Abstract
The secreton (type II secretion) and type IV pilus biogenesis branches of the general secretory pathway in Gram-negative bacteria share many features that suggest a common evolutionary origin. Five components of the secreton, the pseudopilins, are similar to subunits of type IV pili. Here, we report that when the 15 genes encoding the pullulanase secreton of Klebsiella oxytoca were expressed on a high copy number plasmid in Escherichia coli, one pseudopilin, PulG, was assembled into pilus-like bundles. Assembly of the ‘secreton pilus’ required most but not all of the secreton components that are essential for pullulanase secretion, including some with no known homologues in type IV piliation machineries. Two other pseudopilins, pullulanase and two outer membrane-associated secreton components were not associated with pili. Thus, PulG is probably the major component of the pilus. Expression of a type IV pilin gene, the E.coli K-12 gene ppdD, led to secreton-dependent incorporation of PpdD pilin into pili without diminishing pullulanase secretion. This is the first demonstration that pseudopilins can be assembled into pilus-like structures.
Keywords: general secretory pathway/pili/protein secretion/secretin/secreton
Introduction
The general secretory pathway (GSP), which is widespread among Gram-negative bacteria, permits proteins to cross first the cytoplasmic membrane, via the Sec system, and then the outer membrane, via specific terminal branches. The secreton (type II secretory machinery) and type IV pilus biogenesis pathways are examples of GSP terminal branches that share up to 10 homologous proteins, suggesting a common evolutionary origin (Hobbs and Mattick, 1993; Nunn, 1999). In particular, five components of the secreton, the pseudopilins, are homologous to type IV pilins (Bleves et al., 1998; Pugsley, 1993b). The homology between type IV pilins and the pseudopilins is restricted, however, to the N-terminal 30 hydrophobic amino acids that interact to enable the pilus to assemble (Parge et al., 1995). In addition, type IV pilins have two cysteines that form an intramolecular disulfide bridge, four of the pseudopilins (G, H, I and J) lack cysteine residues (Reyss and Pugsley, 1990). Type IV pilins and pseudopilins are processed and N-methylated at their N-terminal ends by the same prepilin peptidase (Nunn and Lory, 1991, 1992; Pugsley, 1993b; Strom et al., 1993). In Pseudomonas aeruginosa, where the secreton and type IV piliation pathways coexist, PilA, the most abundant pilin, can be cross-linked to the pseudopilins and is required for efficient secretion (Lu et al., 1997). Thus, the secreton and the piliation machinery are intimately related and might have overlapping functions.
The role of pseudopilins in secretion is obscure, although they are not involved in the recognition of secreted proteins (Lindeberg et al., 1996; Possot et al., 2000). Pseudopilins may form a pseudopilus that links the cytoplasmic and outer membranes to provide a scaffold for the assembly of other secreton components (Pugsley, 1993a) or to drive secretion (Hobbs and Mattick, 1993). However, there is no direct evidence for the existence of such a structure (Pugsley and Possot, 1993; Pugsley, 1996) and only limited evidence for any interactions between pseudopilins and other secreton components (Kagami et al., 1998; Possot et al., 2000).
Here we investigate the possibility that pseudopilins could assemble into pilus-like structures on the surface of Escherichia coli K-12 cells producing the pullulanase (Pul) secreton of Klebsiella oxytoca (d’Enfert et al., 1987b). The Pul secreton is composed of a maximum of 15 Pul proteins (PulB, PulC–O and PulS), of which 12 are needed for pullulanase A (PulA) secretion in E.coli K-12 (PulB, PulH and PulN are not essential; Possot et al., 2000). Of the five pseudopilins, PulG, PulH, PulI, PulJ and PulK, PulG is the most abundant (Reyss and Pugsley, 1990). By analogy with P.aeruginosa type IV pili, in which the major pilin is the main component but whose biogenesis requires minor pilins (Russel and Darzins, 1994; Alm and Mattick, 1995, 1997), PulG is likely to be the major component of such a structure. This possibility was tested by electron microscopy (EM) and by a shearing technique that removes appendages from the cell surface.
