Summary
Glucocorticoids play an important role in adipogenesis through the glucocorticoid receptor (GR) that forms a heterocomplex with Hsp90•Hsp70 and one high molecular weight immunophilin, either FKBP51 or FKBP52. When 3T3-L1 preadipocytes are induced to differentiate, FKBP51 expression progressively increases, whereas FKBP52 decreases, and Hsp90, Hsp70, p23 and Cyp40 remain unchanged. Interestingly, FKBP51 rapidly translocates from mitochondria to the nucleus where it is retained upon its interaction with chromatin and the nuclear matrix. FKBP51 nuclear localization is transient, and after 48 hours it cycles back to mitochondria. Importantly, this dynamic FKBP51 mitochondrial–nuclear shuttling depends on PKA signaling, because its inhibition by PKI or knockdown of PKA-cα by siRNA, prevented FKBP51 nuclear translocation induced by IBMX. In addition, the electrophoretic pattern of migration of FKBP51 is altered by treatment of cells with PKI or knockdown of PKA-cα, suggesting that FKBP51 is a PKA substrate. In preadipocytes, FKBP51 colocalizes with PKA-cα in mitochondria. When adipogenesis is triggered, PKA-cα also moves to the nucleus colocalizing with FKBP51 mainly in the nuclear lamina. Moreover, FKBP51 and GR interaction increases when preadipocytes are induced to differentiate. GR transcriptional capacity is reduced when cells are incubated in the presence of IBMX, forskolin or dibutyryl-cAMP, compounds that induced FKBP51 nuclear translocation, but not by a specific activator of EPAC. FKBP51 knockdown facilitates adipogenesis, whereas ectopic expression of FKBP51 blocks adipogenesis. These findings indicate that the dynamic mitochondrial–nuclear shuttling of FKBP51 regulated by PKA may be key in fine-tuning the transcriptional control of GR target genes required for the acquisition of adipocyte phenotype.
Key words: FKBP51, Glucocorticoid receptor, PKA, Adipogenesis
Introduction
For a long time, the sole physiological roles attributed to adipose tissue were storage of energy and thermal regulation. However, during the last two decades, it has been demonstrated that this tissue is a complex endocrine organ, secreting many factors, including cytokines, chemokines and the commonly called adipokines, which are biologically active molecules that participate in the control not only of energy balance, but also metabolic homeostasis, the immune response and the cardiovascular system (Klaus, 2004; Rajala and Scherer, 2003; Steinberg, 2007). The importance of the endocrine functions of adipose tissue is demonstrated in overweight and obesity conditions that are associated with increased adipocyte size, as well as increased development of new adipocytes (Flegal et al., 2012). Obesity is a serious health problem world-wide because of the increased prevalence of associated diseases such as type 2 diabetes, atherosclerosis, hypertension coronary artery disease, and even increase in predisposition to certain cancers that are associated with deregulated adipokine expression (Steinberg, 2007; van Kruijsdijk et al., 2009).
Obesity is not a homogeneous condition and differences in regional distribution of adipose tissue deposits are related to disturbances in glucose and lipid metabolism (Wajchenberg, 2000). In this respect, glucocorticoids are key regulators not only of fat redistribution, but also of adipocyte differentiation as demonstrated both in vitro and in vivo (Gaillard et al., 1991; Gregoire et al., 1998). Glucocorticoids are present in the ‘adipogenic cocktail’ that induces the differentiation of 3T3-L1 or 3T3-F442a preadipocytes (Green and Kehinde, 1975). Their adipogenic effect is evident in the development of central obesity in patients with high levels of circulating glucocorticoids, as observed in Cushing's syndrome or in patients that required prolonged administration of this steroid hormone therapeutically (Newell-Price et al., 2006). Furthermore, adipose tissue-dependent amplification of corticosterone production in transgenic mice results in a full metabolic syndrome, including central obesity, insulin resistance and hypertension (Masuzaki et al., 2001). In contrast, glucocorticoid inactivation is associated with resistance to metabolic dysfunction (Kershaw et al., 2005; Morton et al., 2004).
At the molecular level, glucocorticoid effects depend on the hormone binding to glucocorticoid receptor (GR) that is present in the cytoplasm as part of a heterocomplex with Hsp90, Hsp70, p23 and the high molecular weight immunophilins (IMMs), FKBP51 or FKBP52 (Pratt and Toft, 1997). IMMs belong to a family of proteins classified by their ability to bind immunosuppressant drugs, for example cyclophilins bind cyclosporine A, whereas FKBPs (FK506-binding proteins) bind FK506. The high molecular weight IMMs FKBP51 and FKBP52 do not play a role in immunosuppression, but have been related to steroid receptor regulation (Storer et al., 2011). The FKBPs are modular proteins that possess FKBP12-like peptidyl-prolyl isomerase (PPIase) domains 1 and 2 and a tetratricopeptide repeat motif (TPR). The FK1 domain is required for the binding of the immunosuppressive drug FK506, it confers PPIase activity, and it is also the primary domain required for steroid hormone receptor regulation (Pirkl and Buchner, 2001; Riggs et al., 2003; Storer et al., 2011). The TPR domain contains sequences of 34 amino acids repeated in tandem, through which FKBPs interact with Hsp90. FKBP51 and FKBP52 share 60% identity and 70% similarity; however, the former has, so far, been mainly reported to be a negative regulator of steroid hormone receptors while the latter is a positive regulator (Davies et al., 2002; Gallo et al., 2007; Riggs et al., 2003; Storer et al., 2011; Wochnik et al., 2005). Furthermore, fkbp52-deficient male mice display phenotypes related to partial androgen insensitivity syndrome (Cheung-Flynn et al., 2005; Yong et al., 2007). Heterozygous fkbp52-deficient mice show increased susceptibility to high-fat-diet-induced hyperglycemia and hyperinsulinemia that correlates with reduced insulin clearance, hepatic steatosis and glucocorticoid resistance (Warrier et al., 2010). In contrast, fkbp51-deficient mice were initially observed to display no overt phenotypes, but these mice are less vulnerable to the detrimental effects of stress (Hartmann et al., 2012; O'Leary et al., 2011; Touma et al., 2011). However fkbp51-fkbp52 double knockout results in embryonic lethality, demonstrating that these IMMs have some physiological functional redundancies (Sivils et al., 2011). Upon steroid hormone binding to GR, as well as to mineralocorticoid receptor (MR) Hsp90 heterocomplexes, FKBP51 is released from the receptor complex and replaced by FKBP52, which in turn recruits dynein–dynactin motor proteins favoring the cytoplasmic transport of nuclear receptors (NRs) to the nucleus (model in Fig. 8H) (Galigniana et al., 2010; Galigniana et al., 2001). Interestingly, GR and its associated chaperones bind to nuclear pore proteins such as nucleoporins and importin β, and it has been shown that the entire Hsp90 heterocomplex cross-linked to GR translocates intact through the nuclear pore in digitonin-permeabilized cells (Echeverría et al., 2009). Moreover, it has been shown that the whole MR•Hsp90-based heterocomplex can be transiently recovered from the soluble fraction of the nucleus shortly after steroid hormone incubation (Galigniana, 2012; Galigniana et al., 2010; Grossmann et al., 2012). Thus, the steroid-receptor transformation could possibly take place in the nucleus.
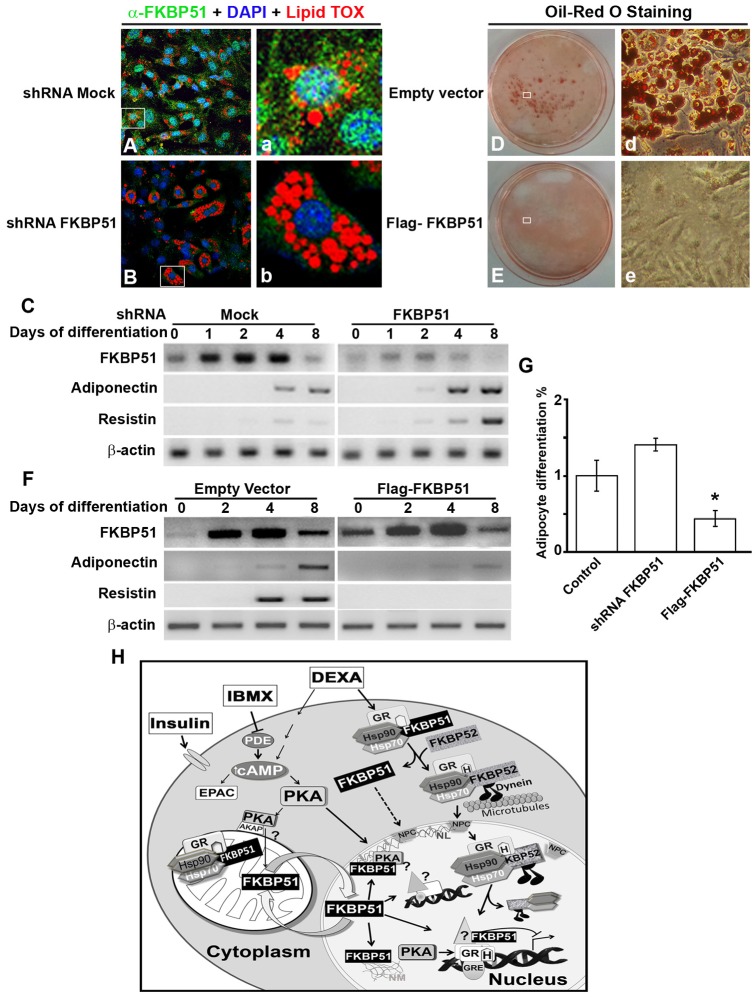
Fig. 8.
FKBP51 restrains differentiation of 3T3-L1 preadipocytes. Plasmids with mock shRNA or shRNA specific for FKBP51 were transfected in 3T3-L1 cells, and 48 hours later cells were induced to differentiate. Adipogenesis was evaluated by IIF using LipidTOX to stain vesicles containing lipids (A,B), and by evaluating mRNA for FKBP51, adiponectin and resistin (C). 3T3-L1 cells were transfected with empty vector or FLAG-FKBP51, and 24 hours later cells were induced to differentiate and adipocyte differentiation was evaluated by Oil Red O staining (D,E), and by detecting mRNA for FKBP51, adiponectin and resistin (F). β-Actin was used as a control of loading in C and F. (G) Fold change in the percentage of cells that differentiated into adipocytes for control cells, and 3T3-L1 cells in which FKBP51 was knocked down (shRNA FKBP51) or overexpressed (Flag-FKBP51), *P<0.01. (H) Model of FKBP51 activity in adipocyte differentiation. In the adipogenic cocktail MDI, IBMX and DEXA are responsible for FKBP51 nuclear translocation. IBMX inhibits the phosphodiesterase (PDE) increasing intracellular cAMP that activates PKA, and induces PKA-cα and mitochondrial FKBP51 nuclear translocation, where they mainly colocalize in the nuclear lamina (NL). In the nucleus, FKBP51 is retained by its interaction with the nuclear matrix (NM) and chromatin, regulating GR-target genes, and possibly other targets.
