Abstract
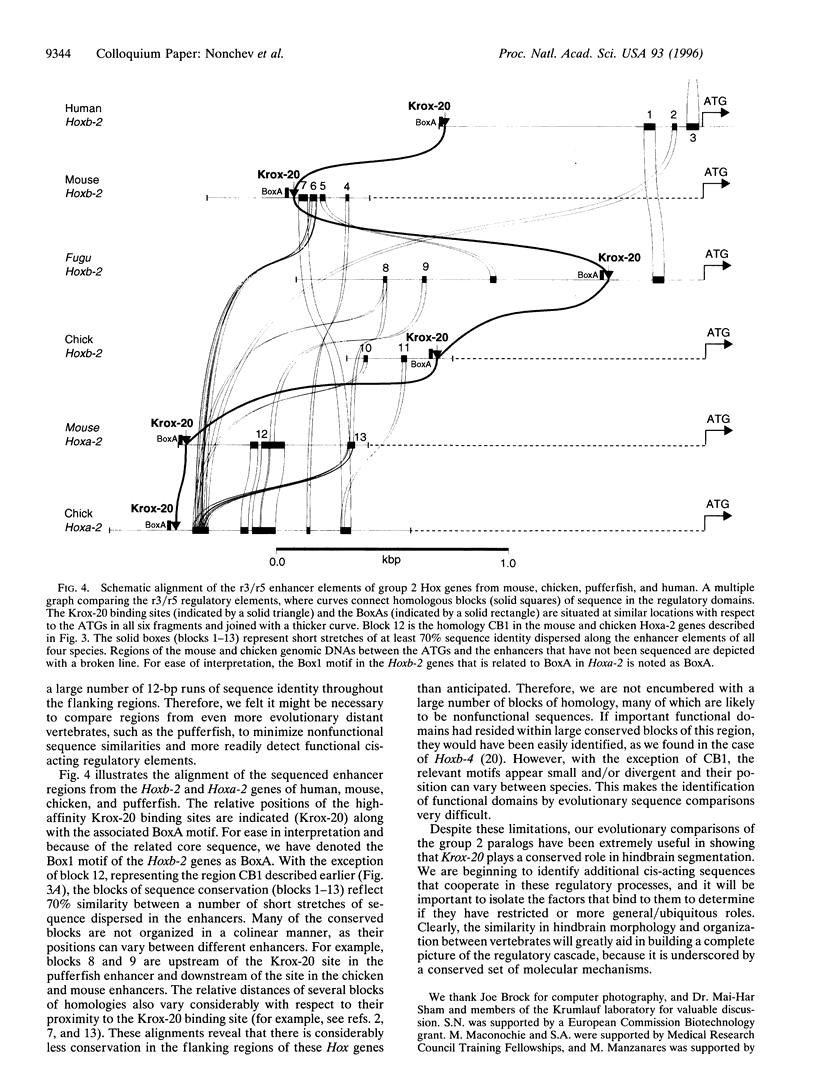
Transient segmentation in the hindbrain is a fundamental morphogenetic phenomenon in the vertebrate embryo, and the restricted expression of subsets of Hox genes in the developing rhombomeric units and their derivatives is linked with regional specification. Here we show that patterning of the vertebrate hindbrain involves the direct upregulation of the chicken and pufferfish group 2 paralogous genes, Hoxb-2 and Hoxa-2, in rhombomeres 3 and 5 (r3 and r5) by the zinc finger gene Krox-20. We identified evolutionarily conserved r3/r5 enhancers that contain high affinity Krox-20. binding sites capable of mediating transactivation by Krox-20. In addition to conservation of binding sites critical for Krox-20 activity in the chicken Hoxa-2 and pufferfish Hoxb-2 genes, the r3/r5 enhancers are also characterized by the presence of a number of identical motifs likely to be involved in cooperative interactions with Krox-20 during the process of hindbrain patterning in vertebrates.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aparicio S., Morrison A., Gould A., Gilthorpe J., Chaudhuri C., Rigby P., Krumlauf R., Brenner S. Detecting conserved regulatory elements with the model genome of the Japanese puffer fish, Fugu rubripes. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1684–1688. doi: 10.1073/pnas.92.5.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxendale S., Abdulla S., Elgar G., Buck D., Berks M., Micklem G., Durbin R., Bates G., Brenner S., Beck S. Comparative sequence analysis of the human and pufferfish Huntington's disease genes. Nat Genet. 1995 May;10(1):67–76. doi: 10.1038/ng0595-67. [DOI] [PubMed] [Google Scholar]
- Becker N., Seitanidou T., Murphy P., Mattéi M. G., Topilko P., Nieto M. A., Wilkinson D. G., Charnay P., Gilardi-Hebenstreit P. Several receptor tyrosine kinase genes of the Eph family are segmentally expressed in the developing hindbrain. Mech Dev. 1994 Jul;47(1):3–17. doi: 10.1016/0925-4773(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Boncinelli E., Simeone A., Acampora D., Mavilio F. HOX gene activation by retinoic acid. Trends Genet. 1991 Oct;7(10):329–334. doi: 10.1016/0168-9525(91)90423-n. [DOI] [PubMed] [Google Scholar]
- Bradley L. C., Snape A., Bhatt S., Wilkinson D. G. The structure and expression of the Xenopus Krox-20 gene: conserved and divergent patterns of expression in rhombomeres and neural crest. Mech Dev. 1993 Jan;40(1-2):73–84. doi: 10.1016/0925-4773(93)90089-g. [DOI] [PubMed] [Google Scholar]
- Brenner S., Elgar G., Sandford R., Macrae A., Venkatesh B., Aparicio S. Characterization of the pufferfish (Fugu) genome as a compact model vertebrate genome. Nature. 1993 Nov 18;366(6452):265–268. doi: 10.1038/366265a0. [DOI] [PubMed] [Google Scholar]
- Duboule D., Morata G. Colinearity and functional hierarchy among genes of the homeotic complexes. Trends Genet. 1994 Oct;10(10):358–364. doi: 10.1016/0168-9525(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Fraser S., Keynes R., Lumsden A. Segmentation in the chick embryo hindbrain is defined by cell lineage restrictions. Nature. 1990 Mar 29;344(6265):431–435. doi: 10.1038/344431a0. [DOI] [PubMed] [Google Scholar]
