Abstract
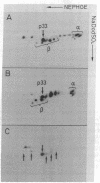
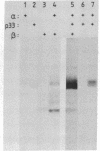
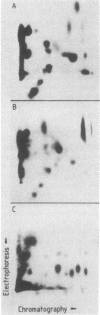
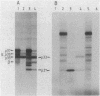
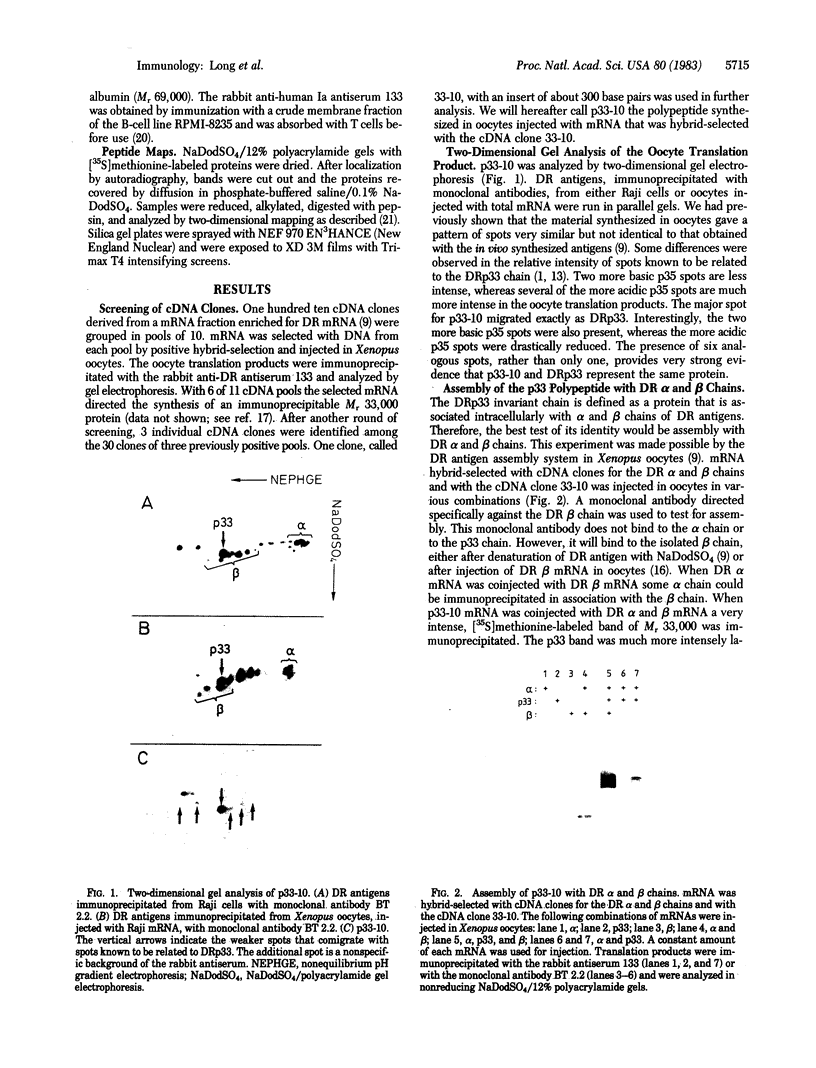
HLA-DR antigens are polymorphic cell surface glycoproteins involved in the control of the immune response in man. They consist of two subunits, the alpha and the beta chains. In addition, an invariant glycoprotein of Mr 33,000 (DRp33) is associated intracellularly with HLA-DR antigens. A cDNA clone for DRp33, called 33-10, was isolated. Because no amino acid sequence has yet been determined for DRp33 the identification of cDNA clone 33-10 was based on selection of mRNA by hybridization, subsequent translation in a rabbit reticulocyte lysate supplemented with microsomes, and translation in microinjected Xenopus oocytes followed by immunoprecipitation with an anti-DR antiserum. The translation products assembled with DR alpha and beta chains in oocytes coinjected with all three mRNAs. Assembly of DR alpha and beta chains was also observed in the absence of DRp33 mRNA. Furthermore, when compared with DRp33 immunoprecipitated from a human B-cell line, translation products of the hybrid-selected mRNA showed (i) identical migration in two-dimensional gel electrophoresis, (ii) identical apparent molecular weight in the absence of N-linked glycosylation, and (iii) a very similar two-dimensional peptide map. Transcription of the DRp33 gene into a mRNA 1,400 nucleotides long was observed in B cells but was undetectable in T-cell lines and was very low in liver. Thus, DRp33 appears to be coordinately expressed with DR alpha and beta chains. Hybridization to DNA of mouse-human somatic cell hybrids showed that DRp33 is encoded by a gene that is located outside the major histocompatibility complex.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accolla R. S., Gross N., Carrel S., Corte G. Distinct forms of both alpha and beta subunits are present in the human Ia molecular pool. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4549–4551. doi: 10.1073/pnas.78.7.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrel S., Gross N., Heumann D., Mach J. P. Expression of "Ia-like" antigens on cells from a human endometrial carcinoma cell line, END-1. Transplantation. 1979 Jun;27(6):431–433. [PubMed] [Google Scholar]
- Charron D. J., Aellen-Schulz M. F., St Geme J., 3rd, Erlich H. A., McDevitt H. O. Biochemical characterization of an invariant polypeptide associated with Ia antigens in human and mouse. Mol Immunol. 1983 Jan;20(1):21–32. doi: 10.1016/0161-5890(83)90101-3. [DOI] [PubMed] [Google Scholar]
