Abstract
Chlamydomonas reinhardtii adapts to copper deficiency by degrading apoplastocyanin and inducing Cyc6 and Cpx1 encoding cytochrome c6 and coproporphyrinogen oxidase, respectively. To identify other components in this pathway, colonies resulting from insertional mutagenesis were screened for copper- conditional phenotypes. Twelve crd (copper response defect) strains were identified. In copper-deficient conditions, the crd strains fail to accumulate photosystem I and light-harvesting complex I, and they contain reduced amounts of light-harvesting complex II. Cyc6, Cpx1 expression and plastocyanin accumulation remain copper responsive. The crd phenotype is rescued by a similar amount of copper as is required for repression of Cyc6 and Cpx1 and for maintenance of plastocyanin at its usual stoichiometry, suggesting that the affected gene is a target of the same signal transduction pathway. The crd strains represent alleles at a single locus, CRD1, which encodes a 47 kDa, hydrophilic protein with a consensus carboxylate-bridged di-iron binding site. Crd1 homologs are present in the genomes of photosynthetic organisms. In Chlamydomonas, Crd1 expression is activated in copper- or oxygen-deficient cells, and Crd1 function is required for adaptation to these conditions.
Keywords: di-iron enzyme/light-harvesting complex/oxygen/photosynthesis/plastocyanin
Introduction
Copper is an essential micronutrient because it functions as a cofactor in enzymes and electron transfer proteins that catalyze oxygen chemistry or redox reactions (e.g. cytochrome oxidase, lysyl oxidase and plastocyanin). Nevertheless, its reactivity in these types of reactions requires that its intracellular abundance be controlled by regulated uptake and distribution so that toxicity is avoided. Copper metabolism has been studied extensively in Saccharomyces cerevisiae, where copper resistance mechanisms involving metallothioneins, copper uptake mechanisms involving reductases and transporters, and intracellular copper delivery pathways to cytosolic Cu/Zn superoxide dismutase, the Fet3p multicopper oxidase in the plasma membrane and cytochrome oxidase in the mitochondrion, have been identified (reviewed by Zhou and Thiele, 1993; Askwith et al., 1994; Eide, 1998; Winge et al., 1998; Pena et al., 1999). In S.cerevisiae, genes encoding the high-affinity copper transporters Ctr1p and Ctr3p, and the copper reductases Fre1p and Fre7p are induced by the transcriptional activator Mac1p in response to copper deficiency (Jungmann et al., 1993; Georgatsou et al., 1997; Labbe et al., 1997; Martins et al., 1998). Conversely, when copper levels are high, a related protein, Ace1p, stimulates transcription of CUP1 and CRS5 encoding metallothioneins that sequester and detoxify excess copper (Thiele, 1988; Welch et al., 1989; Culotta et al., 1994). Copper homeostasis therefore involves a balance between the expression and function of the target genes of two transcription factors, Ace1p and Mac1p, and its purpose is to ensure an adequate but not excessive supply of intracellular copper for metabolic pathways requiring copper.
Much less is understood about the adaptation of copper-requiring metabolic pathways when intracellular copper is inadequate to saturate the biosynthesis of all copper proteins. Such a situation can occur when the nutritional supply of copper is too low (reviewed by Olivares and Uauy, 1996) or when a genetic defect prevents normal copper assimilation, as in Menkes’ disease (reviewed by Danks, 1995). An excellent model system for the study of this aspect of copper biology is Chlamydomonas reinhardtii. This organism, like many green algae and cyanobacteria, displays a highly regulated adaptive response to copper deficiency that includes compensatory modification of a major metabolic pathway (reviewed by Merchant, 1998b). Plastocyanin, present at a stoichiometry of ∼8 × 106 molecules per cell, is the most abundant copper protein in Chlamydomonas and in photosynthetic tissues of vascular plants (Merchant et al., 1991). Plastocyanin transfers electrons from the cytochrome (cyt) b6/f complex to photosystem I (PSI) and hence is an obligatory catalyst in photosynthesis (Gorman and Levine, 1965). Nevertheless, when C.reinhardtii and other algae/cyanobacteria face copper deficiency (<9 × 106 Cu ions/cell for Chlamydomonas) in nature or in the laboratory, they remain photosynthetically competent by replacing plastocyanin function with the heme protein cyt c6 (Wood, 1978; Ho et al., 1979).
The processes induced in copper-deficient C.reinhardtii cells are: transcription of Cyc6 (encoding cyt c6) and Cpx1 (encoding the tetrapyrrole biosynthesis enzyme coproporphyrinogen oxidase), degradation of apoplastocyanin and copper assimilation through a pathway involving a transporter and a cupric reductase (Hill and Merchant, 1995; Li and Merchant, 1995; Quinn and Merchant, 1995; Hill et al., 1996; Quinn et al., 1999). The induction of a transport pathway is precedented by the Mac1p-dependent induction of Ctr1p, Ctr3p, Fre1p and Fre7p in S.cerevisiae. The degradation of apoplastocyanin can be rationalized as a mechanism for ensuring re-distribution of copper from the photosynthetic apparatus to the respiratory apparatus in the mitochondrion. Activation of Cpx1 expression has been attributed to an increased demand for heme, the cofactor of cyt c6, in copper-deficient cells. The Cyc6 and Cpx1 genes are likely to be targets of the same copper-responsive signal transduction pathway (Quinn et al., 2000) and, based on the pattern of induction of the assimilatory pathway, it is proposed that so are the genes for the transporter and reductase (Hill et al., 1996). To identify these candidate target genes, and also to identify additional metabolic changes in copper-deficient cells, we sought a genetic approach to the study of copper deficiency in C.reinhardtii.
Results
The CRD1 locus is defined by a novel phenotype
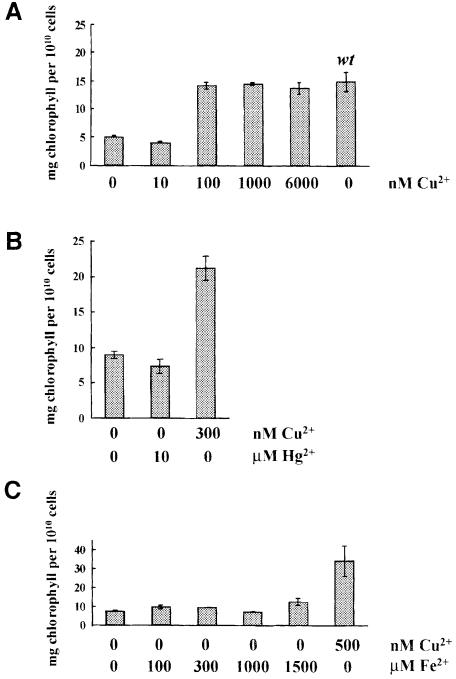
To identify copper response regulators and additional copper-responsive genes besides Cpx1 and Cyc6, insertionally mutagenized cells were screened for copper-conditional phenotypes with the expectation that growth phenotypes might correspond to strains carrying either regulatory mutations or mutations in copper assimilation, while a photosynthesis defect might correspond to a strain in which Cyc6 was disrupted. Either ble (Stevens et al., 1996) or Arg7 (Debuchy et al., 1989) DNA was used for transformation, and 7.5 × 103 zeocin-resistant and 4 × 103 arginine prototrophs were screened after three transfers to +Cu versus –Cu plates. Colonies displaying growth defects were identified as expected, but an unexpected phenotype was also noted at high frequency regardless of whether ble or Arg7 DNA was used for transformation. Approximately 1 in 1 × 103 transformants was yellow/pale-green in copper-deficient conditions but showed wild-type coloration under copper-supplemented conditions (Figure 1), suggesting that chlorophyll content was reduced. Indeed the strains contained less chlorophyll/cell when grown in copper-deficient conditions but as much as wild type when grown in the usual copper-supplemented medium (Figure 2A).
Fig. 1. Phenotype of crd1 strains. A representative tetrad from a cross of crd1-1::ARG7 to arg7 shows that progeny displaying the crd1 phenotype are arginine prototrophs. Cultures were grown for 8 days (21°C, 50 µmol/m2s constant light, 200 r.p.m.). Arginine supplementation (250 µg/ml) is indicated.
Fig. 2. Copper but not mercuric or iron salts rescues the chlorophyll deficiency in –Cu crd1 cells. (A) Erlenmeyer flasks (125 ml) containing 50 ml of copper-free TAP medium supplemented with CuSO4 as indicated were inoculated to an initial density of 1 × 105 cells/ml with copper-deficient crd1 cells, and grown for 5–9 days (21°C, 50 µmol/m2s constant light, 200 r.p.m.). The parental strain cc425 (arg7cw15), wild-type for CRD1, was grown the same way except that the culture was supplemented with 250 µg/ml arginine. Cultures were grown to a density of ∼5–10 × 106 cells/ml. (B) Fifty milliliter cultures of a cell-walled crd1-1 spore were grown for 7 days (–Cu TAP medium, 21°C, 50 µmol/m2s constant light, 200 r.p.m.). Cu or Hg salts were added as indicated and the cultures were returned to the same conditions for another 7 days, after which time cell density and chlorophyll concentrations were determined. (C) One hundred milliliter crd1-1 cultures were grown for 9 days at 24°C, ∼100 µmol/m2s, 250 r.p.m. with shaking, in copper-free TAP medium containing the indicated concentrations of additional FeSO4⋅EDTA or 500 nM CuSO4. The average of two samples from the culture is shown for each experiment. Copper-free TAP medium contains 18 µM FeSO4⋅EDTA, and ∼1 µM chelated iron is adequate for growth of wild-type copper-deficient cells. Thus, 1.5 mM represents a 103-fold excess.