Glucocorticoids regulate adipogenesis as well as the distribution of adipose tissue; therefore, it is relevant to study how they exert their action at the molecular level. When 3T3-L1 preadipocytes are induced to differentiate, GR is one of the NRs expressed in a biphasic manner, with higher levels of mRNA during the first days of adipogenesis, followed by a period of lower level of mRNA that gradually increases back to preadipocytes levels (Fu et al., 2005). In addition, genome-wide studies have shown an increased cooperative binding of GR with other adipogenic transcription factors during the early stages of 3T3-L1 preadipocyte differentiation (Siersbæk et al., 2011; Steger et al., 2010). However, in spite of massive chromatin remodeling and a high level of occupancy by transcription factors in chromatin within hours of adipogenesis being induced, gene expression is kept well controlled, and in many cases gene expression takes place later in the adipogenic process (Siersbæk et al., 2011). These findings emphasize the necessity for precise control of GR action. However, nothing is known about proteins in the heterocomplex that regulate GR activity during the process of adipocyte differentiation. In this study we show for the first time that when 3T3-L1 preadipocytes are induced to differentiate, there are changes in the level of expression of the IMMs that modulate GR responses. We found that the increase of FKBP51 protein level is accompanied by a decrease in FKBP52. Surprisingly, FKBP51 shows dynamic changes in its subcellular distribution throughout the process of adipogenesis. Importantly, mitochondrial–nuclear shuttling of FKBP51 depends on the activation of protein kinase A (PKA) signaling pathway, possibly constituting a mechanism for the fine tuning of the expression of GR-target genes at the onset of the adipocyte differentiation program.
Results
FKBP51 expression level increases during 3T3-L1 preadipocyte differentiation
Glucocorticoids are important mediators of adipocyte differentiation, therefore, we first analyzed the pattern of expression of GR and the components of GR•Hsp90 heterocomplex at different time points post-induction of murine 3T3-L1 preadipocyte differentiation. As shown in Fig. 1A, GR protein expression was biphasic, its lowest level occurring between the fourth to sixth day of 3T3-L1 differentiation, in agreement with a previous study in which GR mRNA was analyzed in similar conditions (Fu et al., 2005). The protein expression level of Hsp90, Hsp70, Cyp40 and p23 remained constant (Fig. 1A). In contrast, FKBP51 and FKBP52 levels varied: FKBP51 progressively increased, whereas FKBP52 gradually decreased as the cells differentiated (Fig. 1A). Furthermore, an increase in FKBP51 mRNA level was observed when 3T3-L1 cells differentiated (Fig. 1B). No change in FKBP52 mRNA level was detected (Fig. 1B), suggesting that changes in FKBP52 protein may possibly depend on control of mRNA translation and/or protein stability. The increased expression of C/EBPα and C/EBPβ (Fig. 1A), as well as the increased mRNA level of the adipogenic markers adiponectin (Fig. 1B), C/EBPβ and resistin (data not shown) demonstrated that 3T3-L1 cells differentiated properly.
Fig. 1.
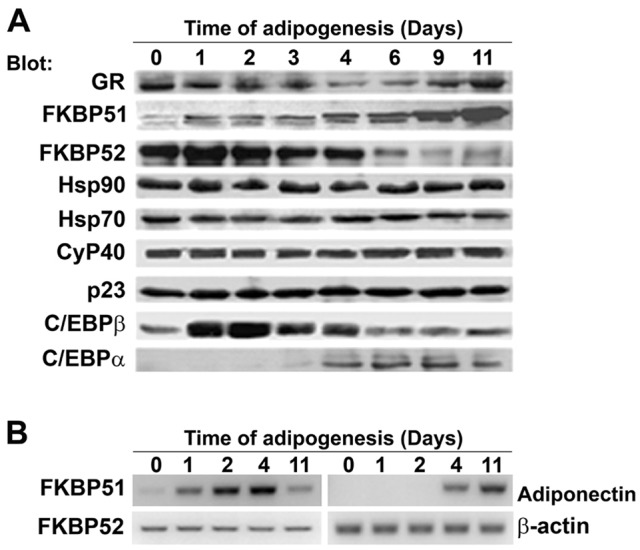
FKBP51 expression increases during the process of 3T3-L1 preadipocyte differentiation. (A) 3T3-L1 cells were differentiated for the indicated periods of time, cell lysates were obtained and proteins were separated by SDS-PAGE. Immunoblotting was performed with the indicated antibodies. (B) mRNA was isolated from 3T3-L1 at the indicated times post-induction of adipogenesis and analyzed as described in Materials and Methods. Similar results were obtained in three different experiments.
FKBP51 rapidly shuttles from mitochondria to the nucleus at the onset of adipogenesis
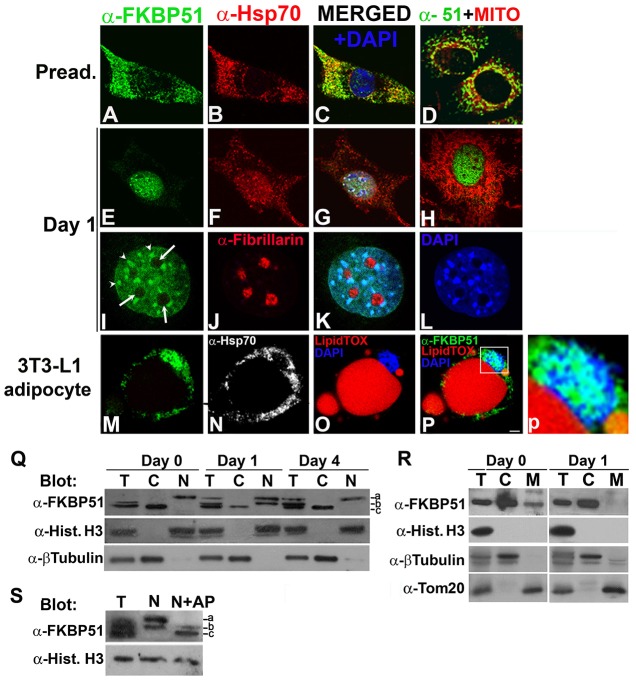
Because the expression of FKBP51 increases throughout the process of adipogenesis, we examined whether the subcellular distribution of FKBP51 is modified when the differentiation program of 3T3-L1 cells is triggered. In preadipocytes, FKBP51 was mainly detected in mitochondria (Fig. 2A), colocalizing with MitoTracker (Fig. 2D) and with Hsp70 (Fig. 2B, and merged images in C), a chaperone also known to be present in mitochondria. FKBP51 also colocalized with Tom20, one of the import receptors required for the insertion of newly synthesized proteins into the outer membrane of the mitochondria (Bains and Lithgow, 1999) (supplementary material Fig. S1A,B and merged image in C), further demonstrating that in 3T3-L1 preadipocytes FKBP51 localizes mainly in mitochondria. Interestingly, when adipogenesis was induced FKBP51 translocated from mitochondria to the nucleus (Fig. 1E), no longer colocalizing with MitoTracker (Fig. 1H) and Tom20 (supplementary material Fig. S1F). Hsp70, albeit to a lesser extent, was also detected in the nucleus (Fig. 2F) and partially colocalized with FKBP51 (Fig. 2G). FKBP51 and Hsp70 nuclear translocation was observed in 84±5% of cells. It was a rapid event as they were detected in the nucleus within 30 minutes of treating 3T3-L1 with MDI [3-isobutyl-1-methylxanthine (IBMX), dexamethasone (DEXA) and insulin] (data not shown). During the first 2 days post-induction of adipogenesis, FKBP51 was detected in the nucleus but was always excluded from the nucleoli (arrows in Fig. 2I; supplementary material Fig. S1D), which were identified by the presence of fibrillarin (Fig. 2J; no colocalization was observed between these two proteins Fig. 2K). Analyzing in detail the nuclear pattern of FKBP51, the IMM was detected diffusely distributed and in speckles (Fig. 2I, arrowheads). The FKBP51 speckles coincided with areas intensely stained by DAPI (Fig. 2L and merged K) that in murine cells correspond to the chromocenters (Guenatri et al., 2004). From the third day of differentiation until cells acquired their final phenotype (easily recognized by the presence of lipid vesicles stained red by LipidTOX; Fig. 2O,P), FKBP51 was detected in mitochondria, as shown by colocalization with MitoTracker (supplementary material Fig. S1I), and in the nucleus (Fig. 2M,P,p). In contrast, Hsp70 was excluded from the nucleus of the 3T3-L1 adipocytes (Fig. 2N). To confirm this change in FKBP51 subcellular distribution, cytosolic and nuclear fractions from 3T3-L1 cells before and 1 and 4 days after initiating the adipogenic program were analyzed by western blotting (WB). In preadipocytes, as expected, FKBP51 was mainly detected in the cytosolic fraction (Fig. 2Q, day 0, C versus N). Importantly, 24 hours after induction of adipogenesis, FKBP51 increased in the nuclear fraction and decreased in the cytosol (Fig. 2Q, day 1, N versus C). After 4 days, FKBP51 was again mainly detected in the cytosolic fraction (Fig. 2Q, day 4, C versus N), which is similar to what was observed in preadipocytes. To further demonstrate that the mitochondrial fraction of FKBP51 is the one that rapidly translocates to the nucleus, the cytoplasmic samples were separated in mitochondrial and cytosolic fractions, proteins were separated by SDS-PAGE and analyzed by WB. As shown in Fig. 2R, in preadipocytes FKBP51 was detected in both fractions (day 0, lanes C and M). In contrast, as expected based on the IFI results, when cells were induced to differentiate the IMM was no longer detected in the mitochondrial fraction (Fig. 2R, day 1 versus day 0, lane M), but still detected in the cytosolic one (Fig. 2R, day 1 versus day 0, lane C). Detection of histone H3, β-tubulin and Tom20 were used as control of the correct isolation of the nuclear, cytosolic and mitochondrial fractions, respectively. We would like to highlight that after induction of adipogenesis FKBP51 in cell lysates was resolved in three bands on WB (Fig. 2Q, days 1 and 4, lane T) suggesting that FKBP51 may be present in forms with different degrees of phosphorylation. Interestingly, FKBP51 bands with slower electrophoretic migration (bands a and b) were detected in the nuclear fractions but not in the cytosolic ones (Fig. 2Q, lane N versus C). To ascertain whether the slow migrating bands corresponded to phosphorylated forms of FKBP51, the nuclear extract from day 1 of adipogenesis was incubated with alkaline phosphatase (AP) for 1 hour before the sample was applied to the gel. Alkaline phosphatase treatment resulted in FKBP51 migrating as the faster mobility form (Fig. 2S, N+AP versus N, band c), consistent with band c representing dephosphorylated FKBP51. These results demonstrate that FKBP51 is present in different cellular compartments in forms with different degrees of phosphorylation, with nuclear FKBP51 corresponding to highly phosphorylated forms. Taken together these results show for the first time dynamic changes in the subcellular distribution of FKBP51 throughout the process of adipogenesis, raising the possibility that FKBP51 mitochondrial–nuclear shuttling is regulated by post-translational modifications of the IMM when adipogenesis is triggered.
Fig. 2.