- Charron D. J., McDevitt H. O. Analysis of HLA-D region-associated molecules with monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6567–6571. doi: 10.1073/pnas.76.12.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day C. E., Jones P. P. The gene encoding the Ia antigen-associated invariant chain (Ii) is not linked to the H-2 complex. Nature. 1983 Mar 10;302(5904):157–159. doi: 10.1038/302157a0. [DOI] [PubMed] [Google Scholar]
- Goodfellow P. N., Banting G., Trowsdale J., Chambers S., Solomon E. Introduction of a human X-6 translocation chromosome into a mouse teratocarcinoma: investigation of control of HLA-A, B, C expression. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1190–1194. doi: 10.1073/pnas.79.4.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. P., Murphy D. B., Hewgill D., McDevitt H. O. Detection of a common polypeptide chain in I--A and I--E sub-region immunoprecipitates. Mol Immunol. 1979 Jan;16(1):51–60. doi: 10.1016/0161-5890(79)90027-0. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Chiller J. M., Sidman C. L. Major histocompatibility complex-restricted cellular interactions determining B cell activation. Eur J Immunol. 1982 Aug;12(8):627–633. doi: 10.1002/eji.1830120802. [DOI] [PubMed] [Google Scholar]
- Koch N., Hämmerling G. J., Szymura J., Wabl M. R. Ia associated Ii chain is not encoded by chromosome 17 of the mouse. Immunogenetics. 1982;16(6):603–606. doi: 10.1007/BF00372029. [DOI] [PubMed] [Google Scholar]
- Kvist S., Wiman K., Claesson L., Peterson P. A., Dobberstein B. Membrane insertion and oligomeric assembly of HLA-DR histocompatibility antigens. Cell. 1982 May;29(1):61–69. doi: 10.1016/0092-8674(82)90090-3. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Trowsdale J., Bodmer W. F. cDNA clones coding for the heavy chain of human HLA-DR antigen. Proc Natl Acad Sci U S A. 1982 Jan;79(2):545–549. doi: 10.1073/pnas.79.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long E. O., Gross N., Wake C. T., Mach J. P., Carrel S., Accolla R., Mach B. Translation and assembly of HLA-DR antigens in Xenopus oocytes injected with mRNA from a human B-cell line. EMBO J. 1982;1(5):649–654. doi: 10.1002/j.1460-2075.1982.tb01222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long E. O., Wake C. T., Strubin M., Gross N., Accolla R. S., Carrel S., Mach B. Isolation of distinct cDNA clones encoding HLA-DR beta chains by use of an expression assay. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7465–7469. doi: 10.1073/pnas.79.23.7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
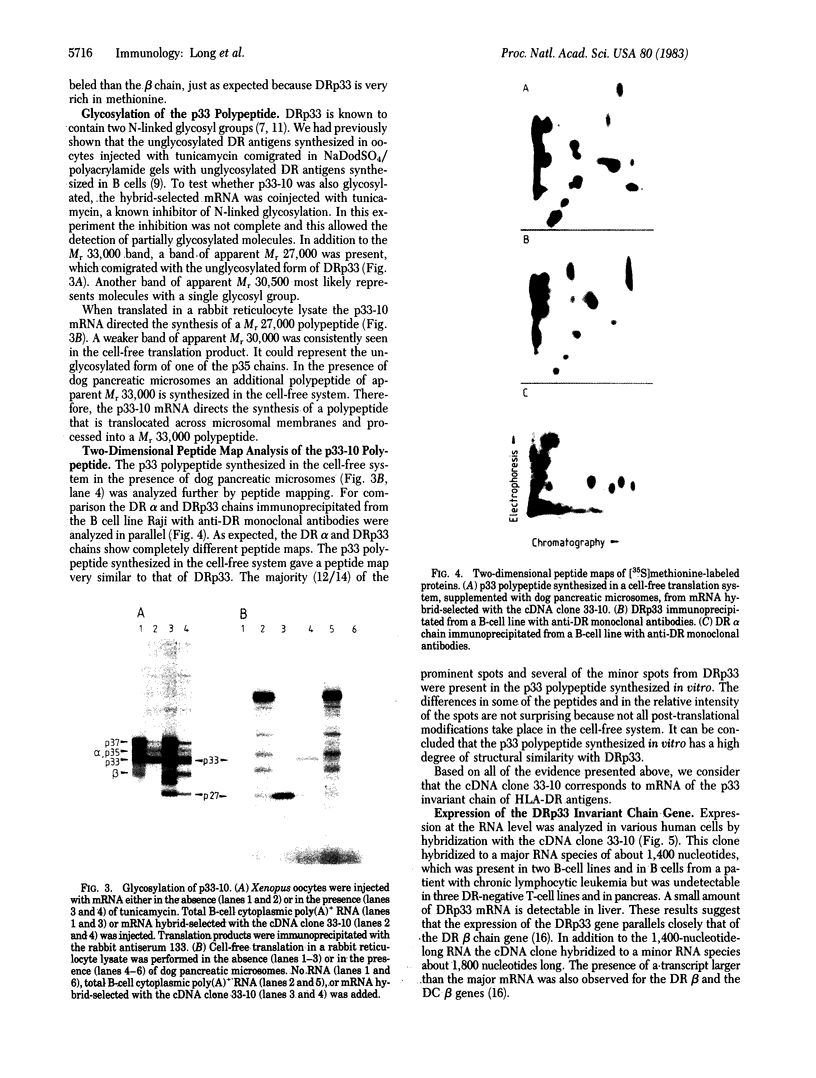
- Machamer C. E., Cresswell P. Biosynthesis and glycosylation of the invariant chain associated with HLA-DR antigens. J Immunol. 1982 Dec;129(6):2564–2569. [PubMed] [Google Scholar]