Nine independently generated strains were tested for: (i) allelism with the reference allele, crd1-1::ARG7 (Table I); and (ii) linkage with the marker used for transformation. As no recombinants were found among the nine strains tested, we conclude that the mutants represent alleles at a single locus, named CRD1 for copper response defect. To examine linkage of the crd1 phenotype with Arg7 or ble as appropriate, crd1-1 was crossed to arg7, and the other eight crd1-2–crd1-9 strains were crossed to the wild-type strain CC124. All 19 crd1 progeny from the crd1-1×arg7 cross were arginine prototrophs, indicating that crd1-1 is tightly linked to ARG7. Similarly, all progeny from crosses of crd1-2, crd1-5, crd1-6 and crd1-7 with CC124 that displayed the mutant phenotype were also resistant to 10 µg/ml zeocin, demonstrating that crd1 is tightly linked to ble in these four strains. Thus, the CRD1 locus is tagged in five out of nine crd1 strains.
Table I. Tight linkage of independently generated crd1 mutants.
| crd1-1 | crd1-2 | crd1-3 | crd1-4 | crd1-5 | crd1-6 | crd1-7 | crd1-8 | crd1-9 | |
|---|---|---|---|---|---|---|---|---|---|
| Recombinants/complete tetrads or octets | 0/8 | 0/11 | 0/12 | 0/6 | 0/10 | 0/6 | 0/2 | 0/7 | 0/6 |
| Recombinants/total progeny | 0/36 | 0/44 | 0/48 | 0/42 | 0/70 | 0/46 | 0/33 | 0/53 | 0/44 |
| (zygotes) | (8) | (11) | (12) | (12) | (20) | (14) | (8) | (10) | (12) |
A mating type minus crd1-1 reference strain was crossed to mating type plus crd1 test strains. Zygotes were germinated and spores were transferred three times to –Cu TAP plates. Segregation of the crd1 phenotype was scored visually (chlorophyll deficiency) and by fluorescence. The first row shows the number of complete tetrads analyzed, while the second row shows the recombinants out of the total number of spores analyzed. The number of zygotes dissected is shown in parentheses.
The chlorophyll content in copper-deficient crd1 strains was only 30–40% of that of equivalently grown wild-type cells, but the chlorophyll a:b ratio was unchanged in –Cu crd1 cells (2.51) versus +Cu cells (2.47). Restoration of chlorophyll content to wild-type abundance required 100 nM CuSO4 (Figure 2A). At this cell density (∼1.5 × 107 cells/ml), a concentration of 100 nM corresponds to 4.1 × 106 Cu ions/cell, which is in the range required to repress Cyc6 and Cpx1 expression by 50% (4.5 × 106 Cu ions/cell) (Merchant et al., 1991; Hill and Merchant, 1995). This result indicated that the Crd1 gene product was required only when Cyc6 and Cpx1 were expressed, i.e. when the cells perceived a copper deficiency, and suggested that the expression of wild-type Crd1 might be regulated by copper—specifically, that it might be a target of the same signal transduction pathway as the one controlling Cyc6 and Cpx1 expression.
Mercuric ions at high concentrations can mimic copper ions in turning off the Cyc6 and Cpx1 genes (Hill et al., 1991; Quinn et al., 2000). If the phenotype resulted from a gain of function in copper deficiency or if expression of the phenotype required the activation of the copper deficiency pathway, it might be rescued by the addition of mercuric ions. However, addition of 10 µM HgCl2, which is enough to repress the Cyc6 and Cpx1 genes completely, did not restore chlorophyll accumulation in –Cu crd1 cultures (Figure 2B). It is rather more likely that the phenotype results from loss of a biochemical function that is required or becomes important only in copper-deficient cells.
Is the chlorotic phenotype a direct or indirect consequence of copper deficiency? We considered two possibilities: first, that the phenotype resulted from a secondary iron deficiency because iron-deficient C.reinhardtii cells are chlorotic (Weger, 1999); and, secondly, that the phenotype resulted from bleaching as a consequence of a photosynthesis defect (Spreitzer and Mets, 1981). In S.cerevisiae and also in humans, copper is required for iron assimilation because it is a cofactor in a multi-copper oxidase involved in iron transport and distribution (Askwith et al., 1994; Harris et al., 1995). Addition of up to 1.5 mM FeSO4 to –Cu cultures of crd1 cells does not restore chlorophyll accumulation, indicating that the loss of chlorophyll in crd1 strains is not a consequence of defective iron uptake (Figure 2C). The phenotype of crd1 strains was also tested in dim light (5 µmol/m2s) and in darkness. Under these conditions, both copper-deficient and copper-supplemented crd1 cells grew at equal rates, but the –Cu cultures still accumulated less chlorophyll (data not shown). At light intensities of 100 µmol/m2s, crd1 strains also showed a growth phenotype, indicating that the growth phenotype, but not the chlorophyll accumulation phenotype, is a consequence of photosensitivity.
A photosynthesis defect in crd1 strains in either copper- or oxygen-deficient medium
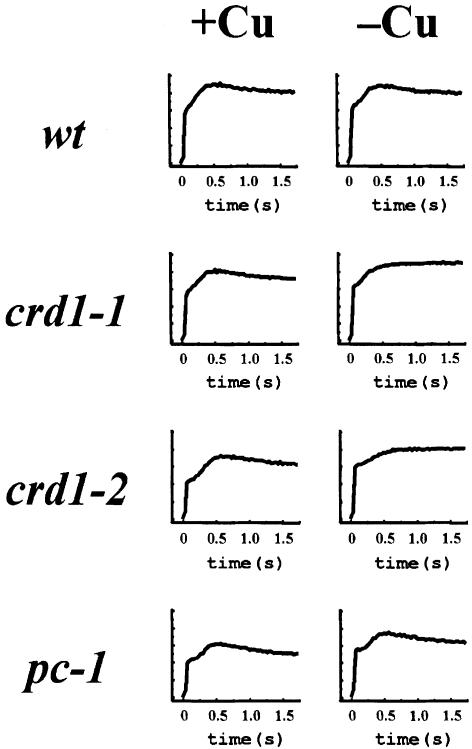
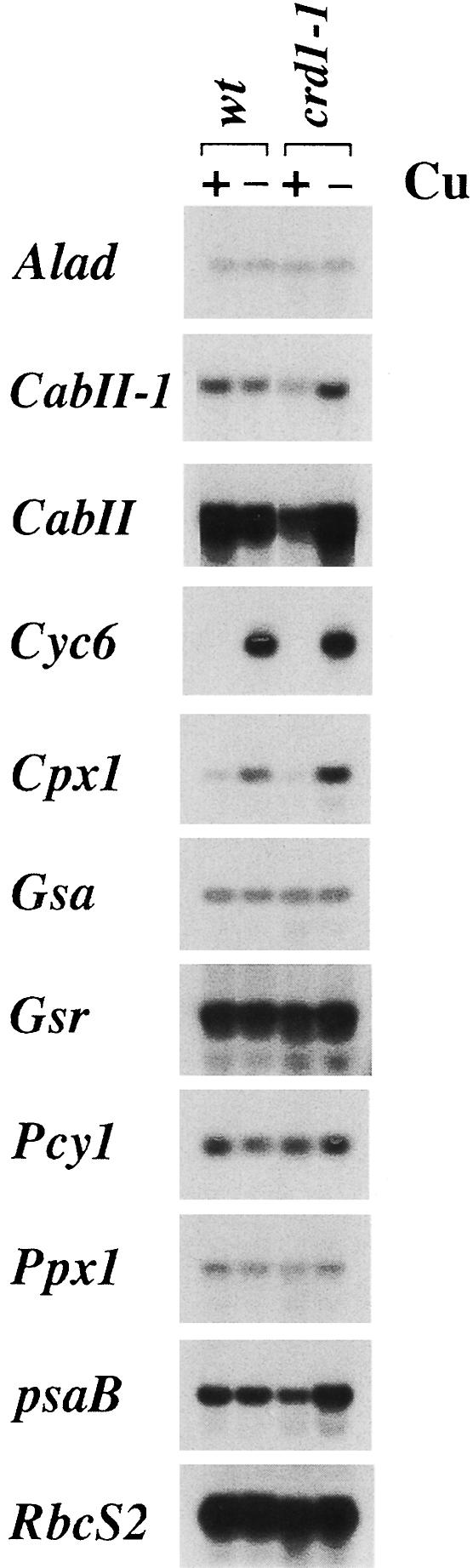
Photosynthetic function was examined by monitoring the fluorescence induction and decay kinetics of crd1 strains grown on +Cu versus –Cu medium (Figure 3). In the wild type, the transient rise phase reflects the reduction of a particular plastoquinone, QA, in PSII, while the decline phase reflects its reoxidation by downstream electron carriers. Wild-type cells display similar fluorescence in both +Cu and –Cu, but the decay phase is not observed in crd1 strains when they are grown on –Cu medium, indicating a defect downstream of PSII. Is the photosynthetic block attributable to a defect in chlorophyll accumulation? The pc-1 mutant that has lost the light-dependent protochlorophyllide reductase has a chlorophyll content similar to that of copper-deficient crd1 strains, ∼40% of the amount in wild type (Li and Timko, 1996). However, pc-1 displays wild-type fluorescence in both copper-supplemented and copper-deficient cells. The photosynthesis defect in –Cu crd1 cells is therefore a specific defect rather than a consequence of reduced chlorophyll content. We also did not find any alterations in the expression of a number of genes functioning in the tetrapyrrole biosynthetic pathway (Figure 6).