Results
PulG forms pilus-like structures
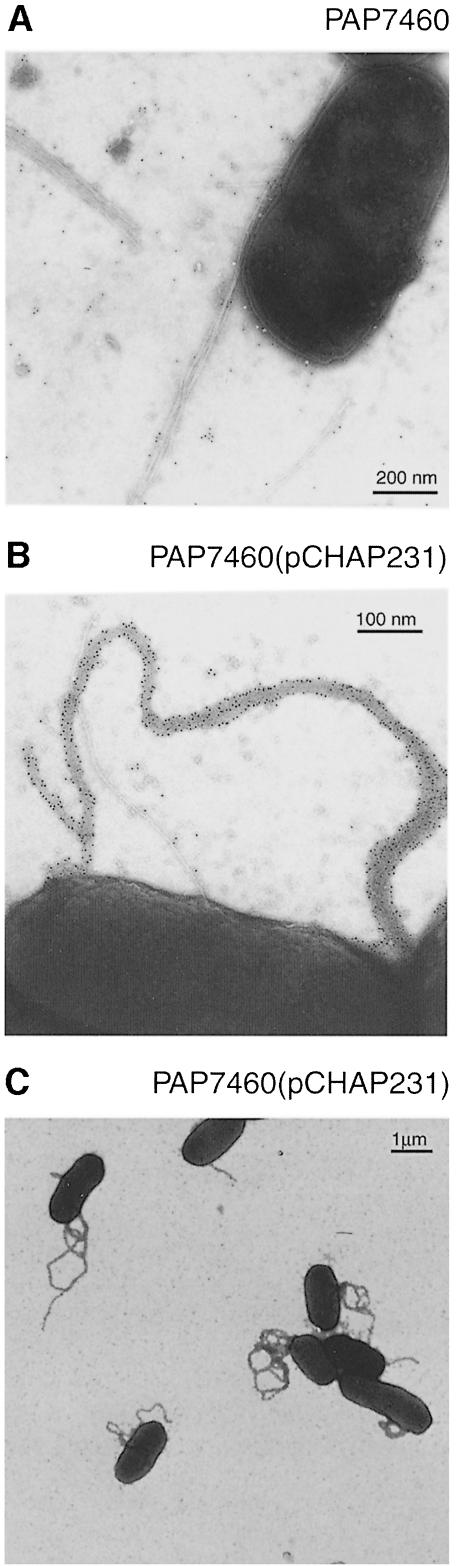
To test whether PulG could be assembled into pilus-like structures, we used E.coli K-12 carrying a multiple copy number plasmid, pCHAP231 (d’Enfert et al., 1987b) encoding all Pul proteins, including the secreted protein PulA. The bacteria harvested from agar plates were immunogold-labelled with PulG antibodies and observed by EM (Sauvonnet et al., 2000). Gold-coated pili were not observed in control cells (Figure 1A), whereas labelled appendages were clearly visible on the surface of bacteria carrying pCHAP231 (Figure 1B), indicating that PulG is incorporated into pili on the cell surface. These pili were organized in a network, resulting in broad fibres (∼15–20 nm thick) resembling bundled pili seen in enteropathogenic E.coli (Bieber et al., 1998) and Aeromonas (Kirov et al., 1999). All of the bacteria appeared to have at least one bundled pilus (Figure 1C) and individual labelled filaments were never observed. Pili were not observed on the surfaces of bacteria grown in shaken flask cultures used to measure pullulanase secretion (Pugsley et al., 1990). However, 80–100% of the pullulanase produced by plate-grown bacteria carrying pCHAP231 was accessible to substrate (pullulan) (Michaelis et al., 1985), indicating that they are fully secretion-proficient. Furthermore, immunogold EM revealed that plate-grown bacteria were covered with pullulanase, as reported previously for broth-grown bacteria (d’Enfert et al., 1987b; Pugsley et al., 1990) but pullulanase was not found associated with the pili (not shown).
Fig. 1. EM analysis of bacteria immunogold-labelled with antibodies raised against PulG. (A) Escherichia coli K-12 strain, PAP7460 (without Pul secreton); (B and C) strain PAP7460 (pCHAP231) (producing Pul secreton). Besides the labelled secreton pili (B), unlabelled type I pili are seen on the surfaces of both strains.
Large amounts (20–30%) of PulG from plate-grown bacteria carrying pCHAP231 could be released by shearing (Figure 2), a method commonly used to release surface-associated proteins (Nunn et al., 1990). The major integral outer membrane protein LamB was not present in the sheared fraction (not shown), indicating that PulG release was not due to membrane perturbation. Indeed, PulG seemed to be only weakly attached to the cell surface because large amounts could be released merely by resuspending bacteria harvested from the plates. Immunoblotting experiments revealed that two other, less abundant pseudopilins, PulI and PulK, were not present in the sheared fraction, although they could be detected in the sheared bacteria. Furthermore, two other secreton components, PulC (Possot et al., 1999) and PulD (the outer membrane-associated secretin; Hardie et al., 1996a; Nouwen et al., 1999) were also not released by shearing (not shown). Therefore, PulG is likely to be the major component of the pilus.
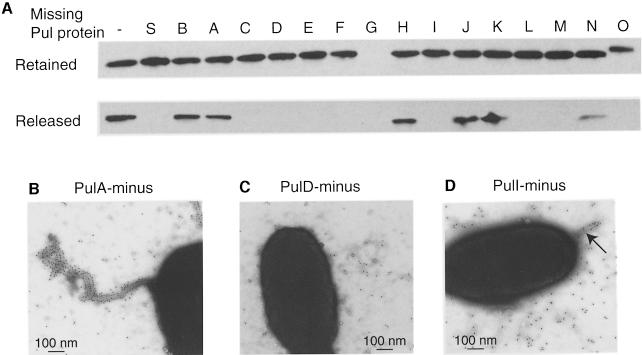
Fig. 2. PulG needs a functional secreton to form pili. (A) SDS--PAGE and immunoblot analysis of PulG released by shearing of E.coli PAP7460 bearing derivatives of pCHAP231 mutated in the pul gene as indicated. All fractions loaded were derived from the same volume of bacterial suspension. (B–D) EM analysis of PAP7460 bearing pCHAP1218, encoding all Pul proteins except PulA (B), pCHAP1226 (lacking pulD) (C) and pCHAP1357 (lacking pulI) (D). The bacteria were immunogold-labelled with anti-PulG antibodies and secondary antibodies labelled with 10 nm gold beads.