- Gendron-Maguire M., Mallo M., Zhang M., Gridley T. Hoxa-2 mutant mice exhibit homeotic transformation of skeletal elements derived from cranial neural crest. Cell. 1993 Dec 31;75(7):1317–1331. doi: 10.1016/0092-8674(93)90619-2. [DOI] [PubMed] [Google Scholar]
- Keynes R., Krumlauf R. Hox genes and regionalization of the nervous system. Annu Rev Neurosci. 1994;17:109–132. doi: 10.1146/annurev.ne.17.030194.000545. [DOI] [PubMed] [Google Scholar]
- Krumlauf R. Evolution of the vertebrate Hox homeobox genes. Bioessays. 1992 Apr;14(4):245–252. doi: 10.1002/bies.950140408. [DOI] [PubMed] [Google Scholar]
- Krumlauf R. Hox genes and pattern formation in the branchial region of the vertebrate head. Trends Genet. 1993 Apr;9(4):106–112. doi: 10.1016/0168-9525(93)90203-t. [DOI] [PubMed] [Google Scholar]
- Krumlauf R. Hox genes in vertebrate development. Cell. 1994 Jul 29;78(2):191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Lumsden A., Keynes R. Segmental patterns of neuronal development in the chick hindbrain. Nature. 1989 Feb 2;337(6206):424–428. doi: 10.1038/337424a0. [DOI] [PubMed] [Google Scholar]
- Marshall H., Studer M., Pöpperl H., Aparicio S., Kuroiwa A., Brenner S., Krumlauf R. A conserved retinoic acid response element required for early expression of the homeobox gene Hoxb-1. Nature. 1994 Aug 18;370(6490):567–571. doi: 10.1038/370567a0. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Krumlauf R. Homeobox genes and axial patterning. Cell. 1992 Jan 24;68(2):283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- Morrison A., Chaudhuri C., Ariza-McNaughton L., Muchamore I., Kuroiwa A., Krumlauf R. Comparative analysis of chicken Hoxb-4 regulation in transgenic mice. Mech Dev. 1995 Sep;53(1):47–59. doi: 10.1016/0925-4773(95)00423-8. [DOI] [PubMed] [Google Scholar]
- Nieto M. A., Bradley L. C., Wilkinson D. G. Conserved segmental expression of Krox-20 in the vertebrate hindbrain and its relationship to lineage restriction. Dev Suppl. 1991;Suppl 2:59–62. [PubMed] [Google Scholar]
- Nieto M. A., Gilardi-Hebenstreit P., Charnay P., Wilkinson D. G. A receptor protein tyrosine kinase implicated in the segmental patterning of the hindbrain and mesoderm. Development. 1992 Dec;116(4):1137–1150. doi: 10.1242/dev.116.4.1137. [DOI] [PubMed] [Google Scholar]
- Noden D. M. Interactions and fates of avian craniofacial mesenchyme. Development. 1988;103 (Suppl):121–140. doi: 10.1242/dev.103.Supplement.121. [DOI] [PubMed] [Google Scholar]
- Noden D. M. The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissues. Dev Biol. 1983 Mar;96(1):144–165. doi: 10.1016/0012-1606(83)90318-4. [DOI] [PubMed] [Google Scholar]
- Nonchev S., Vesque C., Maconochie M., Seitanidou T., Ariza-McNaughton L., Frain M., Marshall H., Sham M. H., Krumlauf R., Charnay P. Segmental expression of Hoxa-2 in the hindbrain is directly regulated by Krox-20. Development. 1996 Feb;122(2):543–554. doi: 10.1242/dev.122.2.543. [DOI] [PubMed] [Google Scholar]