FKBP51 rapidly shuttles from mitochondria to the nucleus at the onset of adipogenesis. (A–P) 3T3-L1 cells were grown on coverslips and induced to differentiate for 1 (E–L) or 8 days (M–P), subjected to IIF with the indicated antibodies, and images were analyzed by confocal microscopy. In O and P lipid vesicles in 3T3-L1 adipocytes were stained with LipidTOX. Nuclei were counterstained with DAPI. Results are representative of five independent experiments. Panel p shows a magnification of the boxed area in P. Arrows indicate nucleoli and arrowheads indicate chromocenters. Scale bar: 5 µm. (Q) Total (T), cytosolic (C) and nuclear (N) fractions from 3T3-L1 cells prior (day 0) and 1 or 4 days post-induction of differentiation were resolved by SDS-PAGE, and analyzed by immunoblotting with the indicated antibodies. Bands representing FKBP51 with different electrophoretic migration (bands a–c) are indicated. (R) Total (T), cytosolic (C) and mitochondrial (M) fractions from 3T3-L1 cells prior (day 0) and 1 day post-induction of differentiation were resolved by SDS-PAGE, and analyzed by immunoblotting with the antibodies indicated in Q, and anti-Tom20 as marker for mitochondria. (S) The nuclear fraction from 3T3-L1 cells induced to differentiate for 1 day was incubated in the absence (N) or the presence of alkaline phosphatase (N+AP) as described in the Materials and Methods. The indicated samples and total 3T3-L1 cell lysates (T) were then subjected to western blot analysis. Blots are representative of at least three independent experiments.
FKBP51 interacts in the nucleus with chromatin and the nuclear matrix
Because we found that FKBP51 and, to a lesser extent, Hsp70 localize in the nucleus when the adipogenic program is triggered, we investigated whether they have the capacity to interact with chromatin and/or the nuclear matrix, a fibrogranular ribonucleoprotein network with which chromatin and many nuclear factors interact, thereby contributing to the spatial organization of the genome (Nickerson, 2001). 3T3-L1 cells were induced to differentiate for 24 hours, and then subjected to in situ extraction. This treatment extracts proteins from the nucleoplasm together with proteins weakly bound to chromatin and/or the nuclear matrix (Fey et al., 1986). When 3T3-L1 cells were subjected to in situ extraction, FKBP51 and Hsp70 were retained in the nucleus in all cells (Fig. 3E versus A, and F versus B, respectively). This implies that FKBP51 and Hsp70 are normally tightly associated with chromatin and/or the nuclear matrix. To analyze whether such interaction depends on ribonucleoproteins and/or RNA, in situ extraction was followed by RNase A treatment. We found that FKBP51 and Hsp70 were extracted at high levels from the nucleus (Fig. 3I,J), indicating that their nuclear localization is stabilized by RNA and/or ribonucleoprotein(s). As a control, C/EBPβ pericentromeric localization was not altered after RNase A treatment (data not shown), as previously reported (Susperreguy et al., 2011). Next, to analyze whether FKBP51 and/or Hsp70 interact not only with chromatin but also with the nuclear matrix, chromatin was completely removed by treatment of cells with DNase I after Triton X-100 treatment, as demonstrated by the lack of DAPI staining (Fig. 3O). The nuclear matrix, however, was not affected by DNase I digestion, shown by the presence of distinct NuMA foci (Fig. 3N) (Nickerson, 2001). Interestingly, some FKBP51 (Fig. 3M) was detected in the nucleus, partially colocalizing with NuMA (Fig. 3P), indicating that FKBP51 can interact with the nuclear matrix. Hsp70 was also detected colocalizing with NuMA (data not shown). Thus, FKBP51 and Hsp70 are retained in the nucleus upon their interaction with chromatin and the nuclear matrix in an RNA- and/or ribonucleoprotein-dependent manner.
Fig. 3.
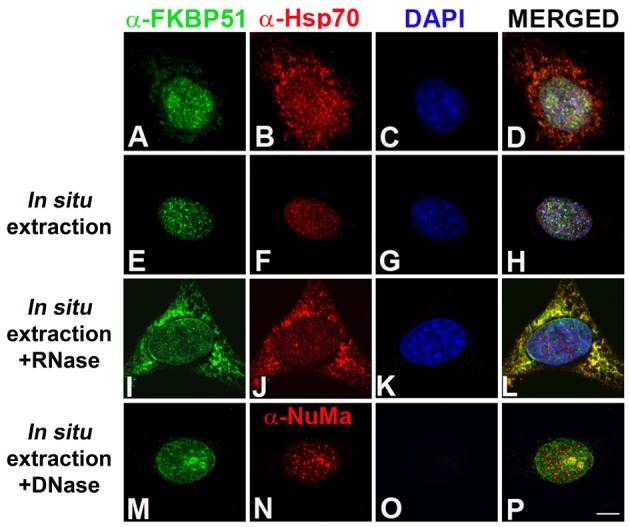
FKBP51 interacts with chromatin and the nuclear matrix. 3T3-L1 cells grown on coverslips were induced to differentiate with MDI for 24 hours and then fixed (A–D) or subjected to in situ extraction (E–H), in situ extraction followed by RNase (I–L) or DNase treatment (M–P). Cells were then fixed, subjected to IIF with the indicated antibodies and samples analyzed by confocal microscopy. Results are representative of four independent experiments, with an average of 50 cells imaged per experiment. Scale bar: 5 µm.
FKBP51 mitochondrial–nuclear shuttling is regulated by cAMP signaling
FKBP51 rapidly re-localizes in the nucleus when 3T3-L1 preadipocytes are induced to differentiate with MDI. Accordingly, we determined which of the component(s) of MDI was responsible for FKBP51 mitochondrial–nuclear shuttling. When 3T3-L1 preadipocytes were incubated in the presence of IBMX, FKBP51 rapidly translocated from the mitochondria to the nucleus (Fig. 4C,D versus A,B) within 15 minutes after IBMX had been added to the medium. DEXA treatment also induced the translocation of the IMM to the nucleus (Fig. 4E,F), but to a lesser extent than when the response is compared with that to IBMX (Fig. 4E versus C). This differential response of FKBP51 to IBMX and DEXA was also observed after 24 hours of treatment (Fig. 4G,H versus I,J, respectively). Furthermore, when 3T3-L1 cells incubated in the presence of IBMX for 24 hours were grown in the absence of IBMX for another 24 hours, FKBP51 shuttled back to mitochondria (Fig. 4K,L versus G,H), supporting the notion that FKBP51 subcellular redistribution is a dynamic event dependent on cAMP. Treatment of 3T3-L1 cells with different concentrations of forskolin or the cell-permeable cAMP analog dibutyryl-cAMP also induced a rapid relocalization of FKBP51 from mitochondria to the nucleus (data not shown). In contrast, insulin, the third component of the adipogenic cocktail, did not promote changes in FKBP51 subcellular localization at any time point tested (data not shown). Taken together these results demonstrate that the dynamic mitochondrial–nuclear shuttling of FKBP51 is mainly regulated by the second messenger cAMP signaling and to a lesser extent by DEXA.
Fig. 4.
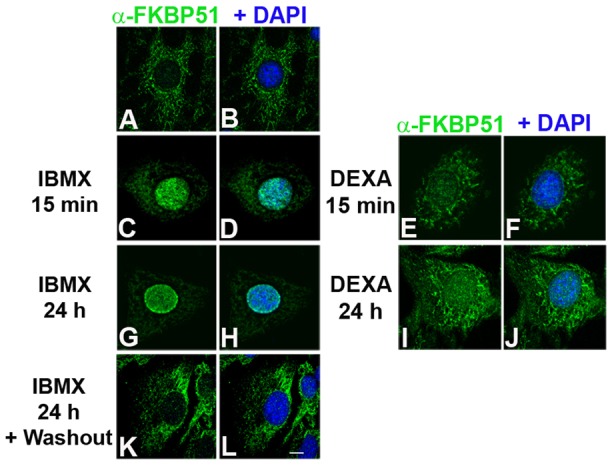
FKBP51 mitochondrial–nuclear shuttling is regulated by cAMP-dependent and steroid hormone signaling. 3T3-L1 cells were grown on coverslips and incubated in the absence (A,B) or the presence of 520 µM IBMX (C,D,G,H) or 1 µM DEXA (E,F,I,J) for the indicated periods of time, and then subjected to IIF with anti-FKBP51. Nuclei were counterstained with DAPI. (K,L) After IBMX treatment cells were grown in the absence of IBMX for another 24 hours prior to IIF. Results are representative of four independent experiments. Scale bar: 5 µm.
FKBP51 mitochondrial–nuclear shuttling is regulated by the PKA signaling pathway
Next, we tested whether the IMM shuttling depends on activation of PKA, a signaling pathway involved in regulation of diverse cellular functions, among them adipocyte differentiation (Rosen and MacDougald, 2006; Taylor et al., 1990). To investigate whether cAMP-dependent FKBP51 redistribution is independent of the cell type, HEK293T cells were treated in the absence or the presence of IBMX, and FKBP51 localization assessed by IIF and confocal microscopy. FKBP51 and PKA-cα were detected in the mitochondria (Fig. 5A and B, respectively), as previously shown (Gallo et al., 2011). When cells were incubated in the presence of IBMX for 24 hours, not only FKBP51 (Fig. 5E,e versus A), but also PKA-cα translocated to the nucleus (Fig. 5F,f versus B), demonstrating that FKBP51 mitochondrial–nuclear redistribution is independent of the cell type. FKBP51 and PKA-cα colocalized mainly in the nuclear periphery (Fig. 5H,h). Importantly, neither FKBP51 nor PKA-cα translocated to the nucleus when HEK293T cells were treated with IBMX in the presence of myristoylated protein kinase inhibitor (PKI), a specific PKA inhibitor (Fig. 5I,J), suggesting that cAMP-dependent activation of PKA regulates FKBP51 mitochondrial–nuclear translocation. The same effect was observed when 3T3-L1 preadipocytes were treated with IBMX in the presence of myristoylated PKI (supplementary material Fig. S2). Furthermore, inhibition of MEK–ERK1/2 by UO126 (a MEK inhibitor) did not affect FKBP51 nuclear translocation induced by IBMX or forskolin (data not shown). Then, the expression of PKA-cα was reduced using specific shRNA. PKA-c was knocked down by 50–70%, as shown by indirect immunofluorescence (Fig. 5N) and WB (Fig. 5Q, lanes 4 and 5). Importantly, the translocation of FKBP51 from mitochondria to the nucleus was prevented when PKA-cα was knocked down (Fig. 5M). Thus these results suggest that FKBP51 is very sensitive to even a moderate decrease in the level of expression of PKA-c. Because PKA inhibition by PKI and PKA-cα knockdown blocked FKBP51 translocation to the nucleus, we hypothesized that phosphorylation of FKBP51 by PKA may regulate the subcellular distribution of the IMM. To test this possibility, lysates from HEK293T cells incubated for 24 hours with IBMX in the absence or the presence of PKI were analyzed by SDS-PAGE and WB. In cell lysates from HEK293T cells treated with IBMX, FKBP51 was resolved in two bands (Fig. 5Q, lane 1, bands a and b). In contrast, when cells were treated with IBMX in the presence of PKI, the upper band a was markedly reduced (Fig. 5Q, lane 2 versus 1). To ascertain whether the slower migrating band a represents phosphorylated form of FKBP51, lysates from IBMX-treated cells were incubated with AP for 1 hour before they were applied to the gel. Alkaline phosphatase caused FKBP51 to migrate mainly as the faster mobility form (Fig. 5Q, lane 3, band b) in a similar manner to what is observed when cells are treated with IBMX and PKI (lane 2), and as shown in Fig. 2S for FKBP51 present in nuclear extracts of preadipocytes induced to differentiate. Furthermore, when PKA-cα was knocked down in HEK293T cells, FKBP51 migrated as the faster mobility form (band b) in any condition (Fig. 5Q, lanes 4–6), demonstrating that the slower migrating form (band a) corresponds to the PKA-dependent phosphorylated forms of FKBP51.