The shearing method was used to determine which other secreton components are essential for formation of pili in strains carrying derivatives of pCHAP231 with mutations in each of the 15 pul genes (Possot et al., 2000). PulG could be released in approximately normal amounts from the bacteria when PulA, PulB, PulH, PulJ, PulK or PulN was absent (Figure 2A) and trace amounts were released from bacteria lacking PulI (not visible in Figure 2). However, PulG could not be released by shearing bacteria lacking PulC, PulD, PulE, PulF, PulI, PulL, PulM, PulO or PulS (Figure 2A). Examination by EM (Figure 2B–D) indicated that release by shearing was perfectly correlated with the presence of pili in the mutants. In particular, cells with single short fibres that were labelled by PulG antibodies were occasionally seen in the PulI– mutant (Figure 2D).
PulB, PulH and PulN are not essential for secretion, at least in E.coli carrying pCHAP231 (Possot et al., 2000). However, PulJ and PulK, which are dispensable for pilus formation (Figure 2A and EM data not shown), are both required for pullulanase secretion (Possot et al., 2000). Both PulJ– and PulK– mutants were found to be completely secretion defective when grown on plates. We conclude that there is an incomplete correlation between ability to assemble the secreton pilus and ability to secrete pullulanase, because the minor pseudopilins PulJ and PulK are needed for secretion but not for piliation.
The major pilin PpdD can be assembled into pili via the Pul secreton
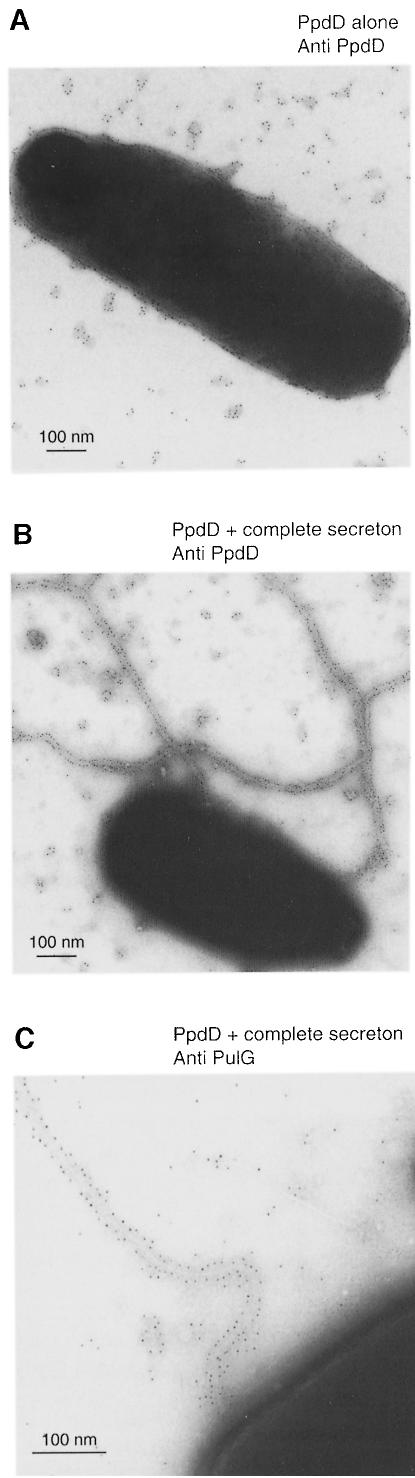
There is ample evidence that major type IV pilins can be assembled by heterologous piliation machineries (Elleman et al., 1986; Mattick et al., 1987; Beard et al., 1990). For example, the major E.coli K-12 type IV pilin, PpdD, can be assembled by the P.aeruginosa piliation machinery (Sauvonnet et al., 2000). To test whether a major pilin could be incorporated into pili via the Pul secreton, we introduced ppdD under lacZp control on a high copy number plasmid (pCHAP3100) into E.coli K-12 with or without the Pul secreton encoded by pCHAP231. Immunogold EM with specific antibodies revealed that PpdD was assembled into pili but only when the Pul secreton was also present (Figure 3A and B). The fibres were similar to those observed with bacteria carrying pCHAP231. All of the fibres were labelled with the PpdD antibodies and no labelling was observed in bacteria lacking pCHAP3100, confirming the absence of immunological cross-reactions between PulG and PpdD. All of the fibres were also labelled by antibodies against PulG (Figure 3C), indicating that both proteins are incorporated into the same fibres and possibly even into the same filaments.
Fig. 3. A major pilin (PpdD) can be assembled into pili by a functional Pul secreton. EM of bacteria that had been immunogold labelled with PpdD (A and B) or with anti-PulG antibodies (C) and then with secondary antibodies labelled with 10 nm gold beads. (A) Strain PAP7460 (pCHAP3100) producing PpdD alone; (B and C), PAP7460 (pCHAP231 pCHAP3100) producing both the Pul secreton and PpdD.
According to the results of shearing assays, assembly of PpdD into pili required the same proteins as those required for PulG assembly, i.e. all secreton components except PulB, PulH, PulJ, PulK and PulN (not shown). Furthermore, PpdD could be released by shearing of bacteria lacking PulG but could not promote pullulanase secretion in the absence of PulG (not shown).