Fig. 3. crd1 strains display mutant fluorescence induction and decay kinetics. Fluorescence transients of cells grown on ±Cu TAP plates. Representative traces are shown from crd1-1 and crd1-2. wt = CC124. The pc-1 mutant lacks the light-dependent protochlorophyllide reductase.
Fig. 6. RNA blot analysis. Total RNA was isolated from wild-type or crd1 cells in medium containing copper or not (as indicated). A 10 µg aliquot of total RNA was loaded in each lane. Alad encodes aminolevulinic acid dehydratase, CabII encodes chlorophyll a/b-binding protein II; the CabII-1 probe is specific for the CabII-1 gene, while the CabII probe recognizes a family of similarly sized mRNAs; Cyc6 encodes cyt c6; Cpx1 encodes coprogen oxidase; Gsa encodes glutamyl-1-semialdehyde aminotransferase; Gsr encodes glutamyl-1-semialdehyde reductase; Pcy1 encodes plastocyanin; Ppx1 encodes protogen oxidase; psaB encodes photosystem I P700 apoprotein Ib; and RbcS2 encodes the Rubisco small subunit.
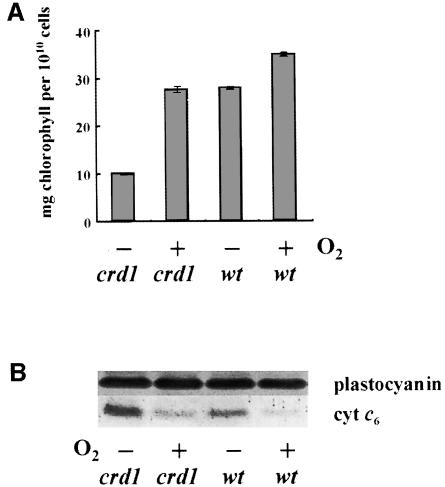
The Cyc6 and Cpx1 genes respond not only to copper deprivation but also to oxygen deprivation (Quinn et al., 2000). If Crd1 is a target of the same pathway, one might expect to induce the crd1 phenotype in oxygen-depleted cultures. Chlamydomonas reinhardtii cells grown in low light become oxygen depleted if they are kept suspended by low basal stirring instead of by vigorous aeration (Wood, 1978; J.Quinn, unpublished). Under these conditions, crd1 strains become chlorophyll deficient, and when such hypoxic cells are transferred to aerated medium, chlorophyll accumulation is restored, indicating that the wild-type Crd1 gene is also required in oxygen-deprived cells (Figure 4A). Induction of the crd1 phenotype was verified as being due to O2 depletion (as opposed to depletion of another gas such as CO2) by showing that bubbling with a mixture of 2% air, 2% CO2 in N2 also induces chlorosis (J.Quinn, unpublished).
Fig. 4. Hypoxic conditions mimic the crd1 phenotype. (A) Chlorophyll deficiency in oxygen-deprived crd1 cultures. +Cu TAP cultures (200 ml) of the arg7 (wt) strain and crd1-1 were grown on the benchtop in 250 ml Erlenmeyer flasks with a magnetic stir-bar revolving at <60 r.p.m. Cultures were inoculated to an initial density of 1 × 106 cells per ml, grown for 3 days and then re-inoculated into new medium. After another 3 days, cells from this second transfer were used to inoculate either a third round of cultures that were grown for 3 days with low basal stirring (–O2) or 100 ml cultures that were agitated vigorously at 250 r.p.m. (+O2). Cells were counted and chlorophyll content was determined. Similar results were obtained when cultures were O2 depleted by bubbling with 2% air, 2% CO2 in N2. (B) Immunoblot analysis for plastocyanin and cyt c6 accumulation.
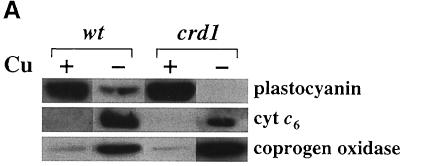
The copper-conditional block in electron transfer in crd1 cells suggested that cyt c6 synthesis or accumulation might be affected, although loss of function of the Cyc6 gene seemed unlikely because mutations affecting this step in photosynthetic electron transfer (as in pcy1 or ccs strains) do not result in such drastic reductions in chlorophyll content (Howe and Merchant, 1992; Li et al., 1996). Indeed the abundance and expression of cyt c6 and coprogen oxidase is normal in crd1 cells (Figures 4B and 5A). We conclude that the crd1 phenotype does not result from disruption of the Cyc6 gene or a putative regulator of copper-responsive expression.
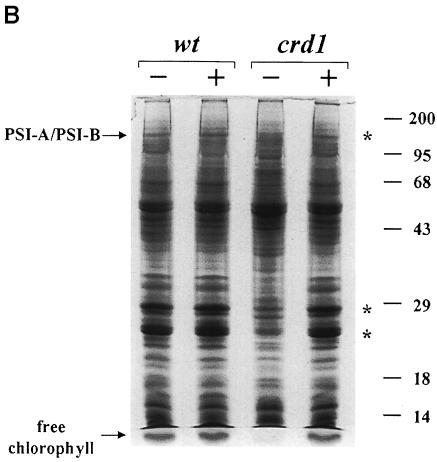
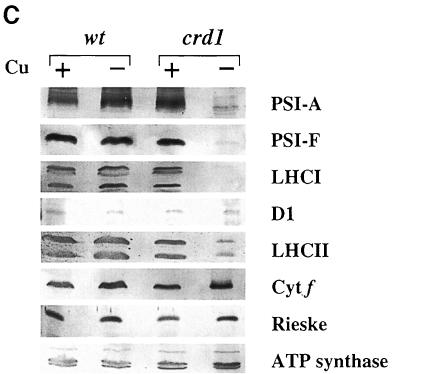
Fig. 5. The crd1 mutant shows (A) normal regulation of plastocyanin, cytochrome c6 and coprogen oxidase abundance but lacks PSI polypeptides (B and C). (A) Immunoblot analysis of cyt c6, plastocyanin and coprogen oxidase abundance. Liquid cultures in ±Cu TAP of a wild-type (cc425::ARG7) strain and crd1-1::ARG7 were grown for 8 days at 21°C, 200 r.p.m., ∼50 µmol/m2s constant light. Ten microliters of soluble protein extract from equal numbers of cells were separated by denaturing electrophoresis (15% acrylamide) and analyzed by immunoblotting. (B) Accumulation of chlorophyll-containing proteins in crd1 versus wild type. Total cell proteins were separated by denaturing electrophoresis (12% acrylamide) and stained with Coomassie Blue R250. Whole-cell extracts were made from equal numbers of cells, and samples were loaded without heating prior to electrophoresis to prevent dissociation of the PSI-A and PSI-B polypeptides. Asterisks indicate the positions of the green PSI and LHC subcomplexes prior to staining. Molecular weights (in kDa) are indicated. (C) Abundance of complex-specific proteins in crd1. Proteins in whole-cell extracts of a wild-type (arg7,Crd1) strain and a crd1-1 spore were separated by denaturing electrophoresis (12% acrylamide) and analyzed by immunoblotting. Whole-cell extracts were made from equal numbers of cells, and samples were boiled for 45 s prior to loading.
Gel electrophoretic analysis of total cellular protein showed that copper-deficient crd1 cells do not accumulate the PSI-A/PSI-B polypeptides of PSI and contain reduced amounts of light-harvesting complex I (LHCI) and LHCII polypeptides (Figure 5B). Immunoblot analysis confirmed that the abundance of PSI-A and PSI-F is drastically reduced in –Cu crd1 cells, as is also the case for the LHCI polypeptides between 20 and 30 kDa (Figure 5C). The major polypeptides of the LHCII trimeric complex accumulate to ∼10% of wild-type levels, accounting for the severe chlorophyll deficiency. On the other hand, the abundance of the D1 protein of PSII is only slightly reduced, and the accumulation of other photosynthetic complexes is normal (as gauged by the abundance of cyt f, Rieske iron–sulfur protein and the α- and β-subunits of ATP synthase). We conclude that the primary electron transfer defect in copper-deficient crd1 cells is attributable to a PSI deficiency. The unusual phenotype of crd1 strains points to a hitherto unrecognized role for copper in PSI maintenance. Since both nucleus- and plastid-encoded proteins are affected in –Cu crd1 cells, we suspect that the mutation affects photosynthetic complex accumulation at a post-transcriptional level. RNA blot analysis indicates that the expression of genes encoding LHC and PSI subunits is not reduced in –Cu crd1 relative to the wild type; in fact, for psaB and CabII, it is enhanced in the mutant (Figure 6).