Fig. 5.
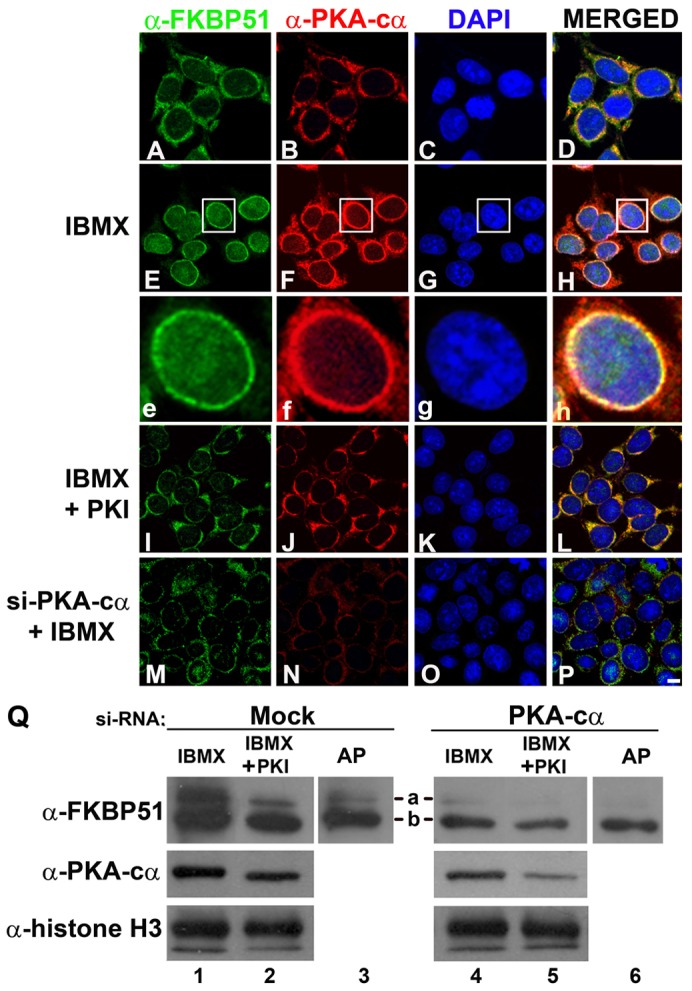
Knockdown of PKA-cα prevents phosphorylation and nuclear translocation of FKBP51. (A–P) HEK293T cells were transfected with a scrambled siRNA (A–L) or a specific siRNA for PKA-cα (M–P). Two days later cells were subjected to the indicated treatments, and subcellular distribution of FKBP51 and PKA-cα were evaluated by IIF and confocal microscopy. Scale bar: 5 µm. (Q) HEK293T cells transfected with scrambled or specific siRNA for PKA-cα were treated with 520 µM IBMX in the absence or presence of 0.5 µM PKI for 24 hours, and cell lysates were analyzed by immunoblotting with the indicated antibodies. AP: cell lysates from HEK293T cells incubated 24 hours with IBMX were treated with alkaline phosphatase before applying them to the gel. Bands a and b correspond to FKBP51 with different electrophoretic migration. Results are representative of at least three independent experiments.
FKBP51 and PKA-cα transiently localize in the nuclear lamina and interact with lamin B
Mitochondrial–nuclear translocation of FKBP51 that takes place during the first 2 days of the process of adipogenesis (Fig. 6F,J versus B) was accompanied by the translocation of PKA-cα to the nucleus (Fig. 6E,I versus A), where a marked colocalization of PKA-cα and FKBP51 was observed (Fig. 6G,H,K,L). FKBP51 and PKA-cα were excluded from the nucleoli and by day 2, in 80±4% of the cells these two proteins concentrated in the periphery of the nucleus. FKBP51 colocalized in patches with lamin B, one of the constituents of the nuclear lamina (Fig. 6O,P), demonstrating that FKBP51 and PKA-cα are present in the nuclear lamina during the early stages of adipogenesis. Careful analysis of lamin B shows that it is fragmented (Fig. 6M, arrowheads), a result that is in agreement with the recently reported reorganization of the nuclear lamina that takes place at the early stages of adipogenesis (Verstraeten et al., 2011). Interestingly, FKBP51 and PKA-cα showed a similar pattern of fragmentation as lamin B (Fig. 6N,J,I arrowheads, respectively). Importantly, when the FKBP51 was immunoprecipitated, PKA-c and lamin B co-immunoprecipitated with the IMM from nuclei of 3T3-L1 cells differentiated for 2 days but not from nuclei of preadipocytes (Fig. 6U), demonstrating that they not only colocalize but interact at the onset of differentiation. When the 3T3-L1 preadipocytes were fully differentiated, PKA-cα was detected in the nucleus and cytoplasm (Fig. 6Q), enriched in vesicles full of lipids stained by LipidTOX (Fig. 6S,T). These results demonstrate for the first time that not only FKBP51 but also PKA-cα shows dynamic changes in its subcellular distribution as adipogenesis proceeds, possibly playing a role in nuclear lamina reorganization that takes place at the early stages of adipogenesis.
Fig. 6.
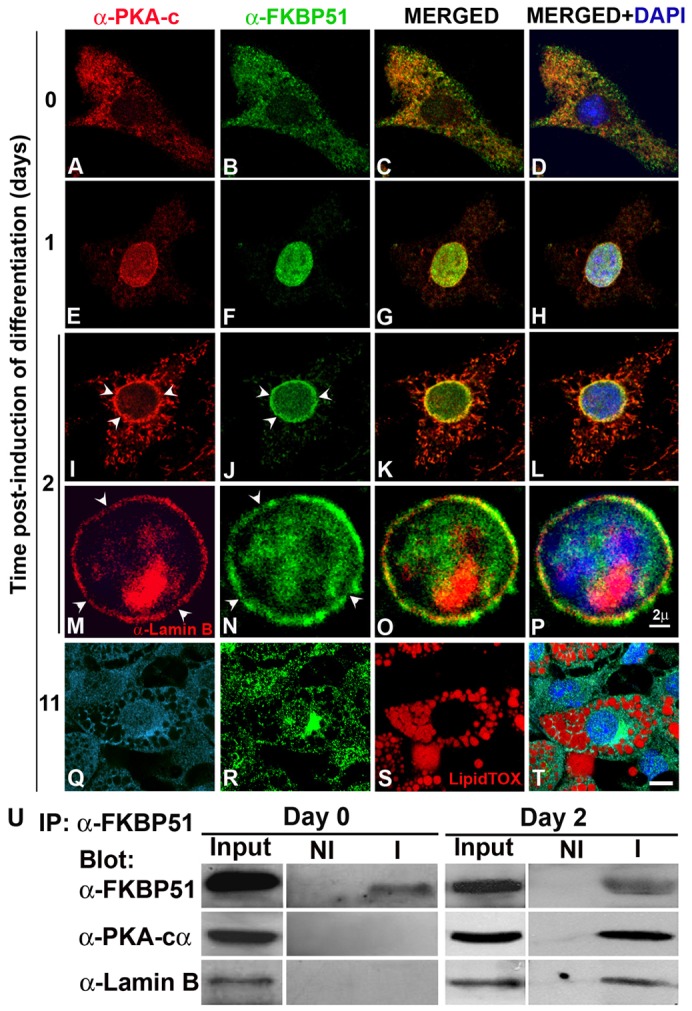
FKBP51 and PKA-cα transiently colocalize in the nuclear lamina at the onset of adipocyte differentiation. (A–T) 3T3-L1 preadipocytes grown on coverslips were induced to differentiate for the indicated periods of time and subcellular localization of PKA-cα and FKBP51 was assessed by IIF and confocal microscopy. Nuclei were counterstained with DAPI. For M, O and P, IIF was performed using anti-lamin B to label the nuclear lamina. Arrowheads in M and N indicate loss of lamin B or FKBP51 in the nuclear rim, respectively. (Q–T) PKA-cα is shown in light blue (Q) to distinguish it from the nucleus stained with DAPI (blue) and the lipid vesicles were stained with LipidTOX (red; S). Scale bars: 2 µm (A–P); 5 µm (Q–T). (U) FKBP51 was immunoprecipitated from 3T3-L1 cells prior to (day 0) and 2 days after induction of differentiation, and immunoprecipitated complexes were resolved by SDS-PAGE and analyzed by immunoblotting with the indicated antibodies. NI, non-immune antibody; I, immune antibody. Results are representative of four independent experiments.