Pili in strains with chromosomal pul genes
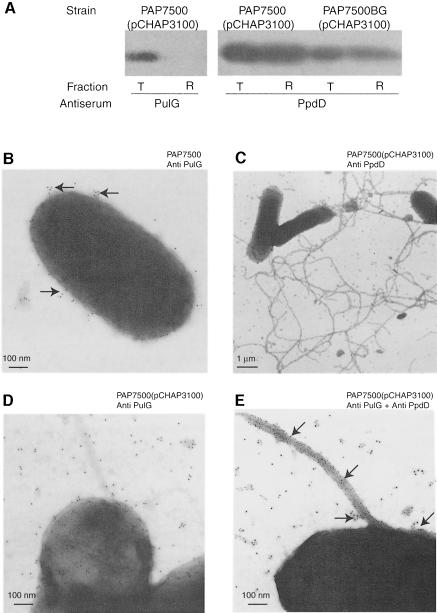
To see whether expression levels affected pilus production, we performed immunogold EM and shearing analyses of K.oxytoca, E.coli K-12 in which the pul genes are integrated in the chromosome (PAP7500) and E.coli K-12 (pCHAP231) in which the plasmid copy number was reduced to one to three per cell by a pcnB mutation (Lopilato et al., 1986). PulG could not be released from these bacteria by shearing (not shown; see Figure 4A) and the limited surface labelling visible in K.oxytoca and E.coli PAP7500 (for example, see arrows in Figure 4B) was difficult to distinguish from the non-specific-labelling of cells without PulG (Figure 1A). In all three strains, the level of expression of the pul genes was ∼5% of that in wild-type E.coli (pCHAP231). Therefore, production of long PulG pili is dependent on high-level production of at least one Pul secreton component. To see whether PulG was the limiting factor, we introduced pulG on a high copy number plasmid (pCHAP162; Pugsley, 1993b) into PAP7500. Even though the level of PulG produced was similar to or even higher than that produced by bacteria carrying pCHAP231, only small amounts of PulG (2–5%) were released by shearing, and immunogold labelling failed to reveal long pili. It should be noted, however, that high-level production of PulG in strains with the pul genes integrated in the chromosome blocks PulA secretion (Pugsley, 1993b), destabilizes several secreton components (Possot et al., 2000) and causes cells to lyse when harvested from plates.
Fig. 4. PpdD can be assembled into long pili by chromosomal secreton genes. (A) Analysis of total cell suspensions (T) of the strains indicated and proteins released by shearing (R) by immunoblotting with antiserum against PulG and PpdD. Three times as much material was loaded in R than in T. (B) EM of E.coli K-12 strain PAP7500 (pul cluster located in the chromosome) after immunogold-labelling with anti-PulG antibodies. Arrows indicate surface labelling by the antibodies. (C) EM of PAP7500 (pCHAP3100) producing PpdD and labelled with PpdD antibodies. (D) Same as (B) except that the bacteria were labelled with PulG antibodies. (E) Double labelling of the same bacteria with antibodies against PulG (arrows) and PpdD. The secondary antibodies were labelled with 10 nm gold beads except for (D), when the antibody applied after the anti-PpdD antibody was labelled with 5 nm gold beads (see Materials and methods).
High-level production of PpdD does not block pullulanase secretion. Therefore, we examined strain PAP7500 expressing ppdD from pCHAP3100 by shearing and immunogold EM. Large amounts of PpdD were released by shearing but virtually no PulG was released (Figure 4A). Extremely long PpdD-labelled fibres were observed on the surface of the bacteria (Figure 4C). These fibres had the same diameter as the PulG pili produced by strains carrying pCHAP231, for example. The fibres were weakly labelled with antibodies against PulG (Figure 4D). Examination of a large number of bacteria that had been double labelled with antibodies against PulG and PpdD revealed that PulG was frequently located in clusters along the fibre, often at sites where it appeared deformed (arrows in Figure 4E). Thus, high-level expression of the secreton is not required for the formation of long pilus fibres if a type IV pilin gene is expressed. As in strains carrying pCHAP231 and pCHAP3100 (see above), the assembly of PpdD into pili in strains with the pul genes integrated into the chromosome was unaffected by the absence of PulG, as determined by both shearing (Figure 4A) and EM (not shown).
Discussion
We have shown that the major pseudopilin component of the Pul secreton can be assembled into pili. Like type IV pili, these pili are apparently composed of one major component, the most abundant pseudopilin (PulG). Moreover, the secreton can also assemble a type IV pilin (PpdD) into pili. These data show that the type IV pilus biogenesis and the secreton pathways are very closely related, as one would expect from the homology between the proteins involved in the two pathways (Hobbs and Mattick, 1993; Pugsley and Possot, 1993; Nunn, 1999; Possot et al., 2000).