Crd1 is a previously unknown protein found in photosynthetic organisms
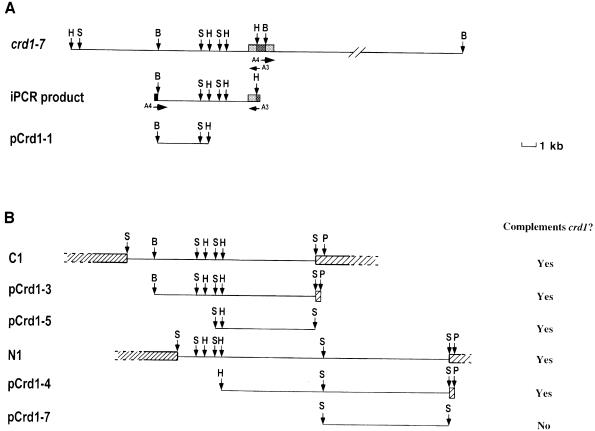
Since five of the nine well characterized crd1 strains appeared to be tagged, we sought to clone Crd1 based on its linkage to the marker genes. Southern analysis of genomic DNA from crd1-7 revealed that the ble gene is present on a 6.5 kb BamHI fragment in this strain (Figure 7A). This fragment was amplified by inverse PCR using divergent primers complementary to sequences within ble. Southern blots with the PCR product as a probe revealed restriction fragment length polymorphisms (RFLPs) between wild-type strains and several crd1 alleles, confirming that the amplified DNA represented sequences that closely flanked the Crd1 gene. A 3 kb DNA fragment of the amplified product was used to probe a wild-type genomic library. Three overlapping λ clones were identified, and each of these complemented the crd1 phenotype when introduced into the crd1-6 background (Figure 7B). A 5.8 kb SalI fragment of λ clone C1 was subcloned (pCrd1-5) and shown to be adequate for rescue of both the chlorophyll deficiency and the non-photosynthetic phenotype of crd1-6 (data not shown). This fragment therefore contained a functional Crd1 gene. A 1.2 kb PstI–XhoI fragment of pCrd1-5 was subsequently used to isolate two cDNA clones containing the entire Crd1 coding region from a –Cu cDNA library (Merchant and Bogorad, 1987a).
Fig. 7. (A) Physical map of the ble insertion in crd1-7 and diagram of the cloned flanking DNA. Probes from the ble gene and the RbcS2 3′-UTR from pSP109 were used to map restriction digests of genomic DNA from crd1-7. The divergent oligonucleotide primers A3 and A4 were used to amplify an ∼6.5 kb BamHI fragment containing the inserted ble gene. This fragment was digested with BamHI and HindIII and a 3 kb fragment was cloned into pBluescript KS+ to create plasmid pCrd1-1. B, BamHI; H, HindIII; S, SalI; medium gray boxes, RbcS2 sequences; dark gray boxes, ble sequences. (B) Genomic clones corresponding to Crd1. The 3 kb BamHI–HindIII insert in pCrd1-1 was used to probe a genomic DNA library. DNA from overlapping λ clones C1, I1 (not shown) and N1 that hybridized to the probe were isolated and subcloned into pBluescript KS+ to create plasmids pCrd1-3, pCrd1-4, pCrd1-5 and pCrd1-7. P, SspI. Hatched boxes, EMBL3 left and right arm sequences. λ clones C1, I1 and N1, and plasmids pCrd1-3, pCrd1-4, pCrd1-5 and pCrd1-7 were co-transformed by the glass bead method with pArg7.8 into an arg2crd1-6 strain and arginine prototrophic colonies were selected on –Cu TAP plates (Kindle, 1990). Colonies in which the crd1 phenotype was rescued were chosen visually and by fluorescence. The column on the right indicates whether or not the DNA fragment shown rescues the crd1 phenotype. ‘Yes’ indicates positive complementation, ‘No’ indicates that the fragment did not rescue.
The C.reinhardtii Crd1 gene encodes a 47.2 kDa soluble polypeptide in a 2.6 kb sequence (Figure 8). The protein occurs in the genomes of plants [>80 expressed sequence tags (ESTs) in >9 species], chromophytic algae, cyanobacteria, a prochlorophyte and a purple photosynthetic bacteria, but not so far in non-photosynthetic organisms (up to March 2000). Its function is not known. A homolog was identified originally as a phytochrome-regulated gene in Pharbitis nil (Zheng et al., 1998). A multiple alignment of the deduced Chlamydomonas protein and the candidate homologs demonstrates that the proteins are highly conserved, with 79% identity between the rice and Arabidopsis sequences, and 40–70% identity between all other pairs (Figure 9A).
Fig. 8. Crd1 genomic sequence. The genomic and cDNA Crd1 sequences have been submitted to the DDBJ/EMBL/GenBank database under accession Nos AF226628 and AF237671, respectively. A possible TATA box is indicated (single underline). A putative transcription start site (→) is suggested based on the 5′ UTR end of a Crd1 cDNA clone. Intron–exon boundaries are indicated by (↓). An asterisk denotes the stop codon, and the TGTAA polyadenylation signal (double underline) was found 15 bp upstream of the Crd1 cDNA poly(A) sequence.
Fig. 9. (A) Conservation of Crd1. The alignment was generated using the CLUSTALV algorithm and Lasergene software (DNASTAR). Residues that are similar in a majority (six) of sequences are shaded gray. Residues that are identical in all sequences are shaded black. The SRDEARHAG and WCQENRHGD motifs that are conserved in stearoyl-ACP desaturases are double-underlined and the prospective Fe-binding ligands are indicated by a downward arrow. The DDBJ/EMBL/GenBank accession numbers of the sequences from Oryza sativa, Arabidopsis thaliana, Porphyra purpurea, Cyanidium caldarium, Synechocystis sp. 6803 PNIL34, Synechocystis sp. 6803 AT103 and Rhodobacter sphaeroides are, AP000815, AF236101, U38804, AFO22186, D90899, D90912 and AF195122 respectively. Anabaena sp. PCC 7120 sequences were retrieved from the Cyanobase database at http://www.kazusa.or.jp/cyano/anabaena/. The Prochlorococcus marinus homolog was found at the Prochlorococcus sequencing homepage at http://bbrp.llnl.gov/jgi/microbial/prochlorococcus_homepage.html. The homolog from Pharbitis nil is not shown as we believe that there is a frameshift error in the 5′ end of the cDNA sequence (DDBJ/EMBL/GenBank accession No. U37437). This introduces a stop codon between the probable true initiator ATG and the next ATG in the sequence, resulting in elimination of the N-terminal leader sequence in the derived open reading frame. (B) The translated sequence of Cth1, a partial Chlamydomonas EST (DDBJ/EMBL/GenBank accession No. AV391947), is similar to the N-terminal region of Crd1.
Crd1 expression is activated in –Cu cells
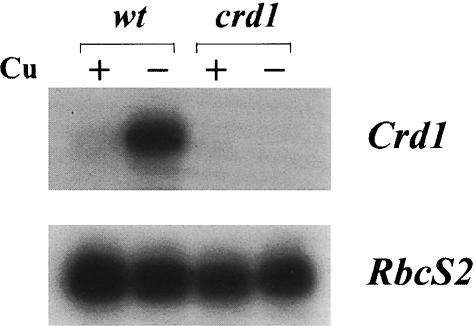
Is Crd1 expressed coordinately with Cyc6 and Cpx1? RNA blot analysis reveals that in +Cu wild-type cells, Crd1 mRNA accumulates at a low, basal level and is induced 15- to 20-fold in –Cu cells (Figure 10). Crd1 expression is activated similarly in oxygen-deficient cells (data not shown). As expected, Crd1 mRNA is not detected in the crd1 mutant. This induction of Crd1 in Cu- and O2-deficient cells suggests an increased requirement for the gene product under these conditions, consistent with the crd1 mutant phenotype.
Fig. 10. Crd1 expression is induced in –Cu. Total RNA was isolated from wild-type and crd1 +Cu and –Cu cells as indicated. An 8 µg aliquot of total RNA was loaded in each lane. A probe made from a Crd1 genomic sequence containing the 3′ UTR was used to detect the Crd1 message. The RbcS2 mRNA encoding the small subunit of Rubisco was used as a loading control.
Discussion
Copper-deficient cells require a new protein, Crd1, for normal photosynthesis. The unexpected phenotype of the crd1 strains reveals: (i) an unrecognized role for copper in photosynthetic metabolism; and (ii) an unrecognized biochemical function in maintenance of PSI and LHCs.
