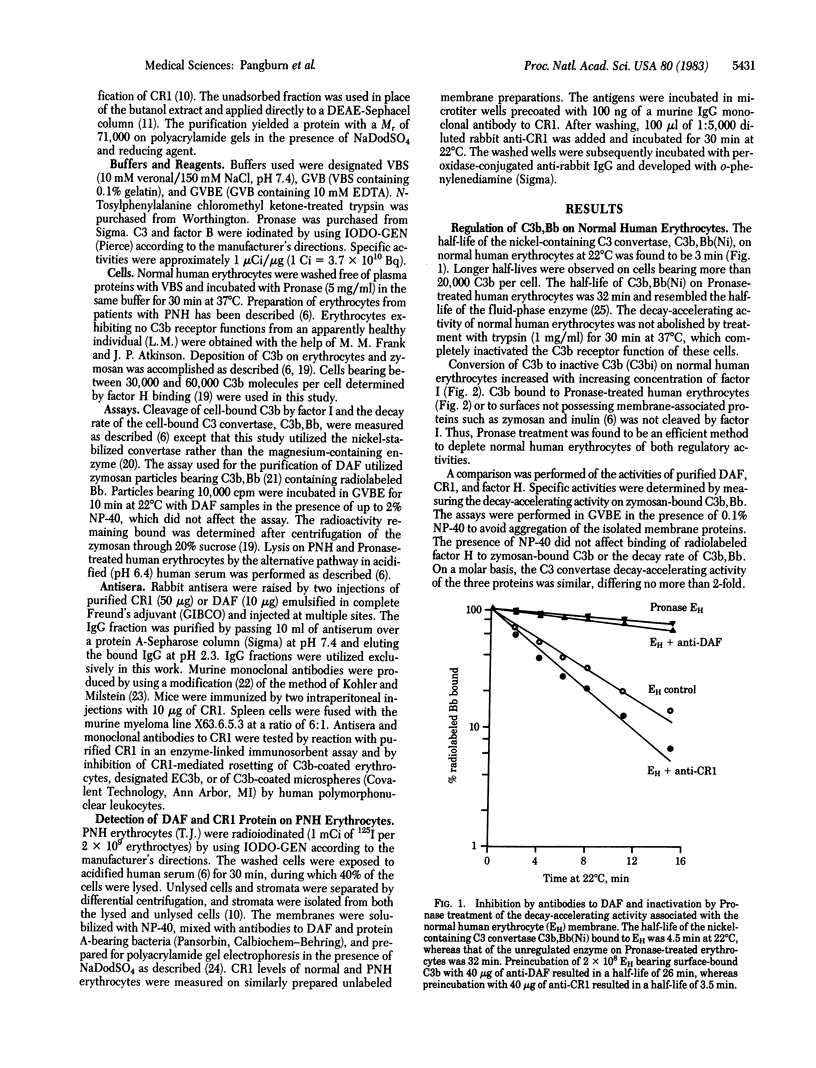
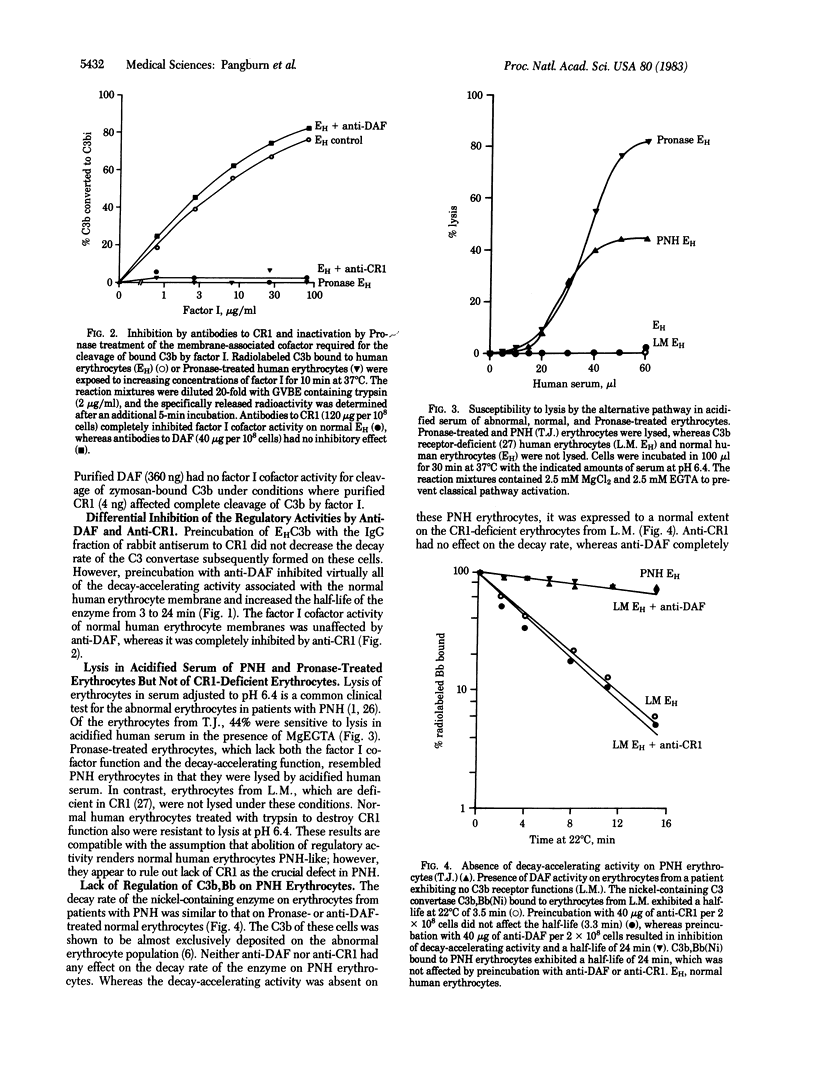
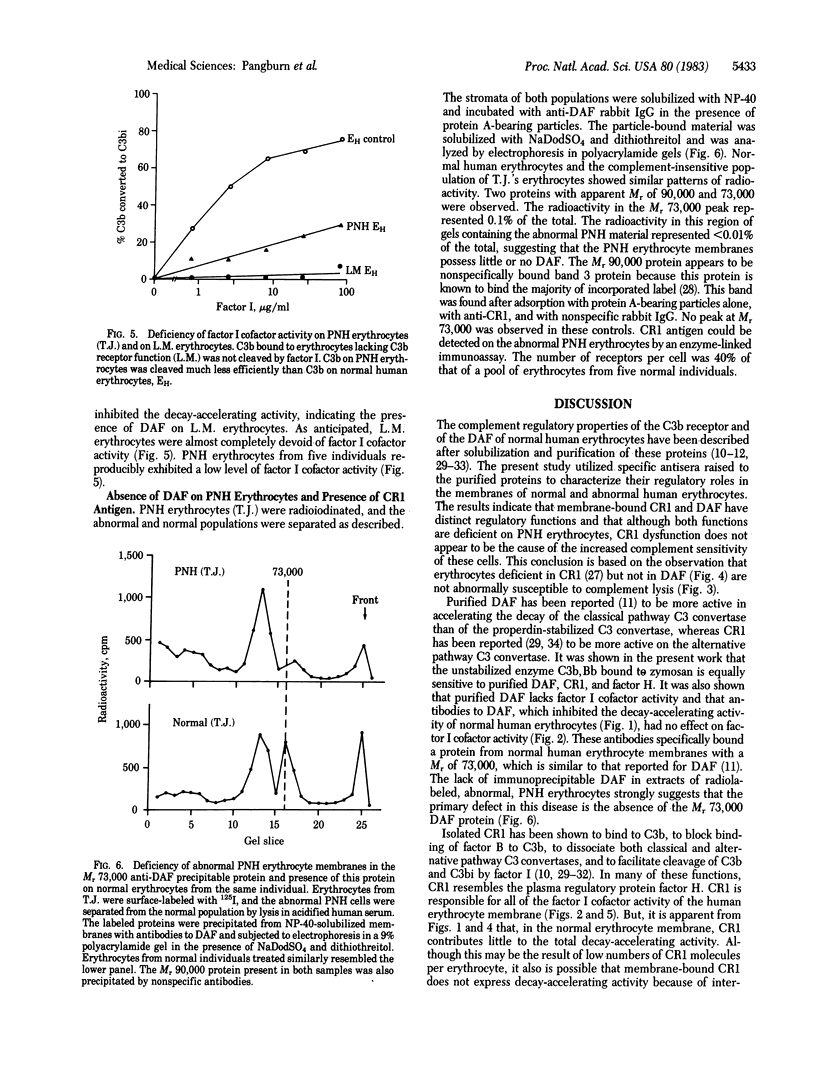
Abstract
Paroxysmal nocturnal hemoglobinuria (PNH) is an acquired hemolytic anemia in which the erythrocytes are abnormally sensitive to lysis by complement. A functional deficiency of membrane-associated complement regulators has been demonstrated on PNH erythrocytes. The two factor H-like proteins, the C3b receptor (CR1) and the decay-accelerating factor (DAF), were isolated from normal human erythrocytes, and specific antisera were prepared. Selective inhibition of the two proteins on normal erythrocytes by the antisera demonstrated (i) that the factor responsible for accelerated decay of erythrocyte-bound C3 convertase is DAF and (ii) that the cofactor required for inactivation of erythrocyte-bound C3b by factor I is CR1. PNH erythrocytes were deficient in both of these activities. Erythrocytes deficient in CR1, which were obtained from an apparently healthy individual, exhibited normal DAF activity but no factor I cofactor activity. These cells were not susceptible to complement-mediated lysis in acidified human serum, whereas PNH erythrocytes and Pronase-treated human erythrocytes (which lack DAF and CR1 activities) were lysed by this treatment. It is suggested that the protein primarily responsible for preventing complement activation on normal human erythrocytes is DAF. AMr 73,000 protein isolated from the normal erythrocyte membranes of one PNH patient by using anti-DAF IgG was largely absent from the abnormal erythrocytes of this individual, suggesting that PNH cells lack the DAF protein. CR1 antigen, however, was present on the abnormal PNH erythrocytes. The results suggest that the primary molecular defect underlying the clinical manifestations of PNH may be the lack of the membrane-associated DAF protein and that the abnormal cells may also exhibit impaired CR1 function.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballow M., Cochrane C. G. Two anticomplementary factors in cobra venom: hemolysis of guinea pig erythrocytes by one of them. J Immunol. 1969 Nov;103(5):944–952. [PubMed] [Google Scholar]
- Chua C., Hoffmann E. M., Adams J. P., Rosse W. F. Inhibitors of complement derived from the erythrocyte membrane in paroxysmal nocturnal hemoglobinuria. Blood. 1980 May;55(5):772–776. [PubMed] [Google Scholar]
- Dobson N. J., Lambris J. D., Ross G. D. Characteristics of isolated erythrocyte complement receptor type one (CR1, C4b-C3b receptor) and CR1-specific antibodies. J Immunol. 1981 Feb;126(2):693–698. [PubMed] [Google Scholar]