From a purely mechanistic point of view, the data presented include some interesting details worthy of further discussion. The first concerns the role of minor pilins in type IV pilus biogenesis. Classical type IV pili appear to be composed of one protein, the major type IV pilin (Parge et al., 1990, 1995). However, several ‘minor’ pilins are also required for pilus biogenesis in P.aeruginosa, although their exact role remains unknown (Russel and Darzins, 1994; Alm and Mattick, 1995, 1997). These minor pilins might be equivalent to the minor pseudopilins (PulH, PulI, PulJ and PulK) in the secreton system. However, only one of the pseudopilins, PulI, is needed for secreton-mediated assembly of the type IV pilin PpdD, although four pseudopilins (PulG, PulI, PulJ and PulK) are needed for secretion (Possot et al., 2000). Thus, PulI must perform an essential structural role in pilus assembly, possibly to initiate polymerization or to anchor the pilus in the cell envelope, with the other pseudopilins performing a role specifically related to pullulanase secretion or having overlapping functions in pilus/pseudopilus assembly. It would be interesting to determine whether minor pilins and minor pseudopilins can be incorporated into pili when overproduced.
Another difference between the two systems concerns other, known components of the two machineries. Secretion and pilus assembly by the secreton are both absolutely dependent on three proteins that do not have known homologues in type IV piliation systems: PulC, PulL and PulM. PulC might be involved in specific recognition of proteins secreted by the secreton (Lindeberg et al., 1996; Possot et al., 2000). PulL, on the other hand, is required for the cytoplasmic membrane association of PulE, a secreton component that does have a homologue involved in type IV piliation (Possot et al., 1992; Possot and Pugsley, 1994), and PulL and PulM form a complex (Possot et al., 2000). Thus, we predict that PulC might have more than one function in the secreton and that homologues of it and of PulL and PulM might exist in the type IV piliation machinery.
Assembly of type IV pili and of secreton pili and protein secretion by the type II secretion pathway are absolutely dependent on an outer membrane secretin (Martin et al., 1993; Drake and Koomey, 1995; Hardie et al., 1996a,b; Drake et al., 1997; Bitter et al., 1998). Since the apparent internal diameter of the P.aeruginosa type IV pilus secretin channel (Bitter et al., 1998) is similar to the width of the pilus filament (Folkhard et al., 1981), it seems reasonable to suppose that the pilus spans the outer membrane inside the secretin structure. The pilus would thereby occlude the secretin channel, preventing protein traffic through it. By analogy, the channel formed by PulD could be the site at which the secreton pilus crosses the outer membrane. However, we proposed that secretin PulD forms the conduit by which pullulanase crosses the outer membrane (Nouwen et al., 1999). The same channel could perform both functions if it is present in excess. Surprisingly, even very high-level production and assembly of PpdD type IV pilin (for example, see Figure 4) did not reduce the level of pullulanase secretion. Therefore, PulD might perform only one of the proposed functions, with either pullulanase or PulG and PpdD crossing the outer membrane by another route. In fact, there does not appear to be any direct evidence that secretins perform either function. However, secretins in the type II secretion pathway appear to determine which substrates are secreted (Lindeberg et al., 1996; Shevchik et al., 1997; Guilvout et al., 1999), which suggests a direct role in secretion. On the other hand, type IV pilins can be assembled by heterologous piliation pathways or by the secreton. Since the type IV piliation and secreton systems are so similar, one might even propose that the former could secrete proteins, as recently demonstrated for the flagellum assembly pathway in Yersinia (Young et al., 1999).
What function, if any, does the PulG pilus play in secretion? The secreton pilus might form an extension at the outer face of the secretin to project the conduit it forms beyond the membrane and across the surface layers (lipopolysaccharide, capsule and S layers). A single pilus filament cannot form such a conduit because its internal channel would not be large enough to accommodate a folded protein the size of pullulanase. The observed bundling of the secreton pili suggests that they might be assembled into a higher-ordered, tube-like structure analogous to that proposed for the Hrp pilus of the type III secretion system of Pseudomonas syringae (Roine et al., 1997). However, we consider this explanation unlikely because pullulanase, unlike other proteins secreted by the type II secretion pathway, is not released directly into the growth medium but remains surface-associated through N-terminal fatty acids that are embedded in the outer membrane (d'Enfert et al., 1987a,b; Pugsley, 1993a). Alternatively, the secreton pili might be involved in adherence to specific ligands, which could be the substrates for the secreted enzymes (like amylopectin for pullulanase secreted by the Pul secreton) or the surfaces of host cells infected by the bacteria, or in the formation of biofilms.
One argument against the idea that the secreton pili are required for secretion is that long pili composed of PulG were only observed when the level of secreton production was high due to expression of the secreton gene cluster from a multiple copy number plasmid. However, even when the pul genes are integrated in the chromosome, bundled pili became visible when the type IV pilin gene ppdD was expressed in trans. This situation might reflect that which normally occurs in bacteria such as P.aeruginosa, which possesses both secreton and type IV piliation systems, i.e. the major pilin is incorporated into both type IV pili and the secreton pilus. This could explain why the major pilin, PilA, is required for optimal secreton function in P.aeruginosa and why PilA could be cross-linked to the PulG homologue XcpT in this bacterium (Lu et al., 1997). However, the P.aeruginosa cells studied were grown in shaken liquid cultures and would be unlikely to have surface pili. It is not known whether K.oxytoca has a type IV piliation system. In E.coli K-12, the type IV pilin structural gene, ppdD, is not expressed (Sauvonnet et al., 2000). Nevertheless, the pullulanase secreton genes function normally when they are integrated into the E.coli chromosome (d’Enfert et al., 1987b), conditions that do not lead to the production of detectable secreton pili or to expression of ppdD (data not shown).