PKA-dependent FKBP51 nuclear translocation restrains GR transcriptional capacity
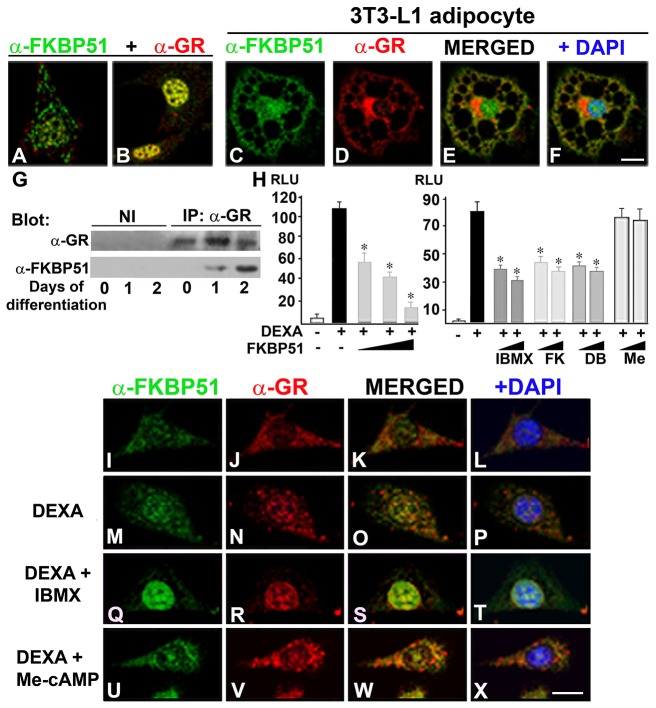
PKA-c was found associated with GR in a ligand-dependent manner, an interaction that potentiates GR-dependent transcription (Doucas et al., 2000). Thus, we hypothesize that PKA may play a dual role in the regulation of GR, i.e. on the one hand, positively modulating GR transcriptional capacity (Doucas et al., 2000) and, on the other hand, restraining it by increasing the nuclear availability of FKBP51, a known GR negative regulator (Wochnik et al., 2005). Therefore, we analyzed by confocal microscopy the subcellular distribution of FKBP51 and GR in preadipocytes before and 24 hours after induction of adipogenesis. FKBP51 and GR were present in negligible amounts in the nucleus of preadipocytes (Fig. 7A). When 3T3-L1 cells were induced to differentiate, both FKBP51 and GR were mainly detected in the nucleus, exhibiting a high level of colocalization (Fig. 7B) during the first 2 days of the differentiation process. By day 4, the subcellular localization of FKBP51 and GR resembled the distribution observed in preadipocytes (data not shown). When the adipocyte phenotype was achieved, GR was mainly cytoplasmic, concentrating close to the nucleus where it is distributed in discrete speckles (Fig. 7D). In 3T3-L1 adipocytes, GR showed only a minor colocalization with FKBP51 (Fig. 7E). When GR–FKBP51 interaction was evaluated, FKBP51 was not detected with GR in preadipocytes (Fig. 7G, day 0). In contrast, when adipogenesis was triggered, FKBP51 co-immunoprecipitated with GR, and more FKBP51 was recovered bound to GR during the second day of differentiation (Fig. 7G, day 1 and 2 versus day 0). Taken together, these results demonstrate that during the early stages of adipogenesis, GR and FKBP51 translocate to the nucleus where they interact, and consequently, FKBP51 restrains GR transcriptional capacity. To test this possibility, reporter gene assays were performed. When GR transcriptional activity was evaluated in the presence of increasing amounts of FKBP51, a decrease in GR transcriptional capacity was detected (Fig. 7H). Interestingly, when GR transcriptional activity was tested in the presence of increasing concentrations of IBMX, forskolin or dibutyryl-cAMP, a decrease in the transcriptional capacity of GR was also measured (Fig. 7H). In contrast, when cells were incubated with 8-(4-chlorophenylthio)-2′O-methyladenosine 3′,5′cyclic monophosphate (Me-cAMP), an agonist that activates the non-classic cAMP pathway (Enserink et al., 2002), GR transcriptional activity was not affected (Fig. 7H, Me). These results suggest that the FKBP51 nuclear translocation induced by IBMX, forskolin and dibutyryl-cAMP, but not Me-cAMP, was responsible, at least in part, for a decrease in GR transcriptional capacity. To test this possibility, GR and FKBP51 subcellular distribution was analyzed by IIF in cells grown in medium supplemented with steroid free-serum and treated with DEXA in the absence or presence of IBMX or Me-cAMP. Upon DEXA treatment, GR translocated to the nucleus (Fig. 7N versus J) along with a small fraction of FKBP51 (Fig. 7M versus I) that partially colocalized with GR (Fig. 7O,P versus K,L). In contrast, when cells were incubated in the presence of DEXA and IBMX, both GR and FKBP51 translocated to the nucleus (Fig. 7R,Q) and exhibited a marked colocalization (Fig. 7S,T), possibly due to GR–FKBP51 complexes with low transcriptional activity. Furthermore, when cells were incubated in the presence of DEXA and Me-cAMP, FKBP51 and GR are detected in the nuclear compartment similarly as was observed with DEXA alone (Fig. 7U,V versus M,N). This result is in agreement with the lack of capacity of Me-cAMP treatment to decrease GR transcriptional activity shown in reporter gene assays (Fig. 7H). Taken together, these results show that cAMP–PKA signaling regulates the glucocorticoid response by inducing the nuclear translocation of FKBP51 that restrains its transcriptional capacity when adipogenesis is triggered.
Fig. 7.
PKA-dependent FKBP51 nuclear translocation restrains GR transcriptional capacity. (A–F) 3T3-L1 preadipocytes grown on coverslips were not induced (A; day 0) or induced to differentiate (B: day 1, C–F: day 8), and then subjected to IIF using anti-FKBP51 and anti-GR. Nuclei were counterstained with DAPI. A, B, E and F are merged confocal images of FKBP51 and GR. Scale bar: 10 µm. (G) GR was immunoprecipitated from 3T3-L1 preadipocytes prior and at the indicated time post-induction of adipogenesis. Immunoprecipitated complexes were resolved by SDS-PAGE and analyzed by immunoblotting using the indicated antibodies. (H) HEK293T cells were transiently transfected with MMTV-Luc and RSV-β-galactosidase plasmids in the absence or the presence of 0.01, 0.1 or 1 µg of pCI-Neo-hFKBP51 and the amount of transfected DNA was normalized to that of the empty vector. After transfection cells were cultured for 24 hours in steroid-free medium, and then incubated for 18 hours with 1 µM DEXA in the absence or the presence of 50 and 520 µM IBMX, 15 and 30 µM forskolin (FK), 50 and 500 µM dibutyryl-cAMP (DB) or 5 and 100 µM Me-cAMP (Me). Luciferase activity was measured and normalized to β-galactosidase activity. Each bar represents the mean ± s.e.m. (P<0.01) for five independent experiments. (I–X) 3T3-L1 cells grown on coverslips were treated with vehicle (I–L) or 1 µM DEXA in the absence (M–P) or presence of 520 µM IBMX (Q–T) or 100 µM Me-cAMP (U–X) for 1 hour, and then subjected to IIF with the indicated antibodies. Nuclei were counterstained with DAPI. Results are representative of three independent experiments. Scale bar: 10 µm.
FKBP51 is a negative regulator of adipogenesis
To ascertain the functional importance of FKBP51 in the process of adipogenesis, the IMM was knocked down in 3T3-L1 preadipocytes using specific shRNA, and after puromycin selection cells were induced to differentiate for 8 days. 3T3-L1 preadipocytes with knocked down FKBP51 were properly differentiated, however, adipocytes exhibited an increased number of lipid vesicles stained by LipidTOX (Fig. 8B versus A). Knockdown of FKBP51 accelerated the process of adipogenesis shown by the earlier increase at day 2, and overall higher levels in adiponectin (fourfold) and resistin (twofold) mRNAs compared to cells treated with mock shRNA (Fig. 8C). In contrast, ectopic expression of FKBP51 blocked adipogenesis evidenced by the absence of cells with lipid vesicles stained by Oil Red O (Fig. 8E versus D). Overexpression of FKBP51 also caused a dramatic decrease of adiponectin and resistin mRNAs (Fig. 8F), results that are in agreement with the significant reduction in the percentage of cells that had the capacity to differentiate (Fig. 8G). Taken together these results show for the first time that FKBP51 is a negative regulator in the process of adipocyte differentiation.
Discussion
We show that FKBP51 dynamically shuttles from mitochondria to the nucleus at the onset of the adipogenic program (Fig. 2). To our knowledge this is the first study that reports a dynamic mitochondrial–nuclear shuttling event during cell differentiation. When 3T3-L1 cells are fully differentiated, FKBP51 is detected both in mitochondria and in the nucleus, but Hsp70 is excluded from the latter. Thus, it is likely that the mechanism controlling the subcellular distribution of FKBP51 and Hsp70, as well as their roles in the nucleus during the process of adipogenesis may diverge at different stages of cell differentiation. Interestingly, FKBP51 rapidly translocates from mitochondria to the nucleus to protect the cells from oxidative stress (Gallo et al., 2011), concentrating in the nucleoli (M.D.G. unpublished results). In contrast, when 3T3-L1 preadipocytes are induced to differentiate FKBP51 is excluded from nucleoli (Fig. 2) and transiently concentrates in the nuclear lamina (Fig. 6). This differential nuclear compartmentalization of FKBP51 during cell differentiation compared with the stress response is an indicator of its differential role in each biological situation, indicating that the IMM can exert different functions in the nucleus in response to different biological cues.
Adipogenesis is controlled by many signaling pathways that modulate the sequential activation of transcription factors required for cells to differentiate (Rosen and MacDougald, 2006). Several reports have shown that the second messenger cAMP is associated with immediate events of adipogenesis through the classic PKA signaling pathway, as well as through the non-classic pathway, the ‘exchange proteins activated by cAMP’ (EPAC) that functions as guanine nucleotide exchange factor for the Ras-like small GTPases Rap1 and Rap2 (Martini et al., 2009; Petersen et al., 2008; Reusch et al., 2000; Xiao et al., 2011). It has been proposed that the role of PKA is to decrease cell tension by converting RhoA to an inactive GTP-free form, which leads to relaxation of microfilaments, in part by inhibition of Rho kinase. However, cAMP–EPAC signaling compensates for the PKA-dependent inhibition of Rho activity for efficient insulin–IGF signaling (Petersen et al., 2008). In the case of FKBP51, we found that its nuclear translocation depends on PKA (Figs 4 and 5) but not on EPAC pathway (Fig. 7), providing new evidence of the differential biological roles of PKA and EPAC/Rap during adipogenesis. We also found that in 3T3-L1 preadipocytes DEXA induces FKBP51 nuclear translocation but to a lesser extent than IBMX (Figs 4 and 7). It has been reported that glucocorticoids, through phospholipase A2, are able to increase cAMP, when required for the differentiation of Ob1771 preadipocytes (Gaillard et al., 1991; Vassaux et al., 1992). DEXA treatment of other cells types, e.g. 3B4.15 T cells, also results in increased levels of intracellular cAMP due to activation of adenylate cyclase and a decrease in phosphodiesterase activity (Aksoy et al., 2002; Baus et al., 2001). Therefore, it is possible that a smaller increase in intracellular cAMP level upon DEXA treatment compared with IBMX treatment might explain the differential degree of FKBP51 nuclear translocation observed in each condition.
A-kinase anchoring proteins (AKAPs) target PKA to distinct subcellular compartments providing spatial and temporal specificity for mediation of the biological effects elicited by this kinase. A pool of PKA is targeted by AKAPs to the mitochondrial membrane (Taskén and Aandahl, 2004), thus PKA is well located to phosphorylate mitochondrial FKBP51 and induce its translocation to the nucleus. We found, by immunoprecipitation assays, that FKBP51 interacts with PKA-cα (Fig. 6U). Importantly, inhibition of PKA by PKI or knockdown of PKA-cα not only blocked the nuclear translocation of FKBP51 but also induced dramatic changes in the electrophoretic pattern of migration of FKBP51 (Fig. 5), supporting the notion that FKBP51 is a PKA substrate. Using NetPhosk 1.0, we found that Ser312 located in the TPR domain of FKBP51 is a candidate PKA phosphoacceptor site. The TPR domain confers to the IMM the ability to bind Hsp90 through the EEVD motif present in the extreme C-terminus of the chaperone. FKBP51 localization to mitochondria also depends on TPR integrity, because FKBP51 TPR-deficient mutants are constitutively nuclear (Gallo et al., 2011). Therefore, changes in phosphorylation of Ser312 in the TPR domain of FKBP51 might have functional consequences in its interaction with Hsp90 and consequently in its subcellular localization. Intriguingly, FKBP52, an IMM that shares 60% identity and 70% similarity with FKBP51, has an alanine in this site, further suggesting that PKA-dependent phosphorylation of Ser312 in FKBP51 could be crucial for the different biological functions of these two highly homologous IMMs, a possibility that is currently under investigation.
The integrity of the TPR domain of FKBP51 is required for its localization in mitochondria, and when its functional interaction with Hsp90 is disrupted by drugs such as radicicol or as a result of mutations in the TPR, FKBP51 exits the mitochondria and concentrates in the nucleus (Gallo et al., 2011). These observations suggest that FKBP51 localizes to mitochondria, in part, as a mechanism to control its nuclear bioavailability. In order to uncover the role of FKBP51 in the nucleus at the onset of adipogenesis, it is necessary to understand how the IMM is retained in the nucleus. By using in situ extraction assays, we found that FKBP51 interacts with chromatin and the nuclear matrix stabilized by ribonucleoproteins and/or RNA (Fig. 3). Lamin B is a component of the nuclear lamina and the nuclear matrix, FKBP51 interacts with lamin B, which may possibly be a scaffold between the IMM and the nucleoskeleton. Different nuclear factors have been shown to be associated to the nuclear matrix, e.g. non-histone chromatin-associated proteins such as HP1α, C/EBPβ, a transcription factor required for adipocyte differentiation, steroid hormone receptors and histone deacetylases among many other factors (Susperreguy et al., 2011; Nickerson, 2001). It has been proposed that active RNA polymerase is located on the nuclear matrix (Cook, 1999) near actively transcribing genes together with bound transcription factors, facilitating their accessibility for binding to the promoter and regulating the expression of their target genes (Sutherland and Bickmore, 2009). FKBP51 is a negative regulator of GR and MR transcriptional capacity (Wochnik et al., 2005; Gallo et al., 2007), thus it is tempting to speculate that in the nuclear matrix compartment the IMM may interact with co-repressors, co-activators, components of the chromatin remodeling machinery and/or transcription factors other than the members of the nuclear receptor family, and in this way exert its control on gene expression.
