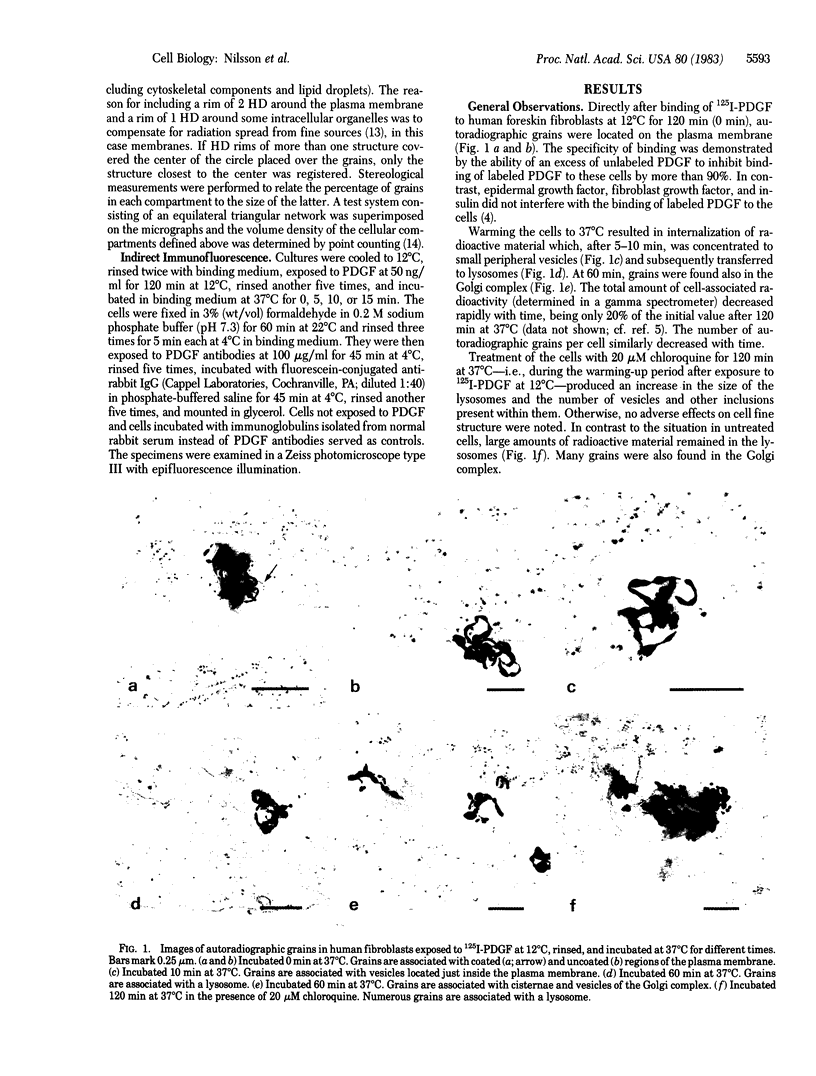
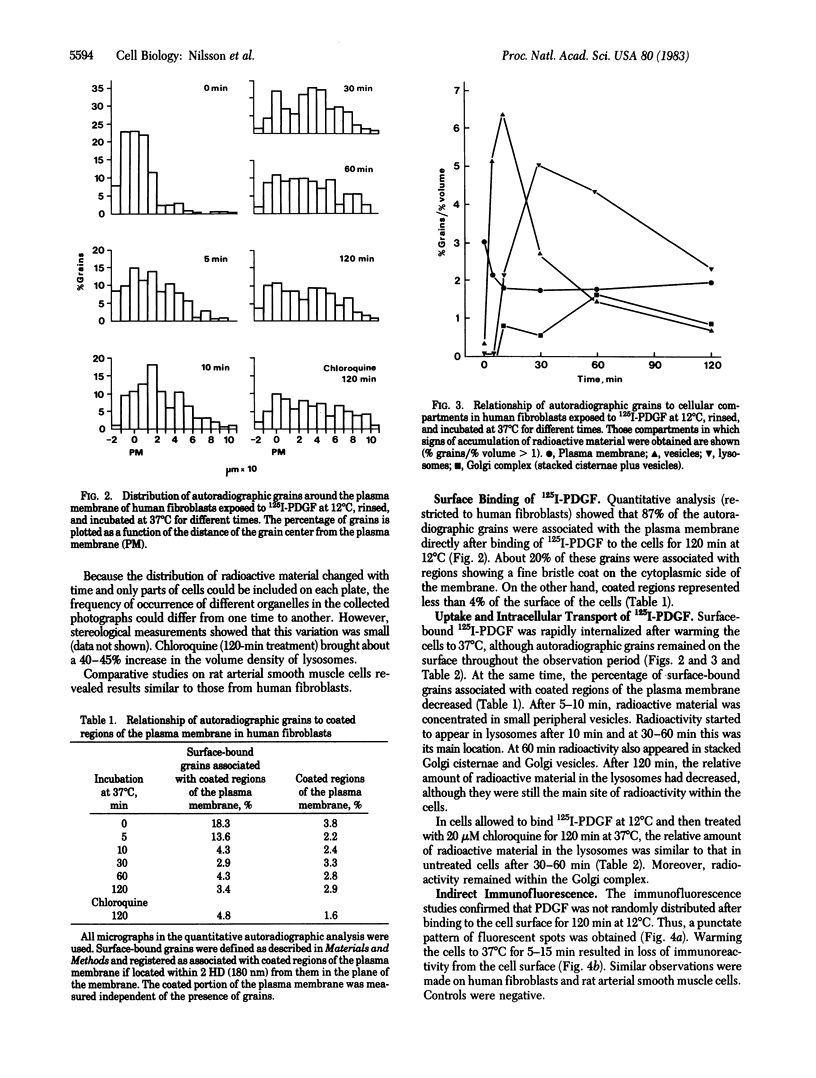
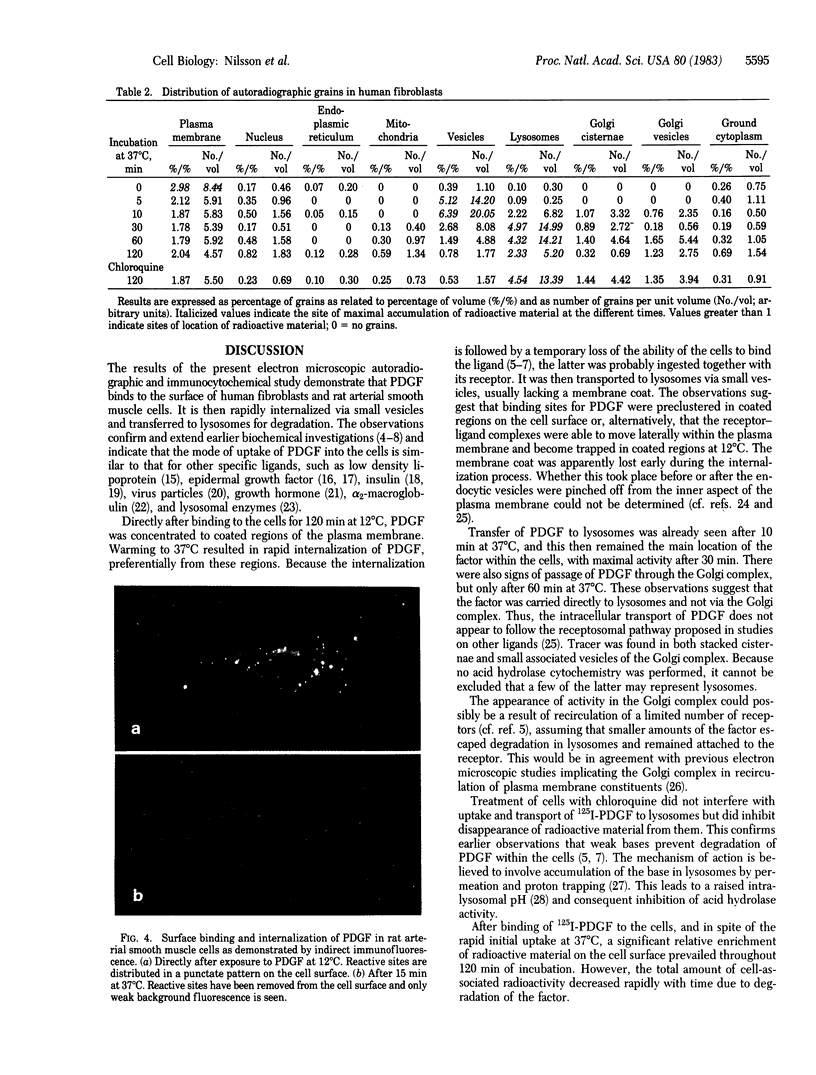
Abstract
Surface binding and uptake of platelet-derived growth factor (PDGF) in human fibroblasts cultivated in vitro were studied by quantitative electron microscopic autoradiography using 125I-labeled PDGF and by indirect immunofluorescence using PDGF antibodies. After 120 min at 12 degrees C PDGF was found preferentially in coated regions of the plasma membrane. Warming the cells to 37 degrees C initiated rapid ingestion of the factor via small vesicles, usually lacking a membrane coat. After 10 min PDGF started to appear in lysosomes, and it showed maximal concentration within these organelles after 30 min. There were also signs of passage of PDGF through the Golgi complex, but only after 60 min. Treatment of the cells with chloroquine, a weak base that inhibits intralysosomal degradation, prevented disappearance of tracer from the lysosomes. The observations indicate that PDGF was internalized via coated regions of the plasma membrane and carried to lysosomes for degradation. Subsequent appearance of tracer within the Golgi complex could reflect receptor-ligand complexes that escaped degradation and were recirculated back to the cell surface.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Brown M. S., Goldstein J. L. Role of the coated endocytic vesicle in the uptake of receptor-bound low density lipoprotein in human fibroblasts. Cell. 1977 Mar;10(3):351–364. doi: 10.1016/0092-8674(77)90022-8. [DOI] [PubMed] [Google Scholar]
- Barazzone P., Lesniak M. A., Gorden P., Van Obberghen E., Carpentier J. L., Orci L. Binding, internalization, and lysosomal association of 125I-human growth hormone in cultured human lymphocytes: a quantitative morphological and biochemical study. J Cell Biol. 1980 Nov;87(2 Pt 1):360–369. doi: 10.1083/jcb.87.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Ross R. Platelet-derived growth factor. II. Specific binding to cultured cells. J Biol Chem. 1982 May 10;257(9):5161–5171. [PubMed] [Google Scholar]