Materials and methods
Strains, plasmids and media
Escherichia coli strains PAP7460 and HS2019, carrying malE and malG mutations to permit induction of pulA and pulC promoters without production of MalE protein, were used previously (Possot et al., 2000). Strain PAP7500 [PAP7460 malP::(pulS pulAB pulC–O)] is a PAP7460 derivative in which the pul cluster is on the chromosome. Strain PAP7500BG is a derivative of this strain carrying a kanamycin-resistance cassette in pulB and an internal deletion in pulG (Pugsley, 1993b). The pcnB::Tn10 mutation (Lopilato et al., 1986) from strain LH1108 was transduced into HS2019 by P1 phage as described by Miller (1972), with selection for tetracyclin resistance to give strain PAP3012. The K.oxytoca strain used was UNF5023 (d’Enfert et al., 1987a,b). Plasmids bearing the pul cluster were pCHAP231 (d’Enfert et al., 1987b) and its derivatives carrying a non-polar mutation in one particular pul gene (Possot et al., 2000). The plasmid encoding PpdD was pCHAP3100 (Sauvonnet et al., 2000).
Bacteria were grown on Luria–Bertini (L) agar containing, where appropriate, the antibiotics ampicillin (100 µg/ml), tetracyclin (16 µg/ml) or chloramphenicol (34 µg/ml), and 0.4% maltose (to induce expression of pul genes). The plates were incubated at 30°C. Transformation was performed as described by Sambrook et al. (1989).
Detection of cell surface pili
For these assays, the strains were grown overnight on L agar containing maltose at 30°C. Immunogold labelling and EM were performed as described by Sauvonnet et al. (2000) using PulG or PpdD antibodies diluted to 1/100 and with 5 or 10 nm gold particles on the secondary (anti-rabbit IgG) antibodies. For double labelling (Figure 4D), grids were treated first with anti-PulG and then with secondary antibody labelled with 10 nm gold beads. The grids were then washed extensively in phosphate-buffered saline (PBS) to remove non-specifically bound antibodies, fixed with 0.1% glutaraldehyde for 5 min, quenched with PBS containing 50 mM NH4Cl and then labelled anti-PpdD and a new secondary antibody (5 nm gold beads). All grids were counterstained with 1% uranyl acetate.
The shearing procedure for releasing cell surface appendages was performed with the same bacteria as used for EM analysis (Sauvonnet et al., 2000). Briefly, bacteria were harvested from the plates, resuspended in L broth to an OD600 of 5.0 and then passed three times through a 26-gauge hypodermic needle on a syringe. The suspensions were then centrifuged twice at 13 000 g in a microcentrifuge for 5 min to separate the bacteria (pellet fraction) from the pilus-enriched supernatant (sheared fraction), which was sometimes precipitated with 10% trichloroacetic acid. Both fractions were loaded on SDS–12% polyacrylamide or SDS–11.3% polyacrylamide–8M urea gels, subjected to electrophoresis and immunoblotted with antibodies raised against PulG, PpdD, LamB, PulC, PulD, PulK or PulI.
Acknowledgments
Acknowledgements
We thank all members of the secretion and maltose laboratories for their constant interest and support. This work was financed by the European Union (Training and Mobility in Research grant number FMRX-CT96-0004) and a French Research Ministry grant (Programme fondamental en Microbiologie et Maladies infectieuses et parasitaires).
References
- Alm R.A. and Mattick,J.S. (1995) Identification of a gene, pilV, required for type 4 fimbrial biogenesis in Pseudomonas aeruginosa, whose product possesses a pre-pilin-like leader sequence. Mol. Microbiol., 16, 485–496. [DOI] [PubMed] [Google Scholar]
- Alm R.A. and Mattick,J.S. (1997) Genes involved in the biogenesis and function of type-4 fimbriae in Pseudomonas aeruginosa. Gene, 192, 89–98. [DOI] [PubMed] [Google Scholar]
- Beard M.K., Mattick,J.S., Moor,M.R., Marrs,C.F. and Egerton,J.R. (1990) Morphogenic expression of Moraxella bovis fimbriae (pili) in Pseudomonas aeruginosa. J. Bacteriol., 172, 2601–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieber D., Ramer,S., Wu,C., Murray,W., Tobe,T., Fernandez,R. and Schoolnik,G. (1998) Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science, 280, 2114–2118. [DOI] [PubMed] [Google Scholar]
- Bitter W., Koster,M., Latijnhouwers,M., de Cock,H. and Tommassen,J. (1998) Formation of oligomeric rings by XcpQ and PilQ, which are involved in protein transport across the outer membrane of Pseudomonas aeruginosa. Mol. Microbiol., 27, 209–219. [DOI] [PubMed] [Google Scholar]
- Bleves S., Lazdunski,A., Tommassen,J. and Filloux,A. (1998) The secretion apparatus of Pseudomonas aeruginosa: identification of a fifth pseudopilin, XcpX. Mol. Microbiol., 27, 31–40. [DOI] [PubMed] [Google Scholar]
- d'Enfert C., Chapon,C. and Pugsley,A.P. (1987a) Export and secretion of the lipoprotein pullulanase by Klebsiella pneumoniae. Mol. Microbiol., 1, 107–116. [DOI] [PubMed] [Google Scholar]