There is growing recognition that the regulatory machinery is compartmentalized in subnuclear domains in which the components for combinatorial control are organized and assembled. Thus, gaining insight into the subnuclear distribution of factors that control transcription is key to understand how the architecture of the nucleus is delineated to sustain the pattern of gene expression required for the acquisition and maintenance of the final phenotype. In this regard, we have recently reported that the homo- and heterodimers formed by the different forms of C/EBPβ, localize to different subnuclear domains (Susperreguy et al., 2011) where they interact with HP1α. When proper nuclear localization of C/EBPβ is altered, adipogenesis is blocked (Susperreguy et al., 2011; Gaya et al., 2013). Here we show that at the onset of the adipogenesis, FKBP51 and PKA-cα are distributed through the nucleus and also concentrate in the nuclear lamina, where they colocalize and interact with lamin B (Fig. 6). Lamins have a scaffolding function for several nuclear processes such as transcription, chromatin organization, DNA replication, and to maintain nuclear and cellular integrity (Verstraeten et al., 2007). Lamin B was found to be fragmented during the second day of 3T3-L1 differentiation, a pattern that has been previously reported as part of the nuclear lamina reorganization that takes place during the process of adipogenesis (Verstraeten et al., 2011). We found that FKBP51 and PKA-cα parallels lamin B in the fragmentation of the nuclear lamina. Several phosphorylation sites, including those for the cyclin B1(CCNB1)–CDC2 complex, PKC and PKA are important in the nuclear lamina disassembly (D'Angelo and Hetzer, 2006; Stuurman, 1997). Therefore, it is possible that enrichment of PKA-cα in the nuclear lamina may facilitate its reorganization by phosphorylation of lamins during adipocyte differentiation. Notably, when cells are treated with DEXA and IBMX, GR is present in the nucleus colocalizing with FKBP51 (Fig. 7), raising the possibility that, dependent on cAMP-signaling, GR nuclear bioavailability may be regulated by FKBP51 possibly by its retention in the nuclear lamina (Fig. 6 and 7) as previously shown for other transcription factors, i.e. sterol regulatory element-binding and cFos (Andrés and González, 2009). Furthermore, it highlights the importance of mapping the subcellular distribution of a protein to gain insight into how it might exert different functions in response to different stimuli.
During the last few years, several studies revealed a dramatic and dynamic modulation of the chromatin landscape during the first hours of adipocyte differentiation (Lefterova et al., 2008; Nielsen et al., 2008; Siersbæk et al., 2011; Steger et al., 2010; Susperreguy et al., 2011; Xiao et al., 2011). These changes coincide with cooperative binding of early adipogenic transcription factors, including GR, within and immediately adjacent to the PPARg locus as well as other genes (Siersbæk et al., 2011; Steger et al., 2010). However, this locus is not transcriptionally activated until later in adipogenesis, as it has been proposed that additional factors and/or signals are required for its later activation (Siersbæk et al., 2011). It is also possible that, in spite of the high level of chromatin relaxation accompanied by increased binding of transcription factors at the early stages of adipogenesis, gene expression is controlled by factors that restrain the transcriptional capacity of complexes already bound to those sites. It was also reported that pretreatment of 3T3-L1 cells with IBMX rendered preadipocytes partially resistant to differentiation induced by MDI (Pantoja et al., 2008). Here we show that ectopic expression of FKBP51 blocked the process of adipogenesis (Fig. 8). Thus an increase in nuclear FKBP51 induced by IBMX prior induction of differentiation may interfere with the process of adipogenesis. Importantly, when adipogenesis is triggered, cAMP–PKA regulates the rapid nuclear translocation of FKBP51, which progressively increases its interaction with GR and restrains its transcriptional capacity (Fig. 7). We propose that in this way PKA may play a dual role in the regulation of GR, i.e. on the one hand, positively modulating GR transcriptional capacity (Doucas et al., 2000) and, on the other hand, restraining it by increasing the nuclear availability of FKBP51, a known GR negative regulator (Wochnik et al., 2005). Importantly, the presence of FKBP51 in the nucleus may be crucial for the control not only of GR but possibly other still unknown transcription factors, bound to target genes that have to be repressed in spite of the high level of chromatin remodeling that takes place within the first hours of adipocyte differentiation. It has to be highlighted that FKBP51 not only exerts its function in the nucleus regulating transcription, but also may also modulate biochemical events that take place in another cellular compartment, the mitochondrion (Gallo et al., 2011), and both the nucleus and mitochondria, are new areas for IMM action that need to be explored.
Materials and Methods
Materials and antibodies
Rabbit polyclonal IgG against FKBP51, the BuGR2 mouse monoclonal IgG against the GR and the JJ3 mouse monoclonal IgG against p23 were from Affinity BioReagents (Golden, CO, USA). The mouse polyclonal IgG against GR and the MG19 mouse monoclonal IgG against FKBP51 have been previously described (Gallo et al., 2011). Mouse polyclonal IgG against C/EBPα, Tom20, fibrillarin and lamin B were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA); mouse monoclonal IgG against Hsp70, Hsp90, Cyp40 were from StressGen (Ann Arbor, MI, USA); mouse polyclonal IgM against NuMA was from BD Transduction Laboratories (Franklin Lakes, NJ, USA); and anti-histone H3 was from Millipore (Billerica, MA, USA). Rabbit polyclonal IgG against PKA-cα and C/EBPβ, as well as the siRNAs for PKA-cα (cat. no. 6406) and control siRNA (cat. no. 6201) were from Cell Signaling Technology, Inc. (Beverly, MA, USA). Secondary antibodies labeled with Alexa Fluor 488, 546 or 647, MitoTracker and LipidTOX were purchased from Molecular Probes (Eugene, OR, USA). HRP-conjugated goat anti-rabbit was from Pierce (Rockford, IL, USA). Dexamethasone (DEXA), insulin, 3-isobutyl-1-methylxanthine (IBMX), forskolin, dibutyryl-cAMP, 8-(4-chlorophenylthio)-2′O-methyladenosine 3′,5′cyclic monophosphate (Me-cAMP), alkaline phosphatase (AP), mouse IgG anti-β tubulin and the HRP-conjugated donkey anti-mouse were from Sigma Chemical Co. (St. Louis, MO, USA). Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were from Gibco (Life Technologies–Invitrogen, Carlsbad, CA, USA). Myristoylated cAMP-dependent protein kinase inhibitor (PKI) was purchased from Enzo Life Sciences (Exeter, UK) and UO126, a MEK inhibitor, was from Promega (Madison, WI, USA).
Cell culture
Murine 3T3-L1 preadipocytes and human embryonic kidney HEK293T cells were obtained from American Type Culture Collection (Manassas, VA). Cells were grown in DMEM 4.5 g/l glucose and supplemented with 10% v/v calf serum in a humidified 5% CO2 atmosphere at 37°C. Differentiation of 3T3-L1 cells was performed as previously described (Susperreguy et al., 2011). FKBP51 was knocked down by transfecting 3T3-L1 cells with plasmids encoding specific shRNAs for FKBP51 (Origene, USA, cat. no. TG5000712) or scrambled shRNA using Lipofectamine 2000. Twenty-four hours later cells were selected for 2 days by using puromycin (4 µg/ml), and selected cells were induced to differentiate for the times indicated in the figure legends. For FKBP51 overexpression, 3T3-L1 cells were transfected with plasmids encoding FLAG-FKBP51 or empty vector using Lipofectamine 2000, as previously described (Susperreguy et al., 2011). Twenty-four hours later, cells were induced to differentiate for the time indicated in figure legends. Adipogenesis was evaluated as the percentage of cells that accumulated lipid droplets (stained by Oil Red O or LipidTOX), and by evaluating the expression of different adipogenic markers.
Cell fractionation
3T3-L1 cells were grown in DMEM supplemented with 10% bovine calf serum. Cells were induced to differentiate for 1 and 4 days, and harvested by trypsinization. Cell fractionation was performed as previously described (Nothwang and Schindler, 2009). Briefly, cells were resuspended in CLB buffer (10 mM HEPES, 10 mM NaCl, 1 mM KH2PO4, 5 mM NaHCO3, 5 mM EDTA, 1 mM CaCl2, 0.5 mM MgCl2 and protease inhibitors) and incubated for 5 minutes on ice. Cells were dounced 50 times with a loose pestle, sucrose was added to reach a final concentration of 0.25 M, and then samples were centrifuged at 6,300 g for 5 minutes at 4°C. The supernatant, which corresponded to the cytosolic fraction, was kept. The pellet (containing the nuclei) was washed once with CLB buffer and 0.25 M sucrose, and then the nuclei were lysed using TSE buffer [10 mM Tris-HCl, 300 mM sucrose, 1 mM EDTA, 0.1% IGEPAL-CA 630 (v/v), pH 7.5]. Total, cytoplasmic and nuclear fractions were resolved by SDS-PAGE and analyzed by immunoblotting, as previously described (Susperreguy et al., 2011). Cell fractionation of cytosol and mitochondria was performed as previously described (Gottlieb and Granville, 2002; Gallo et al., 2011).
Western blot analysis (WB)
3T3-L1 preadipocytes left untreated or induced to differentiate for the indicated times were washed with PBS and scraped into SDS lysis buffer (60 mM Tris-HCl, pH 6.8, 1% SDS) for whole-cell lysates, as described previously (Liao et al., 1999). In some experiments, whole-cell lysates were incubated with 40 units of alkaline phosphatase for 1 hour at 37°C prior to immunoblotting (Liao et al., 1999). The same amount of protein was loaded in all cases, and β-tubulin was used as loading control.
RT-PCR analysis
Total RNA was prepared from 3T3-L1 preadipocytes left untreated or induced to differentiate for different periods of time, using the Trizol reagent (Life Technologies–Invitrogen, Carlsbad, CA, USA) according to manufacturer's instructions, as previously described (Susperreguy et al., 2011). The PCR-amplified products were visualized after ethidium bromide staining on a 2% agarose gel. The mRNA levels of all the genes were normalized using β-actin as an internal control. Primer sequences are available upon request.