Crd1 and PSI
The phenotype of crd1 strains, namely a pleiotropic deficiency in PSI and light-harvesting proteins, should provide a clue to Crd1 function. For instance, Crd1 could be a novel subunit in a photosynthetic complex that is modified in response to copper and oxygen availability. This is precedented by the occurrence of alternative isoforms of respiratory complex subunits in Saccharomyces mitochondria (reviewed by Kwast et al., 1998). Different isoforms of cytochrome oxidase subunits (encoded by COX5a/COX5b) and cytochrome c (encoded by CYC1/CYC7) are expressed in oxygen-replete versus oxygen-deficient S.cerevisiae cells. A similar phenomenon occurs in response to iron deficiency in cyanobacteria, where the CP43′ polypeptide encoded by isiA is induced to high levels (Laudenbach and Straus, 1988; Burnap et al., 1993; Leonhardt and Straus, 1994; Vinnemeier et al., 1998). Interestingly, the organellar and prokaryotic Crd1 homologs are found in clusters of genes encoding reaction center proteins. Thus, Crd1 could be an alternative copper and oxygen deficiency version of a PSI or LHC subunit, and hence its absence in –Cu crd1 cells could result in destabilization of these complexes. There are numerous examples of multisubunit complexes that are degraded if one of the constituent polypeptides is not synthesized (reviewed by Wollman et al., 1999). However, we do not think that this is the situation here. The biosynthesis and maintenance of C.reinhardtii PSI is well characterized (reviewed by Webber and Bingham, 1998), and LHC deficiency has not been observed in strains carrying deletion mutations in the core polypeptides of PSI (Girard-Bascou et al., 1987; Takahashi et al., 1991). Likewise, mutants affected in LHC abundance do not show a linked PSI defect (Plumley and Schmidt, 1995). Finally, the protein and pigment compositions of the reaction center and light-harvesting apparatus differ significantly between purple photosynthetic bacteria and PSI in the oxygenic organisms (cyanobacteria, algae and plants), precluding a specific structural function for Crd1. Instead, it is more likely that Crd1 and its homologs function in a metabolic pathway that is common to each organism and is important for photosynthetic function.
The nature and severity of the crd1 phenotype are dependent on the growth conditions. For example, growth of –Cu crd1 strains at lower temperatures (18°C) partially relieves the chlorophyll deficiency, but does not improve PSI accumulation. In minimal medium the defect is also less pronounced; indeed crd1 cells do grow photoautotrophically (at 50–100 µmol/m2s), indicating that they must contain some PSI. Conversely, cells grown at higher temperatures (25°C) display chlorosis more readily, and the fluorescence transients indicate a reduction in PSII function as well (data not shown). The variation in phenotype can be attributed to the difference in growth rate, as if faster growth exacerbates the phenotype and slower growth ameliorates it. This result is compatible with a defect in cofactor metabolism in crd1. Perhaps the rate of cofactor production becomes more limiting when cell division is occurring more rapidly.
Crd1 function
What does the Crd1 sequence reveal about its function? Two of the most highly conserved sequences, WCQDENRHGD and SRDEARHAG, separated by ∼80 amino acids in the Crd1 proteins, are also present in plastid stearoyl-ACP desaturases (reviewed by Los and Murata, 1998; Shanklin and Cahoon, 1998) and a stearoyl-ACP desaturase from Burkholderia cepacia (Kang et al., 1998), respectively. These sequences contain consensus D/EExxH motifs for the carboxylate-liganded di-iron-binding class of enzyme. The prototypical members of this class are the acyl-ACP desaturases, the R2 subunit of ribonucleotide reductase and methane monooxygenase, and the characteristic feature of these enzymes is that the Fe ions are bridged by carboxylates and oxide or hydroxide ions. Two D/EExxH motifs, usually separated by ∼100 amino acids, provide two carboxylate and two imidazole ligands for the di-iron cluster. Di-iron enzymes catalyze oxidation of a substrate by activation of molecular oxygen at the di-iron cluster. They also require a source of electrons for reducing at least one of the oxygen atoms to water. For the plastid desaturases, reduced ferredoxin provides the electrons. In all Crd1 homologs, the D/EExxH motifs are separated by the right distance and occur in highly conserved regions of the proteins (Figure 9A). Therefore, we propose that Crd1 is a new member of the di-iron-carboxylate-liganded class of oxygen-dependent oxidases.
What might its substrate be? The best characterized plant di-iron enzymes are the plastid acyl-ACP desaturases, but we doubt that Crd1 is a fatty acid desaturase because there is no similarity between Crd1 and four different Chlamydomonas desaturases. Another possibility is that it might be involved in the biosynthesis of an as yet unidentified xanthophyll. This model is attractive because of the structural roles of carotenoids and xanthophylls in the photosystems and LHCs. However, a lor1 npq2 double mutant, which is blocked in the production of the most abundant xanthophylls (presumed precursors for other xanthophylls) (Niyogi et al., 1997, 1998), does not display –Cu induced chlorosis. Hence, the phenotype is unlikely to be caused by a xanthophyll deficiency. A third option is that Crd1 might be involved in some aspect of quinone modification or in generation of a radical in PSI. While we cannot rule out these models, they are inconsistent with the occurrence of Crd1 in Rhodobacter sphaeroides (which has a completely different complement of quinone cofactor and a different type of photosynthetic reaction center). A fourth possibility is that Crd1 function lies in metabolism of a metal cofactor. We favor this model and suggest further that this metal cofactor might be iron because the phenotype of crd1 strains is strikingly similar to that of iron-deficient cells (J.Moseley, unpublished). Although we could not rescue the crd1 strains by provision of extracellular iron, it is possible that the defect is compartment specific (i.e. in the plastid). Alternatively, the defect may not lie in supply but in mobilizing iron for biosynthesis of iron-containing proteins.
In this context, we propose that Crd1 and its homologs are plastid localized. In at least two eukaryotes, the homologous gene is plastid encoded, indicating that in those organisms the Crd1-like protein functions in the plastid. Examination of the C.reinhardtii and vascular plant sequences reveals an N-terminal extension relative to the cyanobacterial and plastid-encoded sequences, supporting the idea that the nucleus-encoded proteins may be targeted to an organelle. Characterization of the crd1 phenotype also suggests a plastid site of action. Specifically, we note reduced accumulation of both nucleus- and plastid-encoded gene products at a post-transcriptional level (Figures 5 and 6).
Response to copper and oxygen deficiency
The induction of cyt c6 in copper-deficient algal cells has been understood for over two decades as a back up mechanism to ensure photosynthetic function when plastocyanin cannot be synthesized (Wood, 1978; Merchant and Bogorad, 1987b). Molecular characterization of copper-deficient C.reinhardtii cells revealed a number of other responses that occur coordinately with activation of the Cyc6 gene, and these have each been rationalized as mechanisms for adaptation to copper deficiency (see Introduction). Recently, we noted that two of the targets of the signal transduction pathway, Cyc6 and Cpx1, are also induced in hypoxic cells (Quinn et al., 2000). The significance of the response to hypoxia is not well understood. One model, proposed initially by Wood (1978), is that oxygen-deficient cells become copper deficient in nature because of the reduced solubility of Cu(I) relative to Cu(II), and we have argued that the response to oxygen might be a way for the organism to anticipate and prepare for copper deficiency in the natural environment (Quinn et al., 2000). Nevertheless, in the laboratory, oxygen-deficient cultures are not copper deficient because they can accumulate holoplastocyanin and provision of excess copper does not repress the hypoxic response (Figure 4B; J.Quinn, unpublished), suggesting that the oxygen deficiency response pathway might operate in parallel to the copper deficiency response pathway rather than through it. The crd1 strains were identified on the basis of a copper-conditional phenotype, and this is phenocopied under hypoxia. This result provides the first indication of a physiological function of the oxygen-responsive signal transduction pathway. The fact that the crd1 mutant phenotype occurs in hypoxic cells tells us that the Crd1 gene product and hence gene expression is required for normal adaptation to O2 deprivation, whereas the induction of the Cyc6 and Cpx1 genes has not yet been shown to be necessary. Furthermore, it suggests a functional link between copper- and oxygen-responsive signal transduction.
How can we rationalize the requirement for Crd1 function in copper-deficient and oxygen-deficient cells? Crd1 expression resembles that of Cpx1, with a basal level of RNA accumulation in copper-supplemented cells and a 15- to 20-fold increase in copper and oxygen deficiency (Figure 10). One possibility is that there is an increased requirement for Crd1, an oxygen-utilizing enzyme, under oxygen-deficient conditions, as is the case for coprogen oxidase in S.cerevisiae (reviewed by Kwast et al., 1998). Alternatively, Crd1 could be the iron substitute for a copper enzyme (comparable to the situation where heme-containing cyt c6 replaces copper-containing plastocyanin). This idea is precedented by the reciprocal copper-responsive expression of iron- versus copper-containing methane monooxygenase in methanotrophic bacteria (Nielsen et al., 1997). The former is a di-iron enzyme while the latter is a multi-copper oxidase.
Crd1 distribution and expression
Crd1 homologs are found exclusively in photosynthetic organisms—plants, cyanobacteria, photosynthetic bacteria and algae (March 2000 database) (Figure 9A). A vascular plant homolog of Crd1, PNZIP, was identified originally as a phytochrome-responsive target gene. Its expression is under circadian control and is restricted to photosynthetic tissue (Zheng et al., 1998), but its function was not tested experimentally. Based on the distribution and expression of Crd1 homologs, their highly related sequences and the phenotype of crd1 mutants, we would argue that they all have a similar biochemical function, probably in assembly of components of the photosynthetic apparatus.