- Fearon D. T. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J Exp Med. 1980 Jul 1;152(1):20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Regulation of the amplification C3 convertase of human complement by an inhibitory protein isolated from human erythrocyte membrane. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5867–5871. doi: 10.1073/pnas.76.11.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishelson Z., Pangburn M. K., Müller-Eberhard H. J. C3 convertase of the alternative complement pathway. Demonstration of an active, stable C3b, Bb (Ni) complex. J Biol Chem. 1983 Jun 25;258(12):7411–7415. [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. Lysis of erythrocytes by complement in the absence of antibody. J Exp Med. 1970 Nov;132(5):898–915. doi: 10.1084/jem.132.5.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. Paroxysmal nocturnal hemoglobinuria. Hemolysis initiated by the C3 activator system. N Engl J Med. 1972 Jan 27;286(4):180–184. doi: 10.1056/NEJM197201272860403. [DOI] [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. The C3-activator system: an alternate pathway of complement activation. J Exp Med. 1971 Sep 1;134(3 Pt 2):90s–108s. [PubMed] [Google Scholar]
- HINZ C. F., Jr, JORDAN W. S., Jr, PILLEMER L. The properdin system and immunity. IV. The hemolysis of erythrocytes from patients with paroxysmal nocturnal hemoglobinuria. J Clin Invest. 1956 May;35(5):453–457. doi: 10.1172/JCI103296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer C. H., Wirtz G. H., Renfer L., Gresham H. D., Tack B. F. Large scale isolation of functionally active components of the human complement system. J Biol Chem. 1981 Apr 25;256(8):3995–4006. [PubMed] [Google Scholar]
- Hoffmann E. M. Inhibition of complement by a substance isolated from human erythrocytes. II. Studies on the site and mechanism of action. Immunochemistry. 1969 May;6(3):405–419. doi: 10.1016/0019-2791(69)90297-3. [DOI] [PubMed] [Google Scholar]
- Iida K., Nussenzweig V. Complement receptor is an inhibitor of the complement cascade. J Exp Med. 1981 May 1;153(5):1138–1150. doi: 10.1084/jem.153.5.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. M., Shin M. L., Mayer M. M. On the lysis of paroxysmal nocturnal hemoglobinuria erythrocytes by complement: dual role of C3b. Blut. 1982 Oct;45(4):249–259. doi: 10.1007/BF00320192. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Lesavre P. H., Hugli T. E., Esser A. F., Müller-Eberhard H. J. The alternative pathway C3/C5 convertase: chemical basis of factor B activation. J Immunol. 1979 Aug;123(2):529–534. [PubMed] [Google Scholar]