- Oxtoby E., Jowett T. Cloning of the zebrafish krox-20 gene (krx-20) and its expression during hindbrain development. Nucleic Acids Res. 1993 Mar 11;21(5):1087–1095. doi: 10.1093/nar/21.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince V., Lumsden A. Hoxa-2 expression in normal and transposed rhombomeres: independent regulation in the neural tube and neural crest. Development. 1994 Apr;120(4):911–923. doi: 10.1242/dev.120.4.911. [DOI] [PubMed] [Google Scholar]
- Pöpperl H., Bienz M., Studer M., Chan S. K., Aparicio S., Brenner S., Mann R. S., Krumlauf R. Segmental expression of Hoxb-1 is controlled by a highly conserved autoregulatory loop dependent upon exd/pbx. Cell. 1995 Jun 30;81(7):1031–1042. doi: 10.1016/s0092-8674(05)80008-x. [DOI] [PubMed] [Google Scholar]
- Rijli F. M., Mark M., Lakkaraju S., Dierich A., Dollé P., Chambon P. A homeotic transformation is generated in the rostral branchial region of the head by disruption of Hoxa-2, which acts as a selector gene. Cell. 1993 Dec 31;75(7):1333–1349. doi: 10.1016/0092-8674(93)90620-6. [DOI] [PubMed] [Google Scholar]
- Schneider-Maunoury S., Topilko P., Seitandou T., Levi G., Cohen-Tannoudji M., Pournin S., Babinet C., Charnay P. Disruption of Krox-20 results in alteration of rhombomeres 3 and 5 in the developing hindbrain. Cell. 1993 Dec 17;75(6):1199–1214. doi: 10.1016/0092-8674(93)90329-o. [DOI] [PubMed] [Google Scholar]
- Sham M. H., Vesque C., Nonchev S., Marshall H., Frain M., Gupta R. D., Whiting J., Wilkinson D., Charnay P., Krumlauf R. The zinc finger gene Krox20 regulates HoxB2 (Hox2.8) during hindbrain segmentation. Cell. 1993 Jan 29;72(2):183–196. doi: 10.1016/0092-8674(93)90659-e. [DOI] [PubMed] [Google Scholar]
- Studer M., Pöpperl H., Marshall H., Kuroiwa A., Krumlauf R. Role of a conserved retinoic acid response element in rhombomere restriction of Hoxb-1. Science. 1994 Sep 16;265(5179):1728–1732. doi: 10.1126/science.7916164. [DOI] [PubMed] [Google Scholar]
- Swiatek P. J., Gridley T. Perinatal lethality and defects in hindbrain development in mice homozygous for a targeted mutation of the zinc finger gene Krox20. Genes Dev. 1993 Nov;7(11):2071–2084. doi: 10.1101/gad.7.11.2071. [DOI] [PubMed] [Google Scholar]
- Vieille-Grosjean I., Huber P. Transcription factor GATA-1 regulates human HOXB2 gene expression in erythroid cells. J Biol Chem. 1995 Mar 3;270(9):4544–4550. doi: 10.1074/jbc.270.9.4544. [DOI] [PubMed] [Google Scholar]
- Whiting J., Marshall H., Cook M., Krumlauf R., Rigby P. W., Stott D., Allemann R. K. Multiple spatially specific enhancers are required to reconstruct the pattern of Hox-2.6 gene expression. Genes Dev. 1991 Nov;5(11):2048–2059. doi: 10.1101/gad.5.11.2048. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bhatt S., Chavrier P., Bravo R., Charnay P. Segment-specific expression of a zinc-finger gene in the developing nervous system of the mouse. Nature. 1989 Feb 2;337(6206):461–464. doi: 10.1038/337461a0. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bhatt S., Cook M., Boncinelli E., Krumlauf R. Segmental expression of Hox-2 homoeobox-containing genes in the developing mouse hindbrain. Nature. 1989 Oct 5;341(6241):405–409. doi: 10.1038/341405a0. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G. Molecular mechanisms of segmental patterning in the vertebrate hindbrain and neural crest. Bioessays. 1993 Aug;15(8):499–505. doi: 10.1002/bies.950150802. [DOI] [PubMed] [Google Scholar]