Interestingly, multiple homologs of Crd1 are found in C.reinhardtii, Synechocystis sp. and Anabaena sp. (∼50–65% identity for homologs in the same species); each of these organisms has a signal transduction pathway for responding to copper deficiency and each replaces plastocyanin with cyt c6 (reviewed by Merchant, 1998a). Possibly one copy of the gene has evolved a specialized pattern of expression that is important primarily for adaptation to copper deficiency, while the other has a so-called ‘housekeeping’ function similar to that of Crd1 homologs in vascular plants. A search of the CyanoMutants database for Synechocystis sp. knockouts indicates that one of the disrupted genes (DDBJ/EMBL/GenBank accession No. D90899) could not be segregated, which is consistent with the possibility that the gene might be essential, while no phenotype is reported for the disruption of the other gene (DDBJ/EMBL/GenBank accession No. D90912). Perhaps the latter gene encodes the functional equivalent of C.reinhardtii Crd1—the strain might well show a phenotype if tested in copper-deficient medium.
In Chlamydomonas, Crd1 is expressed at a very low level in cells grown in copper-supplemented acetate medium, but is highly induced in copper deficiency, consistent with the model that its function is more important when copper is limiting. Accordingly, the cells show a strong phenotype in –Cu medium. A Crd1 homolog, which we have called Cth1 (for copper-target homolog) was found in the Chlamydomonas EST database (Figure 9B, 47% nucleotide identity, 599 bp and 51% amino acid identity), but ESTs corresponding to Crd1 were not found. The ESTs represent sequences expressed under photoautotrophic, copper-supplemented conditions (Asamizu et al., 1999). Preliminary results indicate that expression of Cth1 is not activated in –Cu cells (J.Moseley, unpublished); perhaps Cth1 has the housekeeping function. The unique patterns of expression of Crd1 and Cth1 (which are suggestive of a different level of requirement for Crd1 and Cth1 function under various metabolic conditions) could well explain the variation in penetrance of the phenotype under different growth regimes. The Crd1/Cth1 gene pair in C.reinhardtii undoubtedly arose as a result of gene duplication, and disruption of Crd1 occurred at an unusually high frequency during our mutagenesis (1 per 1000 transformants), suggesting that recombination events are favored at the CRD1 locus. Perhaps in microorganisms faced with highly variable environments, possession of multiple Crd1 homologs has provided a means of adapting expression of the genes to different conditions.
Materials and methods
Strains and culture conditions
Mutants were generated in the background of Chlamydomonas strain CC425 (arg2, cw15) and crossed as described below. The preparation of copper-deficient (–Cu) media for culturing C.reinhardtii strains is described in Quinn and Merchant (1998). Cultures were transferred three times to –Cu medium from a +Cu inoculum in order to eliminate residual intracellular copper. Copper-supplemented medium was either prepared by the standard method (Harris, 1989) or copper salts were added to –Cu medium at the concentrations indicated. Iron was added from a 100 mM copper-free stock solution of chelated FeSO4 that was prepared by boiling 100 mM FeSO4 (Aldrich) with 134 mM EDTA in an acid-washed bottle. The pH was adjusted to 6.5 with KOH while maintaining the temperature above 70°C, and the solution was left at ∼20°C for 2–7 days prior to use. Mercuric salts were added from a stock solution (10 mM) to the final concentration indicated. Liquid cultures were grown usually in 125 or 250 ml Erlenmeyer flasks at 21–24°C with agitation at 200–250 r.p.m. and light intensity between 50 and100 µmol/m2s. Strains were cultured on plates at either 18 or 25°C at light intensities between 5 and 100 µmol/m2s. To generate O2-deprived cultures, 250 ml Erlenmeyer flasks filled with 200 ml of copper-supplemented TAP medium were inoculated to an initial cell density of 1 × 106 cells/ml and agitated by slow stirring on a magnetic stirring plate set at the lowest possible speed. Ambient temperature was 20–24°C and the light intensity was ∼10 µmol/m2s. Samples were collected for analysis after 3 days of growth.
Chlorophyll determination
Cells were collected from duplicate 1 ml aliquots of the culture by centrifugation (14 000 r.p.m. in a microcentrifuge), chlorophyll was extracted from the pellet into 80% acetone in methanol and its concentration was determined spectrophotometrically after removal of protein by centrifugation. Chlorophyll a:b ratios were determined according to the method of Porra et al. (1989).
Fluorescence measurements
A fluorescence imaging system built by Professor David Kramer at the Center for Advanced Biochemical Instrumentation Institute of Biological Chemistry (Washington State University, Pullman, WA) was used to measure the in vivo chlorophyll fluorescence induction kinetics of colonies. Prior to fluorescence measurements, cells grown on solid TAP medium were incubated overnight at 18°C at 10–25 µmol/m2s and dark adapted for at least 5 min. The colonies were illuminated for 1.73 s with 30 µmol/m2s of red light (640 nm) from light-emitting diodes (HLMP C116, Hewlett Packard) and the emitted fluorescence was captured immediately by a CCD camera (Cohu, 2122–1000) in conjunction with a PIXCI-SV4 imaging board and XCIP software (EPIX Incorporated, Buffalo Grove, IL).
Protein analysis
Samples from equal numbers of cells were prepared and analyzed for accumulation of plastocyanin, cyt c6 and coprogen oxidase as described previously (Quinn and Merchant, 1998; Quinn et al., 1999). Proteins were usually transferred to PVDF membranes for 1 h at 100 V in blotting buffer containing 20% methanol. The following solutions were used for processing the blots: wash, Tris-buffered saline with 0.2% Tween-20 (TBS-Tween); block, 30% calf serum in TBS-Tween, primary antibody in 10% calf serum in TBS-Tween. The antibodies were used at the following dilutions: anti-plastocyanin 1:10 000, anti-cyt c6 1:5000, anti-coprogen oxidase 1:3000 and horseradish peroxidase-conjugated goat-anti-rabbit IgG (Biorad) at 1:50 000. The blots were washed three times after each incubation. Bound antibody was detected with chemiluminescent reagents. For analysis of membrane proteins, samples were prepared similarly to the soluble proteins, except that whole cells were used and samples were only boiled for 45 s to avoid aggregation of the PSI core polypeptides. The separating gel contained 12% total acrylamide and the standard Tris-glycine blotting buffer contained 0.01% SDS in addition. Proteins were transferred to PVDF membranes for 2 h at 50 V. Blots were incubated overnight with primary antibodies as follows: 1:1000 anti-PSI-A, 1:1000 anti-PSI-F, 1:1000 anti-PSI/LHCI, 1:5000 anti-D1, 1:800 anti-LHCII, 1:1000 anti-cytochrome f, 1:1000 anti-Reiske iron–sulfur protein and 1:5000 anti-CF1. A 1:3000 dilution of alkaline phosphatase-conjugated goat anti-rabbit IgG (Southern Biotechnology Associates) was used as the secondary antibody, and bound antibody was detected using the alkaline phosphatase color reaction as described (Sambrook et al., 1989).
RNA blot analysis
RNA abundance was estimated by blot analysis as described previously (Quinn et al., 1999). For psaB, a 1 kb BamHI fragment from the EcoRI fragment of chloroplast DNA in plasmid p67A (Chlamydomonas Culture Collection) was used as the probe. The CabII coding probe and the CabII-1 3′-untranslated region (UTR) probe are described in Jacobshagen and Johnson (1994). For Cpx1, the probe was made from the 102 bp HindIII fragment from pCpx1.0 (Quinn et al., 1999). The Crd1 probe was made from a 1.2 kb PstI–XhoI fragment of pCrd1-5. The other probes are described in Quinn et al. (1999). The specific activities ranged from 5 × 108 to 7 × 108 c.p.m./µg DNA. Hybridization signals were visualized after exposure to Kodak X-OMAT Blue XB-1 film at –80°C with two intensifying screens (Alad, Cyc6, Cpx1, Crd1, Gsa, Gsr and Ppx1) or at room temperature without screens (psaB and RbcS2). Exposure times (Figure 6) were 2.5 h for Alad, Cyc6, Cpx1 and Gsa, 24 h for Gsr and Ppx1, 1 h for psaB and RbcS2, and (Figure 10) 16 h for Crd1 and 45 min for RbcS2.
Genetic analysis
Generation of gametes, matings and zygote dissections was performed as described by (Harris, 1989). For linkage analysis, at least six complete tetrads or 12 crd1 progeny were scored for segregation of the mutant phenotype with arginine prototrophy (crd1-1×arg7) or resistance to 10 µg/ml zeocin (Stevens et al., 1996) (crd1-2–crd1-9×CC124). Alleles crd1-2–crd1-9 were each crossed to crd1-1 to test for recombination.