- CARO L. G., VAN TUBERGEN R. P., KOLB J. A. High-resolution autoradiography. I. Methods. J Cell Biol. 1962 Nov;15:173–188. doi: 10.1083/jcb.15.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Amherdt M., Van Obberghen E., Kahn C. R., Orci L. 125I-insulin binding to cultured human lymphocytes. Initial localization and fate of hormone determined by quantitative electron microscopic autoradiography. J Clin Invest. 1978 Apr;61(4):1057–1070. doi: 10.1172/JCI109005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Freychet P., Le Cam A., Orci L. Lysosomal association of internalized 125I-insulin in isolated rat hepatocytes. Direct demonstration by quantitative electron microscopic autoradiography. J Clin Invest. 1979 Jun;63(6):1249–1261. doi: 10.1172/JCI109420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. The Golgi apparatus (complex)-(1954-1981)-from artifact to center stage. J Cell Biol. 1981 Dec;91(3 Pt 2):77s–103s. doi: 10.1083/jcb.91.3.77s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Gorden P., Carpentier J. L., Cohen S., Orci L. Epidermal growth factor: morphological demonstration of binding, internalization, and lysosomal association in human fibroblasts. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5025–5029. doi: 10.1073/pnas.75.10.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler H. T., McKanna J. A., Cohen S. Direct visualization of the binding and internalization of a ferritin conjugate of epidermal growth factor in human carcinoma cells A-431. J Cell Biol. 1979 May;81(2):382–395. doi: 10.1083/jcb.81.