- McMillan M., Frelinger J. A., Jones P. P., Murphy D. B., McDevitt H. O., Hood L. Structure of murine Ia antigens. Two dimensional electrophoretic analyses and high pressure liquid chromatography tryptic peptide maps of products of the I-A and I-E subregions and of an associated invariant polypeptide. J Exp Med. 1981 Apr 1;153(4):936–950. doi: 10.1084/jem.153.4.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen M. J., Kissonerghis A. M., Lodish H. F., Crumpton M. J. Biosynthesis and maturation of HLA-DR antigens in vivo. J Biol Chem. 1981 Sep 10;256(17):8987–8993. [PubMed] [Google Scholar]
- Rabourdin-Combe C., Mach B. Expression of HLA-DR antigens at the surface of mouse L cells co-transfected with cloned human genes. Nature. 1983 Jun 23;303(5919):670–674. doi: 10.1038/303670a0. [DOI] [PubMed] [Google Scholar]
- Scheele G., Dobberstein B., Blobel G. Transfer of proteins across membranes, Biosynthesis in vitro of pretrypsinogen and trypsinogen by cell fractions of canine pancreas. Eur J Biochem. 1978 Jan 16;82(2):593–599. doi: 10.1111/j.1432-1033.1978.tb12055.x. [DOI] [PubMed] [Google Scholar]
- Shackelford D. A., Kaufman J. F., Korman A. J., Strominger J. L. HLA-DR antigens: structure, separation of subpopulations, gene cloning and function. Immunol Rev. 1982;66:133–187. doi: 10.1111/j.1600-065x.1982.tb00437.x. [DOI] [PubMed] [Google Scholar]
- Shackelford D. A., Strominger J. L. Demonstration of structural polymorphism among HLA-DR light chains by two-dimensional gel electrophoresis. J Exp Med. 1980 Jan 1;151(1):144–165. doi: 10.1084/jem.151.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowsdale J., Lee J., Carey J., Grosveld F., Bodmer J., Bodmer W. Sequences related to HLA-DR alpha chain on human chromosome 6: restriction enzyme polymorphism detected with DC alpha chain probes. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1972–1976. doi: 10.1073/pnas.80.7.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake C. T., Long E. O., Mach B. Allelic polymorphism and complexity of the genes for HLA-DR beta-chains--direct analysis by DNA-DNA hybridization. Nature. 1982 Nov 25;300(5890):372–374. doi: 10.1038/300372a0. [DOI] [PubMed] [Google Scholar]
- Wake C. T., Long E. O., Strubin M., Gross N., Accolla R., Carrel S., Mach B. Isolation of cDNA clones encoding HLA-DR alpha chains. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6979–6983. doi: 10.1073/pnas.79.22.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. Isolation and sequence organization of human ribosomal DNA. J Mol Biol. 1979 Mar 5;128(3):289–303. doi: 10.1016/0022-2836(79)90089-5. [DOI] [PubMed] [Google Scholar]