Inverse PCR
Genomic DNA from strain crd1-7 was prepared by a modified miniprep procedure and 5 µg of DNA was digested [6–10 h, 37°C, 100 U of BamHI (NEB)]. The restriction enzyme was denatured (80°C, 20 min), and the DNA was ligated [overnight, 15°C, 450 µl volume containing 1× ligase buffer (Gibco-BRL), 1 mM ATP and 2 U of T4 DNA ligase (Gibco-BRL)]. The DNA was extracted with phenol–chloroform–isoamyl alcohol (25:24:1), chloroform, recovered by precipitation with ethanol and dissolved in 10 µl of water. A 6.5 kb inverse PCR product was obtained by amplification of 2 µl of ligated DNA with divergent primers A3 (GAAGTCGTCCTCCACGAAGT) and A4 (GACGTGACCCTGTTCATCAG) complementary to sequences within the ble gene (Stevens et al., 1996) and Pfx polymerase from Gibco-BRL.
Cloning and sequencing of Crd1
A 3 kb BamHI–HindIII fragment of the inverse PCR product was cloned into pBluescript KS– (Stratagene) and used to probe a Chlamydomonas genomic library in λEMBL3 by plaque hybridization. Three clones whose inserts contained overlapping DNA were obtained, each of which complemented the crd1 phenotype of an arg2crd1-6 strain when introduced into the nuclear genome along with the Arg7 gene by glass bead transformation. The inserts were subcloned into pBluescript KS+ (Stratagene) to generate plasmids pCrd1-3, pCrd1-4, pCrd1-5 and pCrd1-7 (Figure 7B), and the smallest complementing fragment was identified (pCrt1-5). The presence of introduced copies of Crd1 in rescued strains was verified by PCR amplification using primers P9 (CCGTTACAATTCCCTCCC) and P20 (AGCTCCTTGTACAGCAGG) within the Crd1 sequence that flank the site of ble insertion. The sequence of a 4.2 kb region within the 5.8 kb genomic DNA in pCrd1-5 spanning the Crd1 gene was determined on both strands, except for the last 75 bp, which were sequenced twice on one strand. A 1.2 kb PstI–XhoI fragment of pCrd1-5 was used to screen a Chlamydomonas –Cu cDNA library in λgt11 by plaque hybridization, and two Crd1 cDNA clones containing the entire coding sequence were isolated. The sequence of the gene product was deduced by alignment of the translated sequence with the sequences of candidate Crd1 homologs in the database, by prediction of intron–exon boundaries based on the Chlamydomonas gene consensus (Silflow, 1998) and by comparison of the genomic and cDNA sequences. The BLAST algorithm was used to search the nucleotide and protein databases, and sequence alignments were generated with CLUSTAL V in the Lasergene software package (DNASTAR).
Acknowledgments
Acknowledgements
We thank all the members of this laboratory for their input into the project: Ms Paola Barraco for her assistance in isolating mutants, Ms Rebecca Nelson for assistance in library screening, Ms Luisita Dolfini for assistance in sequencing and Dr Beth Dreyfuss for her comments on the manuscript. We also thank Drs Kevin Redding, Jean-David Rochaix, Stephen Mayfield, Catherine de Vitry and Roberto Bassi for their kind donation of antibodies, Dr Krishna Niyogi for providing the lor1 npq2 mutant strain and Dr Michael Goldschmidt-Clermont for the genomic library. This work was supported by the National Institutes of Health, GM 42143. J.M. was supported, in part, by a Dissertation Year Fellowship from the Graduate Division at UCLA and M.E. by a long-term EMBO fellowship.
References
- Asamizu E., Nakamura,Y., Sato,S., Fukuzawa,H. and Tabata,S. (1999) A large scale structural analysis of cDNAs in a unicellular green alga, Chlamydomonas reinhardtii. I. Generation of 3433 non-redundant expressed sequence tags. DNA Res., 6, 369–373. [DOI] [PubMed] [Google Scholar]
- Askwith C., Eide,D., Van Ho,A., Bernard,P.S., Li,L., Davis-Kaplan,S., Sipe,D.M. and Kaplan,J. (1994) The FET3 gene of S.cerevisiae encodes a multicopper oxidase required for ferrous iron uptake. Cell, 76, 403–410. [DOI] [PubMed] [Google Scholar]
- Burnap R.L., Troyan,T. and Sherman,L.A. (1993) The highly abundant chlorophyll–protein complex of iron-deficient Synechococcus sp. PCC7942 (CP43′) is encoded by the isiA gene. Plant Physiol., 103, 893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culotta V.C., Howard,W.R. and Liu,X.F. (1994) CRS5 encodes a metallothionein-like protein in Saccharomyces cerevisiae. J. Biol. Chem., 269, 25295–25302. [PubMed] [Google Scholar]
- Danks D.M. (1995) Disorders of copper transport. In Scriver,C.R. Beaudet,A.L., Sly,W.M. and Valle,D. (eds), The Metabolic and Molecular Basis of Inherited Disease. McGraw Hill, New York, NY, pp. 2211–2235. [Google Scholar]
- Debuchy R., Purton,S. and Rochaix,J.D. (1989) The argininosuccinate lyase gene of Chlamydomonas reinhardtii: an important tool for nuclear transformation and for correlating the genetic and molecular maps of the ARG7 locus. EMBO J., 8, 2803–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide D.J. (1998) The molecular biology of metal ion transport in Saccharomyces cerevisiae. Annu. Rev. Nutr., 18, 441–469. [DOI] [PubMed] [Google Scholar]
- Georgatsou E., Mavrogiannis,L.A., Fragiadakis,G.S. and Alexandraki,D. (1997) The yeast Fre1p/Fre2p cupric reductases facilitate copper uptake and are regulated by the copper-modulated Mac1p activator. J. Biol. Chem., 272, 13786–13792. [DOI] [PubMed] [Google Scholar]
- Girard-Bascou J., Choquet,Y., Schneider,M., Delosme,M. and Dron,M. (1987) Characterization of a chloroplast mutation in the psaA2 gene of Chlamydomonas reinhardtii. Curr. Genet., 12, 489–495. [DOI] [PubMed] [Google Scholar]
- Gorman D.S. and Levine,R.P. (1965) Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc. Natl Acad. Sci. USA, 54, 1665–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E.H. (1989) The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use. Academic Press, San Diego, CA. [DOI] [PubMed] [Google Scholar]
- Harris Z.L., Takahashi,Y., Miyajima,H., Serizawa,M., MacGillivray,R.T. and Gitlin,J.D. (1995) Aceruloplasminemia: molecular characterization of this disorder of iron metabolism. Proc. Natl Acad. Sci. USA, 92, 2539–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K.L. and Merchant,S. (1995) Coordinate expression of coproporphyrinogen oxidase and cytochrome c6 in the green alga Chlamydomonas reinhardtii in response to changes in copper availability. EMBO J., 14, 857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K.L., Li,H.H., Singer,J. and Merchant,S. (1991) Isolation and structural characterization of the Chlamydomonas reinhardtii gene for cytochrome c6. Analysis of the kinetics and metal specificity of its copper-responsive expression. J. Biol. Chem., 266, 15060–15067. [PubMed] [Google Scholar]
- Hill K.L., Hassett,R., Kosman,D. and Merchant,S. (1996) Regulated copper uptake in Chlamydomonas reinhardtii in response to copper availability. Plant Physiol., 112, 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho K.K., Ulrich,E.L., Krogmann,D.W. and Gomez-Lojero,C. (1979) Isolation of photosynthetic catalysts from cyanobacteria. Biochim. Biophys. Acta, 545, 236–248. [DOI] [PubMed] [Google Scholar]
- Howe G. and Merchant,S. (1992) The biosynthesis of membrane and soluble plastidic c-type cytochromes of Chlamydomonas reinhardtii is dependent on multiple common gene products. EMBO J., 11, 2789–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobshagen S. and Johnson,C.H. (1994) Circadian rhythms of gene expression in Chlamydomonas reinhardtii: circadian cycling of mRNA abundances of cab II and possibly of β-tubulin and cytochrome c. Eur. J. Cell Biol., 64, 142–152. [PubMed] [Google Scholar]
- Jungmann J., Reins,H.A., Lee,J., Romeo,A., Hassett,R., Kosman,D. and Jentsch,S. (1993) MAC1, a nuclear regulatory protein related to Cu-dependent transcription factors is involved in Cu/Fe utilization and stress resistance in yeast. EMBO J., 12, 5051–5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y., Carlson,R., Tharpe,W. and Schell,M.A. (1998) Characterization of genes involved in biosynthesis of a novel antibiotic from Burkholderia cepacia BC11 and their role in biological control of Rhizoctonia solani. Appl. Environ. Microbiol., 64, 3939–3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindle K.L. (1990) High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc. Natl Acad. Sci. USA, 87, 1228–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwast K.E., Burke,P.V. and Poyton,R.O. (1998) Oxygen sensing and the transcriptional regulation of oxygen-responsive genes in yeast. J. Exp. Biol., 201, 1177–1195. [DOI] [PubMed] [Google Scholar]
