Abstract
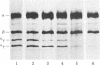
An activity that induces Hb F to Hb A switching in human cells is present in fetal sheep serum. To test directly the role of cell-to-environment interactions in hemoglobin switching and to define the level of erythroid cell differentiation at which this activity operates, colony transfer experiments were done. Clones grown in the presence of switching activity-containing medium (fetal sheep serum) or control medium (fetal calf serum) were transferred, at the 16- to 30-cell stage, to either fetal sheep serum or fetal calf serum plates and Hb F synthesis was determined in the fully mature erythroid bursts. Fetal calf serum-to-fetal calf serum transfers produced colonies with the high Hb F levels characteristic of undisturbed fetal calf serum-grown clones. Fetal sheep serum-to-fetal calf serum transfers resulted in significant decrease in Hb F synthesis, revealing an interaction between hemoglobin switching activity and cells at an early stage of progenitor cell development. The reduction of Hb F synthesis in fetal calf serum-to-fetal sheep serum transfers indicated that hemoglobin switching activity interacts with cells at later stages of progenitor cell development. Maximal decrease in Hb F synthesis was observed in fetal sheep serum-to-fetal sheep serum transfers, indicating that optimal effects on Hb switching are obtained when the environment that induces Hb switching is present throughout the development of progenitor cells. By splitting single early clones into two parts and transferring them to either a fetal sheep serum or a fetal calf serum environment, these interactions were further demonstrated in the progeny of a single erythroid burst-forming unit. Since all clone transfers were done on cell-free plates, the results of fetal calf serum-to-fetal sheep serum and of fetal sheep serum-to-fetal sheep serum transfers indicated that the switching activity does not require helper cells for its action. These studies show directly that (i) Hb F synthesis is controlled at the level of progenitors and (ii) it involves interactions between progenitor cells and their environment.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter B. P., Jackson B. T., Lipton J. M., Piasecki G. J., Jackson P. L., Kudisch M., Nathan D. G. Control of the simian fetal hemoglobin switch at the progenitor cell level. J Clin Invest. 1981 Feb;67(2):458–466. doi: 10.1172/JCI110054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer S. H., Belding T. K., Margolet L., Noyes A. N. Fetal hemoglobin restriction to a few erythrocytes (F cells) in normal human adults. Science. 1975 Apr 25;188(4186):361–363. doi: 10.1126/science.804182. [DOI] [PubMed] [Google Scholar]
- Clarke B. J., Nathan D. G., Alter B. P., Forget B. G., Hillman D. G., Housman D. Hemoglobin synthesis in human BFU-E and CFU-E-derived erythroid colonies. Blood. 1979 Oct;54(4):805–817. [PubMed] [Google Scholar]
- Darbre P. D., Adamson J. W., Wood W. G., Weatherall D. J., Robinson J. S. Patterns of globin chain synthesis in erythroid colonies grown from sheep marrow of different developmental stages. Br J Haematol. 1979 Apr;41(4):459–475. doi: 10.1111/j.1365-2141.1979.tb05884.x. [DOI] [PubMed] [Google Scholar]
- Farquhar M. N., Turner P. A., Papayannopoulou T., Brice M., Nienhuis A. W., Stamatoyannopoulos G. The asynchrony of gamma- and beta-chain synthesis during human erythroid cell maturation. III. gamma- and beta-mRNA in immature and mature erythroid clones. Dev Biol. 1981 Jul 30;85(2):403–408. doi: 10.1016/0012-1606(81)90271-2. [DOI] [PubMed] [Google Scholar]
- Fauser A. A., Messner H. A. Fetal hemoglobin in mixed hemopoietic colonies (CFU-GEMM), erythroid bursts (BFU-E) and erythroid colonies (CFU-E): assessment by radioimmune assay and immunofluorescence. Blood. 1979 Dec;54(6):1384–1394. [PubMed] [Google Scholar]
- Iscove N. N., Sieber F. Erythroid progenitors in mouse bone marrow detected by macroscopic colony formation in culture. Exp Hematol. 1975 Jan;3(1):32–43. [PubMed] [Google Scholar]
- Kidoguchi K., Ogawa M., Karam J. D. Hemoglobin biosynthesis in individual erythropoietic bursts in culture. Studies of adult peripheral blood. J Clin Invest. 1979 Apr;63(4):804–806. doi: 10.1172/JCI109366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidoguchi K., Ogawa M., Karam J. D., Martin A. G. Augmentation of fetal hemoglobin (HbF) synthesis in culture by human erythropoietic precursors in the marrow and peripheral blood: studies in sickle cell anemia and nonhemoglobinopathic adults. Blood. 1978 Dec;52(6):1115–1124. [PubMed] [Google Scholar]
- Nienhuis A. W., Benz E. J., Jr Regulation of hemoglobin synthesis during the development of the red cell (first of three parts). N Engl J Med. 1977 Dec 15;297(24):1318–1328. doi: 10.1056/NEJM197712152972404. [DOI] [PubMed] [Google Scholar]
- Nienhuis A. W., Benz E. J., Jr Regulation of hemoglobin synthesis during the development of the red cell (third of three parts). N Engl J Med. 1977 Dec 29;297(26):1430–1436. doi: 10.1056/NEJM197712292972604. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T. H., Brice M., Stamatoyannopoulos G. Stimulation of fetal hemoglobin synthesis in bone marrow cultures from adult individuals. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2033–2037. doi: 10.1073/pnas.73.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Brice M., Stamatoyannopoulos G. Hemoglobin F synthesis in vitro: evidence for control at the level of primitive erythroid stem cells. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2923–2927. doi: 10.1073/pnas.74.7.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Kalmantis T., Stamatoyannopoulos G. Cellular regulation of hemoglobin switching: evidence for inverse relationship between fetal hemoglobin synthesis and degree of maturity of human erythroid cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6420–6424. doi: 10.1073/pnas.76.12.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Kurachi S., Brice M., Nakamoto B., Stamatoyannopoulos G. Asynchronous synthesis of HbF and HbA during erythroblast maturation. II. Studies of G gamma, A gamma, and beta chain synthesis in individual erythroid clones from neonatal and adult BFU-E cultures. Blood. 1981 Mar;57(3):531–536. [PubMed] [Google Scholar]
- Papayannopoulou T., Kurachi S., Nakamoto B., Zanjani E. D., Stamatoyannopoulos G. Hemoglobin switching in culture: evidence for a humoral factor that induces switching in adult and neonatal but not fetal erythroid cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6579–6583. doi: 10.1073/pnas.79.21.6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatoyannopoulos G., Kurnit D. M., Papayannopoulou T. Stochastic expression of fetal hemoglobin in adult erythroid cells. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7005–7009. doi: 10.1073/pnas.78.11.7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. G., Stamatoyannopoulos G., Lim G., Nute P. E. F-cells in the adult: normal values and levels in individuals with hereditary and acquired elevations of Hb F. Blood. 1975 Nov;46(5):671–682. [PubMed] [Google Scholar]