- d'Enfert C., Ryter,A. and Pugsley,A.P. (1987b) Cloning and expression in Escherichia coli of the Klebsiella pneumoniae genes for production, surface localization and secretion of the lipoprotein pullulanase. EMBO J., 6, 3531–3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake S.L. and Koomey,M. (1995) The product of the pilQ gene is essential for biogenesis of type IV pili in Neisseria gonorrhoeae. Mol. Microbiol., 18, 975–986. [DOI] [PubMed] [Google Scholar]
- Drake S.L., Sandstedt,S.A. and Koomey,M. (1997) PilP, a pilus biogenesis lipoprotein in Neisseria gonorrhoeae, affects expression of PilQ as a high-molecular-mass multimer. Mol. Microbiol., 23, 657–668. [DOI] [PubMed] [Google Scholar]
- Elleman T.C., Hoyne,P.A., Stewart,D.J., McKern,N.M. and Peterson,J.E. (1986) Expression of pili from Bacteroides nodosus in Pseudomonas aeruginosa. J. Bacteriol., 168, 574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkhard W., Marvin,D.A., Watts,T.H. and Paranchych,W. (1981) Structure of polar pili from Pseudomonas aeruginosa strains K and O. J. Mol. Biol. 149, 79–93. [DOI] [PubMed] [Google Scholar]
- Guilvout I., Hardie,K.R., Sauvonnet,N. and Pugsley,A.P. (1999) Genetic dissection of the outer membrane secretin PulD: are there distinct domains for multimerization and secretion specificity? J. Bacteriol., 181, 7212–7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie K.R., Lory,S. and Pugsley,A.P. (1996a) Insertion of an outer membrane protein in Escherichia coli requires a chaperone-like protein. EMBO J., 15, 978–988. [PMC free article] [PubMed] [Google Scholar]
- Hardie K.R., Seydel,A., Guilvout,I. and Pugsley,A.P. (1996b) The secretin-specific, chaperone-like protein of the general secretory pathway: separation of proteolytic protection and piloting functions. Mol. Microbiol., 22, 967–976. [DOI] [PubMed] [Google Scholar]
- Hobbs M. and Mattick,J.S. (1993) Common components in the assembly of type 4 fimbriae, DNA transfer systems, filamentous phage and protein-secretion apparatus: a general system for the formation of surface-associated protein complexes. Mol. Microbiol., 10, 233–243. [DOI] [PubMed] [Google Scholar]
- Kagami Y., Ratliff,M., Surber,M., Martinez,A. and Nunn,D. (1998) Type II protein secretion by Pseudomonas aeruginosa: genetic suppression of a conditional mutation in the pilin-like component XcpT by the cytoplasmic component XcpR. Mol. Microbiol., 27, 221–233. [DOI] [PubMed] [Google Scholar]
- Kirov S.M., O’Donovan,L.M. and Sanderson,K. (1999) Functional characterization of type IV pili expressed on diarrhoea-associated isolates of Aeromonas species. Infect. Immun., 67, 5447–5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeberg M., Salmond,G.P.C. and Collmer,A. (1996) Complementation of deletion mutations in a cloned functional cluster of Erwinia chrysanthemi out genes with Erwinia carotovora out homologs reveals OutC and OutD as candidate gatekeepers of species-specific secretion of proteins via the type II pathway. Mol. Microbiol., 20, 175–190. [DOI] [PubMed] [Google Scholar]
- Lopilato J., Bortner,S. and Beckwith,J. (1986) Mutations in a new chromosomal gene of Escherichia coli K-12, pcnB, reduce plasmid copy number of pBR322 and its derivatives. Mol. Gen. Genet., 205, 285–290. [DOI] [PubMed] [Google Scholar]
- Lu H.-M., Motley,S.T. and Lory,S. (1997) Interactions of the components of the general secretion pathway: role of Pseudomonas aeruginosa type IV pilin subunits in complex formation and extracellular protein secretion. Mol. Microbiol., 25, 247–259. [DOI] [PubMed] [Google Scholar]
- Martin P.R., Hobbs,M., Free,P.D., Jeske,Y. and Mattick,J.S. (1993) Characterization of pilQ, a new gene required for the biogenesis of type 4 fimbriae in Pseudomonas aeruginosa. Mol. Microbiol., 9, 857–868. [DOI] [PubMed] [Google Scholar]
- Mattick J.S., Bills,M.M., Anderson,B.J., Dalrymple,B., Mott,M.R. and Egerton,J.R. (1987) Morphogenic expression of Bacteroides nodosus fimbriae in Pseudomonas aeruginosa. J. Bacteriol., 169, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis S., Chapon,C., d’Enfert,C., Pugsley,A.P. and Schwartz,M. (1985) Characterization and expression of the structural gene for pullulanase, a maltose-inducible secreted protein of Klebsiella pneumoniae. J. Bacteriol., 164, 633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.H. (1972) Experiments in Molecular Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Nouwen N., Ranson,N., Saibil,H., Wolpensinger,B., Engel,A., Ghazi,A. and Pugsley,A.P. (1999) Secretin PulD: association with pilot protein PulS, structure and ion-conducting channel formation. Proc. Natl Acad. Sci. USA, 96, 8173–8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn D. (1999) Bacterial type II protein export and pilus biogenesis: more than just homologies? Trends Cell Biol., 9, 402–408. [DOI] [PubMed] [Google Scholar]