- Logue G. L., Rosse W. F., Adams J. P. Mechanisms of immune lysis of red blood cells in vitro. I. Paroxysmal nocturnal hemoglobinuria cells. J Clin Invest. 1973 May;52(5):1129–1137. doi: 10.1172/JCI107279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Prince G. M., Mold C. Release of soluble immune complexes from immune adherence receptors on human erythrocytes is mediated by C3b inactivator independently of Beta 1H and is accompanied by generation of C3c. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5047–5051. doi: 10.1073/pnas.79.16.5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Prince G. M., Oger J. J. Kinetics of interaction of immune complexes with complement receptors on human blood cells: modification of complexes during interaction with red cells. Clin Exp Immunol. 1982 Jun;48(3):715–725. [PMC free article] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Götze O. C3 proactivator convertase and its mode of action. J Exp Med. 1972 Apr 1;135(4):1003–1008. doi: 10.1084/jem.135.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson-Weller A., Burge J., Fearon D. T., Weller P. F., Austen K. F. Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertases of the complement system. J Immunol. 1982 Jul;129(1):184–189. [PubMed] [Google Scholar]
- Pangburn M. K. Activation of complement via the alternative pathway. Fed Proc. 1983 Jan;42(1):139–143. [PubMed] [Google Scholar]
- Pangburn M. K., Morrison D. C., Schreiber R. D., Müller-Eberhard H. J. Activation of the alternative complement pathway: recognition of surface structures on activators by bound C3b. J Immunol. 1980 Feb;124(2):977–982. [PubMed] [Google Scholar]
- Pangburn M. K., Müller-Eberhard H. J. Complement C3 convertase: cell surface restriction of beta1H control and generation of restriction on neuraminidase-treated cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2416–2420. doi: 10.1073/pnas.75.5.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Müller-Eberhard H. J. Relation of putative thioester bond in C3 to activation of the alternative pathway and the binding of C3b to biological targets of complement. J Exp Med. 1980 Oct 1;152(4):1102–1114. doi: 10.1084/jem.152.