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Wasteson A., Westermark B. Interaction of platelet-derived growth factor with its fibroblast receptor. Demonstration of ligand degradation and receptor modulation. J Biol Chem. 1982 Apr 25;257(8):4216–4221. [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Demonstration of an antibody against platelet-derived growth factor. Exp Cell Res. 1981 Dec;136(2):255–261. doi: 10.1016/0014-4827(81)90003-3. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Specific receptors for platelet-derived growth factor on cells derived from connective tissue and glia. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3664–3668. doi: 10.1073/pnas.78.6.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Kartenbeck J., Simons K., Fries E. On the entry of Semliki forest virus into BHK-21 cells. J Cell Biol. 1980 Feb;84(2):404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. S., Huang S. S., Kennedy B., Deuel T. F. Platelet-derived growth factor. Specific binding to target cells. J Biol Chem. 1982 Jul 25;257(14):8130–8136. [PubMed] [Google Scholar]
- Johnsson A., Heldin C. H., Westermark B., Wasteson A. Platelet-derived growth factor: identification of constituent polypeptide chains. Biochem Biophys Res Commun. 1982 Jan 15;104(1):66–74. doi: 10.1016/0006-291x(82)91941-6. [DOI] [PubMed] [Google Scholar]
- Nilsson J., Ksiazek T., Heldin C. H., Thyberg J. Demonstration of stimulatory effects of platelet-derived growth factor on cultivated rat arterial smooth muscle cells. Differences between cells from young and adult animals. Exp Cell Res. 1983 May;145(2):231–237. doi: 10.1016/0014-4827(83)90001-0. [DOI] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I. H., Willingham M. C. Receptor-mediated endocytosis of hormones in cultured cells. Annu Rev Physiol. 1981;43:239–250. doi: 10.1146/annurev.ph.43.030181.001323. [DOI] [PubMed] [Google Scholar]