- Nunn D. and Lory,S. (1991) Product of the Pseudomonas aeruginosa gene pilD is a prepilin leader peptidase. Proc. Natl Acad. Sci. USA, 88, 3281–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn D. and Lory,S. (1992) Components of the protein excretion apparatus of Pseudomonas aeruginosa are processed by the type IV prepilin peptidase. Proc. Natl Acad. Sci. USA, 89, 47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn D., Bergman,S. and Lory,S. (1990) Products of three accessory genes, pilB, pilC, and pilD, are required for biogenesis of Pseudomonas aeruginosa pili. J. Bacteriol., 172, 2911–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parge H.E. et al. (1990) Biochemical purification and crystallographic characterization of the fiber-forming protein pilin from Neisseria gonorrhoeae. J. Bacteriol., 265, 2278–2285. [PubMed] [Google Scholar]
- Parge H.E., Forest,K.T., Hickey,M.J., Christensen,D.A., Getzoff,E.D. and Tainer,J.A. (1995) Structure of the fibre-forming protein pilin at 2.6 Å resolution. Nature, 378, 32–38. [DOI] [PubMed] [Google Scholar]
- Possot O. and Pugsley,A.P. (1994) Molecular characterization of PulE, a protein required for pullulanase secretion. Mol. Microbiol., 12, 287–299. [DOI] [PubMed] [Google Scholar]
- Possot O., d’Enfert,C., Reyss,I. and Pugsley,A.P. (1992) Pullulanase secretion in Escherichia coli K12 requires a cytoplasmic protein and a putative polytopic cytoplasmic membrane protein. Mol. Microbiol., 6, 95–105. [DOI] [PubMed] [Google Scholar]
- Possot O.M., Gérard,M. and Pugsley,A.P. (1999) Membrane association and multimerization of secreton component PulC. J. Bacteriol., 181, 4004–4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possot O., Vignon,G., Bomchil,N., Ebel,F. and Pugsley,A.P. (2000) Multiple interactions between pullulanase secreton components involved in stabilization and cytoplasmic membrane association of PulE. J. Bacteriol., 182, 2142–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A.P. (1993a) The complete general secretory pathway in gram-negative bacteria. Microbiol. Rev., 57, 50–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A.P. (1993b) Processing and methylation of PulG, a pilin-like component of the general secretory pathway of Klebsiella oxytoca. Mol. Microbiol., 9, 295–308. [DOI] [PubMed] [Google Scholar]
- Pugsley A.P. (1996) Multimers of the precursor of a type IV pilin-like component of the general secretory pathway are unrelated to pili. Mol. Microbiol., 20, 1235–1245. [DOI] [PubMed] [Google Scholar]
- Pugsley A.P. and Possot,O. (1993) The general secretory pathway of Klebsiella oxytoca: no evidence for relocalization or assembly of pilin-like PulG protein into a multiprotein complex. Mol. Microbiol., 10, 665–674. [DOI] [PubMed] [Google Scholar]
- Pugsley A.P., Kornacker,M.G. and Ryter,A. (1990) Analysis of the subcellular location of pullulanase produced by Escherichia coli carrying the pulA gene from Klebsiella pneumoniae strain UNF5023. Mol. Microbiol., 4, 59–72. [DOI] [PubMed] [Google Scholar]
- Reyss I. and Pugsley,A.P. (1990) Five additional genes in the pulC–O operon of the Gram-negative bacterium Klebsiella oxytoca UNF5023 that are required for pullulanase secretion. Mol. Gen. Genet., 222, 176–184. [DOI] [PubMed] [Google Scholar]
- Roine E., Wei,W., Yuan,J., Nurmiaho-Lassila,E.L., Kalkkinen,N., Romantschuk,M. and He,S.Y. (1997) Hrp pilus: an hrp-dependent bacterial surface appendage produced by Pseudomonas syringae pv. tomato DC3000. Proc. Natl Acad. Sci. USA, 94, 3459–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel M.M. and Darzins,A. (1994) The pilE gene of Pseudomonas aeruginosa, required for pilus biogenesis, shares amino acid sequence identity with the N-termini of type 4 prepilin proteins. Mol. Microbiol., 13, 973–985. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning. A Laboratory Manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Sauvonnet N., Gounon,P. and Pugsley,A.P. (2000) PpdD type IV pilin of Escherichia coli K-12 can be assembled into pili in Pseudomonas aeruginosa. J. Bacteriol., 182, 848–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchik V.E., Robert-Badouy,J. and Condemine,G. (1997) Specific interaction between OutD, an Erwinia chrysanthemi outer membrane protein of the general secretory pathway, and secreted proteins. EMBO J., 16, 3007–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom M.S., Nunn,D.N. and Lory,S. (1993) A single bifunctional enzyme, PilD, catalyzes cleavage and N-methylation of proteins belonging to the type IV pilin family. Proc. Natl Acad. Sci. USA, 90, 2404–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young G.M., Schmiel,D.H. and Miller,V.L. (1999) A new pathway for the secretion of virulence factors by bacteria: the flagellar export apparatus functions as a protein-secretion system. Proc. Natl Acad. Sci. USA, 96, 6456–6461. [DOI] [PMC free article] [PubMed] [Google Scholar]