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Formation of the initial C3 convertase of the alternative complement pathway. Acquisition of C3b-like activities by spontaneous hydrolysis of the putative thioester in native C3. J Exp Med. 1981 Sep 1;154(3):856–867. doi: 10.1084/jem.154.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J Exp Med. 1977 Jul 1;146(1):257–270. doi: 10.1084/jem.146.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Trombold J. S., Müller-Eberhard H. J. Paroxysmal nocturnal hemoglobinuria: deficiency in factor H-like functions of the abnormal erythrocytes. J Exp Med. 1983 Jun 1;157(6):1971–1980. doi: 10.1084/jem.157.6.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. J., Baker P. J., Rosse W. F. Increased enzymatic activity of the alternative pathway convertase when bound to the erythrocytes of paroxysmal nocturnal hemoglobinuria. J Clin Invest. 1982 Feb;69(2):337–346. doi: 10.1172/JCI110457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. D., Lambris J. D., Cain J. A., Newman S. L. Generation of three different fragments of bound C3 with purified factor I or serum. I. Requirements for factor H vs CR1 cofactor activity. J Immunol. 1982 Nov;129(5):2051–2060. [PubMed] [Google Scholar]
- Rosse W. F. Paroxysmal nocturnal haemoglobinuria in aplastic anaemia. Clin Haematol. 1978 Oct;7(3):541–553. [PubMed] [Google Scholar]
- Rothman I. K., Gelfand J. A., Fauci A. S., Frank M. M. The immune adherence receptor: dissociation between the expression of erythrocyte and mononuclear cell C3b receptors. J Immunol. 1975 Nov;115(5):1312–1315. [PubMed] [Google Scholar]
- Schreiber R. D., Medicus R. G., Gïtze O., Müller-Eberhard H. J. Properdin- and nephritic factor-dependent C3 convertases: requirement of native C3 for enzyme formation and the function of bound C3b as properdin receptor. J Exp Med. 1975 Sep 1;142(3):760–772. doi: 10.1084/jem.142.3.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamerius J. D., Pangburn M. K., Müller-Eberhard H. J. Selective inhibition of functional sites of cell-bound C3b by hybridoma-derived antibodies. J Immunol. 1982 Jan;128(1):512–514. [PubMed] [Google Scholar]
- Weiler J. M., Daha M. R., Austen K. F., Fearon D. T. Control of the amplification convertase of complement by the plasma protein beta1H. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3268–3272. doi: 10.1073/pnas.73.9.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaley K., Ruddy S. Modulation of the alternative complement pathways by beta 1 H globulin. J Exp Med. 1976 Nov 2;144(5):1147–1163. doi: 10.1084/jem.144.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]