Indirect immunofluorescence assays and Oil Red O staining
Indirect immunofluorescence (IIF) was performed as previously described (Piwien Pilipuk et al., 2003; Susperreguy et al., 2011). All IIF conditions were tested to avoid non-specific reactions. The specificity of anti-FKBP51 had been tested already (Gallo et al., 2011). Mitochondria were labeled with MitoTracker according to the manufacturer's instructions. Nuclei were stained with DAPI and coverslips were mounted in Vectashield. For the staining of lipid vesicles, after DAPI staining coverslips were inverted onto 25 µl of LipidTOX (diluted 1/300 in PBS) and incubated for 30 minutes at room temperature prior to mounting the coverslips in Vectashield. For Oil Red O staining, cells were washed with PBS, fixed with 4% formaldehyde at room temperature for 1 hour, washed twice with water, and stained with Oil Red O, as previously described (Susperreguy et al., 2011). Laser-scanning confocal microscopy was performed with LSM5 Pascal or a Meta microscope (Carl Zeiss. Oberkochen, Germany), using a C-Apochromat 63×/1.4 NA oil-immersion objective and images were taken in the middle section of the cell nucleus. Percentage of cells with the pattern of protein distribution presented in the figures was obtained by observation of 200–300 cells per condition and per experiment, made independently by two observers.
In situ cell extraction
The extractions were performed directly on cells grown on coverslips (in situ extraction) as previously described (Susperreguy et al., 2011), using a protocol adapted from Fey et al. (Fey et al., 1986).
Immunoprecipitation assays
3T3-L1 cells were harvested in ice-cold Earle's balanced saline, washed twice, and ruptured by Dounce homogenization in 1 volume of HEM buffer at pH 7.4 (10 mM HEPES, 1 mM EDTA, 20 mM Na2MoO4). GR was immunoprecipitated as previously described (Echeverría et al., 2009). Proteins in the immune pellet were resolved by SDS-PAGE and analyzed by WB.
Reporter gene assays
HEK293T cells were transiently transfected by the calcium phosphate co-precipitation assay with MMTV-Luc (0.5 µg), and RSV-β-galactosidase (0.1 µg), pCIneo-FLAG-hFKBP51 and empty vector in order to transfect the same amount of DNA in all conditions. Twenty-four hours after transfection, cells were grown in medium supplemented with steroid-free serum and treated or not with 1 µM DEXA in the absence or presence of IBMX, forskolin, dibutyryl-cAMP or Me-cAMP, as indicated in the figure legends. Luciferase and β-galactosidase activity were measured using a Veritas™ microplate luminometer (Turner Biosystems, USA). Luciferase values were normalized to β-galactosidase activity. Each condition was tested in duplicate in each experiment. A two-sample t-test (SigmaStat) was used to judge statistical significance where a value of P<0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We are very grateful to Dr David F. Smith (Mayo Clinic, Scottsdale, AZ, USA), Dr Marc B. Cox (The Border Biomedical Research Center and Department of Biological Sciences, University of Texas, El Paso, TX, USA) and Dr Theo Rein (Max Planck Institute of Psychiatry, Munich, Germany) for generously providing plasmids.
Footnotes
Author contributions
J.T. and S.G performed the majority of experiments; N.L.C. and S.G. performed cell fractionation and gene reporter assays; G.P.P. conceived the project and analysed data with J.T., M.D.G. and J.S.; G.P.P. designed the figures and wrote the manuscript with comments from co-authors.
Funding
This work was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica [grant numbers PICT-26495, PICT-00640 to G.P.P. and PICT-1170, PICT-1715 to M.D.G.]; Universidad de Buenos Aires [grant number UBACYT-W237 to M.D.G.]; National Institutes of Health - the Fogarty International Research Collaboration Award [grant number R03TW008143-01A1 to G.P.P. and J.S.]. J.T. is a recipient of a postdoctoral fellowship from CONICET and N.L.C. is a recipient of a doctoral fellowship from CONICET. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.125799/-/DC1
References
- Aksoy M. O., Mardini I. A., Yang Y., Bin W., Zhou S., Kelsen S. G. (2002). Glucocorticoid effects on the beta-adrenergic receptor-adenylyl cyclase system of human airway epithelium. J. Allergy Clin. Immunol. 109, 491–497 10.1067/mai.2002.122154 [DOI] [PubMed] [Google Scholar]
- Andrés V., González J. M. (2009). Role of A-type lamins in signaling, transcription, and chromatin organization. J. Cell Biol. 187, 945–957 10.1083/jcb.200904124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bains G., Lithgow T. (1999). The Tom channel in the mitochondrial outer membrane: alive and kicking. Bioessays 21, 1–4 [DOI] [PubMed] [Google Scholar]
- Baus E., Van Laethem F., Andris F., Rolin S., Urbain J., Leo O. (2001). Dexamethasone increases intracellular cyclic AMP concentration in murine T lymphocyte cell lines. Steroids 66, 39–47 10.1016/S0039-128X(00)00137-9 [DOI] [PubMed] [Google Scholar]
- Cheung-Flynn J., Prapapanich V., Cox M. B., Riggs D. L., Suarez-Quian C., Smith D. F. (2005). Physiological role for the cochaperone FKBP52 in androgen receptor signaling. Mol. Endocrinol. 19, 1654–1666 10.1210/me.2005-0071 [DOI] [PubMed] [Google Scholar]
- Cook P. R. (1999). The organization of replication and transcription. Science 284, 1790–1795 10.1126/science.284.5421.1790 [DOI] [PubMed] [Google Scholar]
- D'Angelo M. A., Hetzer M. W. (2006). The role of the nuclear envelope in cellular organization. Cell. Mol. Life Sci. 63, 316–332 10.1007/s00018-005-5361-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies T. H., Ning Y. M., Sánchez E. R. (2002). A new first step in activation of steroid receptors: hormone-induced switching of FKBP51 and FKBP52 immunophilins. J. Biol. Chem. 277, 4597–4600 10.1074/jbc.C100531200 [DOI] [PubMed] [Google Scholar]
- Doucas V., Shi Y., Miyamoto S., West A., Verma I., Evans R. M. (2000). Cytoplasmic catalytic subunit of protein kinase A mediates cross-repression by NF-kappa B and the glucocorticoid receptor. Proc. Natl. Acad. Sci. USA 97, 11893–11898 10.1073/pnas.220413297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverría P. C., Mazaira G., Erlejman A., Gomez-Sanchez C., Piwien Pilipuk G., Galigniana M. D. (2009). Nuclear import of the glucocorticoid receptor-hsp90 complex through the nuclear pore complex is mediated by its interaction with Nup62 and importin beta. Mol. Cell. Biol. 29, 4788–4797 10.1128/MCB.00649-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enserink J. M., Christensen A. E., de Rooij J., van Triest M., Schwede F., Genieser H. G., Døskeland S. O., Blank J. L., Bos J. L. (2002). A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat. Cell Biol. 4, 901–906 10.1038/ncb874 [DOI] [PubMed] [Google Scholar]
- Fey E. G., Krochmalnic G., Penman S. (1986). The nonchromatin substructures of the nucleus: the ribonucleoprotein (RNP)-containing and RNP-depleted matrices analyzed by sequential fractionation and resinless section electron microscopy. J. Cell Biol. 102, 1654–1665 10.1083/jcb.102.5.1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal K. M., Carroll M. D., Kit B. K., Ogden C. L. (2012). Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 307, 491–497 10.1001/jama.2012.39 [DOI] [PubMed] [Google Scholar]
- Fu M., Sun T., Bookout A. L., Downes M., Yu R. T., Evans R. M., Mangelsdorf D. J. (2005). A Nuclear Receptor Atlas: 3T3-L1 adipogenesis. Mol. Endocrinol. 19, 2437–2450 10.1210/me.2004-0539 [DOI] [PubMed] [Google Scholar]
- Gaillard D., Wabitsch M., Pipy B., Négrel R. (1991). Control of terminal differentiation of adipose precursor cells by glucocorticoids. J. Lipid Res. 32, 569–579 [PubMed] [Google Scholar]
- Galigniana M. D. (2012). Steroid receptor coupling becomes nuclear. Chem. Biol. 19, 662–663 10.1016/j.chembiol.2012.06.001 [DOI] [PubMed] [Google Scholar]
- Galigniana M. D., Radanyi C., Renoir J. M., Housley P. R., Pratt W. B. (2001). Evidence that the peptidylprolyl isomerase domain of the hsp90-binding immunophilin FKBP52 is involved in both dynein interaction and glucocorticoid receptor movement to the nucleus. J. Biol. Chem. 276, 14884–14889 10.1074/jbc.M010809200 [DOI] [PubMed] [Google Scholar]
- Galigniana M. D., Erlejman A. G., Monte M., Gomez-Sanchez C., Piwien-Pilipuk G. (2010). The hsp90-FKBP52 complex links the mineralocorticoid receptor to motor proteins and persists bound to the receptor in early nuclear events. Mol. Cell. Biol. 30, 1285–1298 10.1128/MCB.01190-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo L. I., Ghini A. A., Piwien Pilipuk G., Galigniana M. D. (2007). Differential recruitment of tetratricorpeptide repeat domain immunophilins to the mineralocorticoid receptor influences both heat-shock protein 90-dependent retrotransport and hormone-dependent transcriptional activity. Biochemistry 46, 14044–14057 10.1021/bi701372c [DOI] [PubMed] [Google Scholar]
- Gallo L. I., Lagadari M., Piwien-Pilipuk G., Galigniana M. D. (2011). The 90-kDa heat-shock protein (Hsp90)-binding immunophilin FKBP51 is a mitochondrial protein that translocates to the nucleus to protect cells against oxidative stress. J. Biol. Chem. 286, 30152–30160 10.1074/jbc.M111.256610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaya M., Repetto V., Toneatto J., Anessini C., Piwien-Pilipuk G., Moreno S. (2013). Antiadipogenic effect of carnosic acid, a natural compound present in Rosmarinus officinalis, is exerted through the C/EBPs and PPARgamma pathways at the onset of the differentiation program. Biochim. Biophys. Acta 1830, 3796–3806 [DOI] [PubMed] [Google Scholar]
- Gottlieb R. A., Granville D. J. (2002). Analyzing mitochondrial changes during apoptosis. Methods 26, 341–347 10.1016/S1046-2023(02)00040-3 [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. (1975). An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell 5, 19–27 10.1016/0092-8674(75)90087-2 [DOI] [PubMed] [Google Scholar]
- Gregoire F. M., Smas C. M., Sul H. S. (1998). Understanding adipocyte differentiation. Physiol. Rev. 78, 783–809 [DOI] [PubMed] [Google Scholar]
- Grossmann C., Ruhs S., Langenbruch L., Mildenberger S., Strätz N., Schumann K., Gekle M. (2012). Nuclear shuttling precedes dimerization in mineralocorticoid receptor signaling. Chem. Biol. 19, 742–751 10.1016/j.chembiol.2012.04.014 [DOI] [PubMed] [Google Scholar]