- Ross R., Vogel A. The platelet-derived growth factor. Cell. 1978 Jun;14(2):203–210. doi: 10.1016/0092-8674(78)90107-1. [DOI] [PubMed] [Google Scholar]
- Salpeter M. M., McHenry F. A., Salpeter E. E. Resolution of electron microscope autoradiography. IV. Application to analysis of autoradiographs. J Cell Biol. 1978 Jan;76(1):127–145. doi: 10.1083/jcb.76.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher C. D., Shepard R. C., Antoniades H. N., Stiles C. D. Platelet-derived growth factor and the regulation of the mammalian fibroblast cell cycle. Biochim Biophys Acta. 1979 Aug 10;560(2):217–241. doi: 10.1016/0304-419x(79)90020-9. [DOI] [PubMed] [Google Scholar]
- Weibel E. R. Stereological principles for morphometry in electron microscopic cytology. Int Rev Cytol. 1969;26:235–302. doi: 10.1016/s0074-7696(08)61637-x. [DOI] [PubMed] [Google Scholar]
- Williams L. T., Tremble P., Antoniades H. N. Platelet-derived growth factor binds specifically to receptors on vascular smooth muscle cells and the binding becomes nondissociable. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5867–5870. doi: 10.1073/pnas.79.19.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. H., Sahagian G. G., Jourdian G. W., Neufeld E. F. Morphologic study of the internalization of a lysosomal enzyme by the mannose 6-phosphate receptor in cultured Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6967–6971. doi: 10.1073/pnas.78.11.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. The receptosome: an intermediate organelle of receptor mediated endocytosis in cultured fibroblasts. Cell. 1980 Aug;21(1):67–77. doi: 10.1016/0092-8674(80)90115-4. [DOI] [PubMed] [Google Scholar]
- de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974 Sep 15;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]