- Labbe S., Zhu,Z. and Thiele,D.J. (1997) Copper-specific transcriptional repression of yeast genes encoding critical components in the copper transport pathway. J. Biol. Chem., 272, 15951–15958. [DOI] [PubMed] [Google Scholar]
- Laudenbach D.E. and Straus,N.A. (1988) Characterization of a cyanobacterial iron stress-induced gene similar to psbC. J. Bacteriol., 170, 5018–5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt K. and Straus,N.A. (1994) Photosystem II genes isiA, psbDI and psbC in Anabaena sp. PCC 7120: cloning, sequencing and the transcriptional regulation in iron-stressed and iron-repleted cells. Plant Mol. Biol., 24, 63–73. [DOI] [PubMed] [Google Scholar]
- Li H.H. and Merchant,S. (1995) Degradation of plastocyanin in copper-deficient Chlamydomonas reinhardtii. J. Biol. Chem., 270, 23504–23510. [DOI] [PubMed] [Google Scholar]
- Li H.H., Quinn,J., Culler,D., Girard-Bascou,J. and Merchant,S. (1996) Molecular genetic analysis of plastocyanin biosynthesis in Chlamydomonas reinhardtii. J. Biol. Chem., 271, 31283–31289. [DOI] [PubMed] [Google Scholar]
- Li J. and Timko,M.P. (1996) The pc-1 phenotype of Chlamydomonas reinhardtii results from a deletion mutation in the nuclear gene for NADPH:protochlorophyllide oxidoreductase. Plant Mol. Biol., 30, 15–37. [DOI] [PubMed] [Google Scholar]
- Los D.A. and Murata,N. (1998) Structure and expression of fatty acid desaturases. Biochim. Biophys. Acta, 1394, 3–15. [DOI] [PubMed] [Google Scholar]
- Martins L.J., Jensen,L.T., Simon,J.R., Keller,G.L., Winge,D.R. and Simons,J.R. (1998) Metalloregulation of FRE1 and FRE2 homologs in Saccharomyces cerevisiae. J. Biol. Chem., 273, 23716–23721. [DOI] [PubMed] [Google Scholar]
- Merchant S. (1998a) Reciprocal, copper-responsive accumulation of plastocyanin and cytochrome c6 in algae and cyanobacteria: a model for metalloregulation of metalloprotein synthesis. In Silver,S. and Walden,W. (eds), Metal Ions in Gene Regulation. Chapman & Hall, New York, NY, pp. 450–467. [Google Scholar]
- Merchant S. (1998b) Synthesis of metalloproteins involved in photosynthesis: plastocyanin and cytochromes. In Rochaix,J.-D., Goldschmidt-Clermont,M. and Merchant,S. (eds), The Molecular Biology of Chloroplasts and Mitochondria in Chlamydomonas. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp. 597–611. [Google Scholar]
- Merchant S. and Bogorad,L. (1987a) The Cu (II)-repressible plastidic cytochrome c. Cloning and sequence of a complementary DNA for the pre-apoprotein. J. Biol. Chem., 262, 9062–9067. [PubMed] [Google Scholar]
- Merchant S. and Bogorad,L. (1987b) Metal ion regulated gene expression: use of a plastocyanin-less mutant of Chlamydomonas reinhardtii to study the Cu(II)-dependent expression of cytochrome c-552. EMBO J., 6, 2531–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant S., Hill,K. and Howe,G. (1991) Dynamic interplay between two copper-titrating components in the transcriptional regulation of cyt c6. EMBO J., 10, 1383–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen A.K., Gerdes,K. and Murrell,J.C. (1997) Copper-dependent reciprocal transcriptional regulation of methane monooxygenase genes in Methylococcus capsulatus and Methylosinus trichosporium. Mol. Microbiol., 25, 399–409. [DOI] [PubMed] [Google Scholar]
- Niyogi K.K., Björkman,O. and Grossman,A.R. (1997) The roles of specific xanthophylls in photoprotection. Proc. Natl Acad. Sci. USA, 94, 14162–14167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi K.K., Grossman,A.R. and Björkman,O. (1998) Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell, 10, 1121–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares M. and Uauy,R. (1996) Copper as an essential nutrient. Am. J. Clin. Nutr., 63, 791S–796S. [DOI] [PubMed] [Google Scholar]
- Pena M.M.O., Lee,J. and Thiele,D.J. (1999) A delicate balance: homeostatic control of copper uptake and distribution. J. Nutr., 129, 1251–1260. [DOI] [PubMed] [Google Scholar]
- Plumley F.G. and Schmidt,G.W. (1995) Light-harvesting chlorophyll a/b complexes: interdependent pigment synthesis and protein assembly. Plant Cell, 7, 689–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra R.J., Thompson,W.A. and Kriedmann,P.E. (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentrantion of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta, 975, 384–394. [Google Scholar]
- Quinn J.M. and Merchant,S. (1995) Two copper-responsive elements associated with the Chlamydomonas Cyc6 gene function as targets for transcriptional activators. Plant Cell, 7, 623–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn J.M. and Merchant,S. (1998) Copper-responsive gene expression during adaptation to copper deficiency. Methods Enzymol., 297, 263–279. [DOI] [PubMed] [Google Scholar]
- Quinn J.M., Nakamoto,S.S. and Merchant,S. (1999) Induction of coproporphyrinogen oxidase in Chlamydomonas chloroplasts occurs via transcriptional regulation of Cpx1 mediated by copper response elements and increased translation from a copper deficiency-specific form of the transcript. J. Biol. Chem., 274, 14444–14454. [DOI] [PubMed] [Google Scholar]
- Quinn J.M., Barraco,P., Eriksson,M. and Merchant,S. (2000) Coordinate copper- and oxygen-responsive Cyc6 and Cpx1 expression in Chlamydomonas is mediated by the same element. J. Biol. Chem., 275, 6080–6089. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Shanklin J. and Cahoon,E.B. (1998) Desaturation and related modifications of fatty acids. Annu. Rev. Plant Physiol. Plant Mol. Biol., 49, 611–641. [DOI] [PubMed] [Google Scholar]
- Silflow C.D. (1998) Organization of the nuclear genome. In Rochaix,J.-D., Goldschmidt-Clermont,M. and Merchant,S. (eds), The Molecular Biology of Chloroplasts and Mitochondria in Chlamydomonas. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp. 25–40. [Google Scholar]
- Spreitzer R.J. and Mets,L. (1981) Photosynthesis-deficient mutants of Chlamydomonas reinhardtii with associated light-sensitive phenotypes. Plant Physiol., 67, 565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens D.R., Rochaix,J.D. and Purton,S. (1996) The bacterial phleomycin resistance gene ble as a dominant selectable marker in Chlamydomonas. Mol. Gen. Genet., 251, 23–30. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Goldschmidt-Clermont,M., Soen,S.Y., Franzen,L.G. and Rochaix,J.D. (1991) Directed chloroplast transformation in Chlamydomonas reinhardtii: insertional inactivation of the psaC gene encoding the iron sulfur protein destabilizes photosystem I. EMBO J., 10, 2033–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele D.J. (1988) ACE1 regulates expression of the Saccharomyces cerevisiae metallothionein gene. Mol. Cell. Biol., 8, 2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinnemeier J., Kunert,A. and Hagemann,M. (1998) Transcriptional analysis of the isiAB operon in salt-stressed cells of the cyanobacterium Synechocystis sp. PCC 6803. FEMS Microbiol. Lett., 169, 323–330. [DOI] [PubMed] [Google Scholar]
- Webber A.N. and Bingham,S.E. (1998) Structure and function of photosystem I. In Rochaix,J.-D., Goldschmidt-Clermont,M. and Merchant,S. (eds), The Molecular Biology of Chloroplasts and Mitochondria in Chlamydomonas. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp. 323–348. [Google Scholar]
- Weger H.G. (1999) Ferric and cupric reductase activities in the green alga Chlamydomonas reinhardtii: experiments using iron-limited chemostats. Planta, 207, 377–384. [Google Scholar]
- Welch J., Fogel,S., Buchman,C. and Karin,M. (1989) The CUP2 gene product regulates the expression of the CUP1 gene, coding for yeast metallothionein. EMBO J., 8, 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winge D.R., Jensen,L.T. and Srinivasan,C. (1998) Metal-ion regulation of gene expression in yeast. Curr. Opin. Chem. Biol., 2, 216–221. [DOI] [PubMed] [Google Scholar]
- Wollman F.-A., Minai,L. and Nechushtai,R. (1999) The biogenesis and assembly of photosynthetic proteins in thylakoid membranes. Biochim. Biophys. Acta, 1411, 21–85. [DOI] [PubMed] [Google Scholar]
- Wood P.M. (1978) Interchangeable copper and iron proteins in algal photosynthesis. Studies on plastocyanin and cytochrome c-552 in Chlamydomonas. Eur. J. Biochem., 87, 9–19. [DOI] [PubMed] [Google Scholar]
- Zheng C.C., Porat,R., Lu,P. and O’Neill,S.D. (1998) PNZIP is a novel mesophyll-specific cDNA that is regulated by phytochrome and a circadian rhythm and encodes a protein with a leucine zipper motif. Plant Physiol., 116, 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P. and Thiele,D.J. (1993) Copper and gene regulation in yeast. Biofactors, 4, 105–115. [PubMed] [Google Scholar]