- Guenatri M., Bailly D., Maison C., Almouzni G. (2004). Mouse centric and pericentric satellite repeats form distinct functional heterochromatin. J. Cell Biol. 166, 493–505 10.1083/jcb.200403109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann J., Wagner K. V., Liebl C., Scharf S. H., Wang X. D., Wolf M., Hausch F., Rein T., Schmidt U., Touma C. et al. (2012). The involvement of FK506-binding protein 51 (FKBP5) in the behavioral and neuroendocrine effects of chronic social defeat stress. Neuropharmacology 62, 332–339 10.1016/j.neuropharm.2011.07.041 [DOI] [PubMed] [Google Scholar]
- Kershaw E. E., Morton N. M., Dhillon H., Ramage L., Seckl J. R., Flier J. S. (2005). Adipocyte-specific glucocorticoid inactivation protects against diet-induced obesity. Diabetes 54, 1023–1031 10.2337/diabetes.54.4.1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus S. (2004). Adipose tissue as a regulator of energy balance. Curr. Drug Targets 5, 241–250 10.2174/1389450043490523 [DOI] [PubMed] [Google Scholar]
- Lefterova M. I., Zhang Y., Steger D. J., Schupp M., Schug J., Cristancho A., Feng D., Zhuo D., Stoeckert C. J., Jr, Liu X. S. et al. (2008). PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 22, 2941–2952 10.1101/gad.1709008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J., Piwien-Pilipuk G., Ross S. E., Hodge C. L., Sealy L., MacDougald O. A., Schwartz J. (1999). CCAAT/Enhancer-binding protein beta (C/EBP beta) and C/EBPδ contribute to growth homone-regulated transcription of c-fos. J. Biol. Chem. 274, 31597–31604 10.1074/jbc.274.44.31597 [DOI] [PubMed] [Google Scholar]
- Martini C. N., Plaza M. V., Vila M. C. (2009). PKA-dependent and independent cAMP signaling in 3T3-L1 fibroblasts differentiation. Mol. Cell. Endocrinol. 298, 42–47 10.1016/j.mce.2008.10.023 [DOI] [PubMed] [Google Scholar]
- Masuzaki H., Paterson J., Shinyama H., Morton N. M., Mullins J. J., Seckl J. R., Flier J. S. (2001). A transgenic model of visceral obesity and the metabolic syndrome. Science 294, 2166–2170 10.1126/science.1066285 [DOI] [PubMed] [Google Scholar]
- Morton N. M., Paterson J. M., Masuzaki H., Holmes M. C., Staels B., Fievet C., Walker B. R., Flier J. S., Mullins J. J., Seckl J. R. (2004). Novel adipose tissue-mediated resistance to diet-induced visceral obesity in 11 beta-hydroxysteroid dehydrogenase type 1-deficient mice. Diabetes 53, 931–938 10.2337/diabetes.53.4.931 [DOI] [PubMed] [Google Scholar]
- Newell-Price J., Bertagna X., Grossman A. B., Nieman L. K. (2006). Cushing's syndrome. Lancet 367, 1605–1617 10.1016/S0140-6736(06)68699-6 [DOI] [PubMed] [Google Scholar]
- Nickerson J. (2001). Experimental observations of a nuclear matrix. J. Cell Sci. 114, 463–474 [DOI] [PubMed] [Google Scholar]
- Nielsen R., Pedersen T. A., Hagenbeek D., Moulos P., Siersbæk R., Megens E., Denissov S., Børgesen M., Francoijs K. J., Mandrup S. et al. (2008). Genome-wide profiling of PPARgamma:RXR and RNA polymerase II occupancy reveals temporal activation of distinct metabolic pathways and changes in RXR dimer composition during adipogenesis. Genes Dev. 22, 2953–2967 10.1101/gad.501108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwang H. G., Guillemin I., Schindler J. (2009). Heidelberg: Springer [Google Scholar]
- O'Leary J. C., 3rd, Dharia S., Blair L. J., Brady S., Johnson A. G., Peters M., Cheung-Flynn J., Cox M. B., de Erausquin G., Weeber E. J. et al. (2011). A new anti-depressive strategy for the elderly: ablation of FKBP5/FKBP51. PLoS ONE 6, e24840 10.1371/journal.pone.0024840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoja C., Huff J. T., Yamamoto K. R. (2008). Glucocorticoid signaling defines a novel commitment state during adipogenesis in vitro. Mol. Biol. Cell 19, 4032–4041 10.1091/mbc.E08-04-0420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R. K., Madsen L., Pedersen L. M., Hallenborg P., Hagland H., Viste K., Døskeland S. O., Kristiansen K. (2008). Cyclic AMP (cAMP)-mediated stimulation of adipocyte differentiation requires the synergistic action of Epac- and cAMP-dependent protein kinase-dependent processes. Mol. Cell. Biol. 28, 3804–3816 10.1128/MCB.00709-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirkl F., Buchner J. (2001). Functional analysis of the Hsp90-associated human peptidyl prolyl cis/trans isomerases FKBP51, FKBP52 and Cyp40. J. Mol. Biol. 308, 795–806 10.1006/jmbi.2001.4595 [DOI] [PubMed] [Google Scholar]
- Piwien Pilipuk G., Galigniana M. D., Schwartz J. (2003). Subnuclear localization of C/EBP beta is regulated by growth hormone and dependent on MAPK. J. Biol. Chem. 278, 35668–35677 10.1074/jbc.M305182200 [DOI] [PubMed] [Google Scholar]
- Pratt W. B., Toft D. O. (1997). Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr. Rev. 18, 306–360 10.1210/er.18.3.306 [DOI] [PubMed] [Google Scholar]
- Rajala M. W., Scherer P. E. (2003). Minireview: the adipocyte – at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology 144, 3765–3773 10.1210/en.2003-0580 [DOI] [PubMed] [Google Scholar]
- Reusch J. E., Colton L. A., Klemm D. J. (2000). CREB activation induces adipogenesis in 3T3-L1 cells. Mol. Cell. Biol. 20, 1008–1020 10.1128/MCB.20.3.1008-1020.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs D. L., Roberts P. J., Chirillo S. C., Cheung-Flynn J., Prapapanich V., Ratajczak T., Gaber R., Picard D., Smith D. F. (2003). The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. EMBO J. 22, 1158–1167 10.1093/emboj/cdg108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen E. D., MacDougald O. A. (2006). Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 7, 885–896 10.1038/nrm2066 [DOI] [PubMed] [Google Scholar]
- Siersbæk R., Nielsen R., John S., Sung M. H., Baek S., Loft A., Hager G. L., Mandrup S. (2011). Extensive chromatin remodelling and establishment of transcription factor ‘hotspots’ during early adipogenesis. EMBO J. 30, 1459–1472 10.1038/emboj.2011.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivils J. C., Storer C. L., Galigniana M. D., Cox M. B. (2011). Regulation of steroid hormone receptor function by the 52-kDa FK506-binding protein (FKBP52). Curr. Opin. Pharmacol. 11, 314–319 10.1016/j.coph.2011.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger D. J., Grant G. R., Schupp M., Tomaru T., Lefterova M. I., Schug J., Manduchi E., Stoeckert C. J., Jr, Lazar M. A. (2010). Propagation of adipogenic signals through an epigenomic transition state. Genes Dev. 24, 1035–1044 10.1101/gad.1907110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg G. R. (2007). Inflammation in obesity is the common link between defects in fatty acid metabolism and insulin resistance. Cell Cycle 6, 888–894 10.4161/cc.6.8.4135 [DOI] [PubMed] [Google Scholar]
- Storer C. L., Dickey C. A., Galigniana M. D., Rein T., Cox M. B. (2011). FKBP51 and FKBP52 in signaling and disease. Trends Endocrinol. Metab. 22, 481–490 10.1016/j.tem.2011.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuurman N. (1997). Identification of a conserved phosphorylation site modulating nuclear lamin polymerization. FEBS Lett. 401, 171–174 10.1016/S0014-5793(96)01464-0 [DOI] [PubMed] [Google Scholar]
- Susperreguy S., Prendes L. P., Desbats M. A., Charó N. L., Brown K., MacDougald O. A., Kerppola T., Schwartz J., Piwien-Pilipuk G. (2011). Visualization by BiFC of different C/EBPβ dimers and their interaction with HP1α reveals a differential subnuclear distribution of complexes in living cells. Exp. Cell Res. 317, 706–723 10.1016/j.yexcr.2010.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland H., Bickmore W. A. (2009). Transcription factories: gene expression in unions? Nat. Rev. Genet. 10, 457–466 10.1038/nrg2592 [DOI] [PubMed] [Google Scholar]
- Taskén K., Aandahl E. M. (2004). Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol. Rev. 84, 137–167 10.1152/physrev.00021.2003 [DOI] [PubMed] [Google Scholar]
- Taylor S. S., Buechler J. A., Yonemoto W. (1990). cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu. Rev. Biochem. 59, 971–1005 10.1146/annurev.bi.59.070190.004543 [DOI] [PubMed] [Google Scholar]
- Touma C., Gassen N. C., Herrmann L., Cheung-Flynn J., Büll D. R., Ionescu I. A., Heinzmann J. M., Knapman A., Siebertz A., Depping A. M. et al. (2011). FK506 binding protein 5 shapes stress responsiveness: modulation of neuroendocrine reactivity and coping behavior. Biol. Psychiatry 70, 928–936 10.1016/j.biopsych.2011.07.023 [DOI] [PubMed] [Google Scholar]
- van Kruijsdijk R. C., van der Wall E., Visseren F. L. (2009). Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol. Biomarkers Prev. 18, 2569–2578 10.1158/1055-9965.EPI-09-0372 [DOI] [PubMed] [Google Scholar]
- Vassaux G., Gaillard D., Ailhaud G., Négrel R. (1992). Prostacyclin is a specific effector of adipose cell differentiation. Its dual role as a cAMP- and Ca(2+)-elevating agent. J. Biol. Chem. 267, 11092–11097 [PubMed] [Google Scholar]
- Verstraeten V. L. R. M., Broers J. L., Ramaekers F. C., van Steensel M. A. (2007). The nuclear envelope, a key structure in cellular integrity and gene expression. Curr. Med. Chem. 14, 1231–1248 10.2174/092986707780598032 [DOI] [PubMed] [Google Scholar]
- Verstraeten V. L. R. M., Renes J., Ramaekers F. C., Kamps M., Kuijpers H. J., Verheyen F., Wabitsch M., Steijlen P. M., van Steensel M. A., Broers J. L. (2011). Reorganization of the nuclear lamina and cytoskeleton in adipogenesis. Histochem. Cell Biol. 135, 251–261 10.1007/s00418-011-0792-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajchenberg B. L. (2000). Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr. Rev. 21, 697–738 10.1210/er.21.6.697 [DOI] [PubMed] [Google Scholar]
- Warrier M., Hinds T. D., Jr, Ledford K. J., Cash H. A., Patel P. R., Bowman T. A., Stechschulte L. A., Yong W., Shou W., Najjar S. M. et al. (2010). Susceptibility to diet-induced hepatic steatosis and glucocorticoid resistance in FK506-binding protein 52-deficient mice. Endocrinology 151, 3225–3236 10.1210/en.2009-1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wochnik G. M., Rüegg J., Abel G. A., Schmidt U., Holsboer F., Rein T. (2005). FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J. Biol. Chem. 280, 4609–4616 10.1074/jbc.M407498200 [DOI] [PubMed] [Google Scholar]
- Xiao H., Leblanc S. E., Wu Q., Konda S., Salma N., Marfella C. G., Ohkawa Y., Imbalzano A. N. (2011). Chromatin accessibility and transcription factor binding at the PPARγ2 promoter during adipogenesis is protein kinase A-dependent. J. Cell. Physiol. 226, 86–93 10.1002/jcp.22308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong W., Yang Z., Periyasamy S., Chen H., Yucel S., Li W., Lin L. Y., Wolf I. M., Cohn M. J., Baskin L. S. et al. (2007). Essential role for Co-chaperone Fkbp52 but not Fkbp51 in androgen receptor-mediated signaling and physiology. J. Biol. Chem. 282, 5026–5036 10.1074/jbc.M609360200 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.