Abstract
Background
Information on malignant pancreatic neuroendocrine neoplasms (pNENs) is mostly from retrospective studies in highly selected patients. The aim of this prospective, multicentre study was to assess treatment and outcomes of malignant pNENs in clinical practice.
Patients and methods
Consecutive patients with newly diagnosed, histologically-proven pNENs were included and followed-up for 2 years. Tumours were defined as malignant when nodal or distant metastases were present or invasion of extrapancreatic structures/organs was evident.
Results
A total of 140 patients with malignant pNENs were included. Ninety-eight patients (70.0%) underwent a surgical resection (76 radical and 22 palliative). Other non-surgical treatments were used in 101 patients (72.1%): somatostatin analogues (n = 63), chemotherapy (n = 30), ablative treatments (n = 15) and peptide-receptor radionuclide therapy (n = 14). No relationship was observed between the 2010 WHO classification and type of treatment. A surgical resection was more often performed in incidentally detected tumours located in the pancreas body tail. Two-year progression-free survival was 63.8%: 82% after a radical resection, 44% after a palliative resection and 41% without a resection. A radical resection and Ki67 proliferative index >5% and >10% were the only significant prognostic determinants in multivariate analysis.
Conclusions
A radical resection is the cornerstone treatment of malignant pNENs and represents, together with Ki67 assessment, the most powerful prognostic factor for 2-year outcomes.
Introduction
Pancreatic neuroendocrine neoplasms (pNENs) are rare and heterogeneous entities, ranging from small asymptomatic lesions to highly malignant metastatic tumours. Although known to be uncommon, knowledge of their real incidence remains limited, and there is also a lack of data on the natural history of the disease, the different treatments used and therapeutic outcomes.1,2 Whereas a complete surgical resection is thought to be the only curative treatment for pNENs,3,4 many other treatments have been proposed and are currently utilized in different stages of malignant disease. These include medical therapy with somatostatin analogues,5 chemotherapy,6 new biological drugs (such as everolimus and sunitinib),7,8 peptide-receptor radionuclide therapy (PRRT),9 ablative therapy of liver metastases (embolization, chemoembolization and radiofrequency ablation),10 and palliative surgical resection (debulking).11,12 However, available data on the frequency of use of treatments and associated outcomes are limited, with evidence usually from specialized centres with highly selected patients that cannot readily be applied to the more general patient population.
To help address this lack of data, in 2004 the Italian Association for the Study of the Pancreas (AISP) initiated a prospective, observational, multicentre study on the clinicopathological features and management of pNENs. A total of 310 patients with newly diagnosed, histologically-proven pNENs were identified with data at diagnosis having been previously reported.13 The present study reports the different treatments utilized in the subgroup of 140 patients with malignant tumours and analyses middle-term (2 years) outcomes associated with different clinicopathological features and therapeutic options.
Patients and methods
All newly diagnosed adult patients with pNENs observed consecutively from June 2004 to March 2007 in the 24 participating centres (listed in Appendix A1) were included in the study.
Criteria for recruitment, histological diagnosis and classification have been previously reported in detail.13 Tumours were defined as malignant when nodal or distant metastases were present or invasion of extrapancreatic structures/organs were evident. The Ki67 proliferative index was expressed as a percentage based on the count of Ki67-positive cells in 2000 tumour cells in areas with the highest immunostaining using the MIB1 antibody (DBA, Milan, Italy) and were stratified into three groups: Ki67 < 2%, Ki67 ≥ 2% and ≤20% and Ki67 > 20%. Patients were also categorized using Ki67 cut-off values of 5% and 10% according to Scarpa et al.14 After inclusion, each patient was followed-up for a minimum of 2 years, with clinical and biochemical evaluations at 6-month intervals as well as a contrast-enhanced total body CT scan in patients for whom disease progression was suspected. The study had an observational non-interventional design as each diagnostic investigation and medical or surgical treatment was performed according to the current clinical practice of each individual centre.
Parameters recorded at inclusion in the study and methods of data recording have been previously described.13 A surgical resection was considered complete if neither a gross residual tumour nor metastases were detectable at the end of the procedure (radical resection); otherwise, a resection was considered to be palliative. At each follow-up observation, the following data were recorded: modifications in symptoms, biochemical data, imaging data, variations in medical treatment, new surgical treatments, other new treatments and disease status (disease-free, stable residual disease and disease progression). Progression was defined according to RECIST criteria.15Death of the patient and cause of death were recorded. Data were collected and tabulated centrally. During the study period, a careful monitoring process was implemented and at the end of the study additional quality control (concerning completeness and congruence of each chart) was performed. The study was approved by the Ethical Committee of each participating centre and informed consent was obtained from all patients.
Statistical analysis
Data are presented as median, mean, standard deviation (SD) and 95% confidence intervals (95% CI). Additional statistical tests (Student's t-test, chi-squared test, Pearson's test, Fisher's exact test, the Levene test and anova) were utilized when appropriate. Relationships between the variables were tested using regression analysis. The difference was considered significant for a P-value < 0.05. Statistical analysis was performed using SPSS software version 10 (SPSS Inc., Chicago, IL, USA). Parameters were included in multivariate analysis if statistically significant at univariate analysis, with a P-value less than 0.05.
Results
A total of 310 patients with pNENs were eligible for study inclusion. Twenty-seven patients (8.7%) were lost at follow-up and were excluded from the study. Of the 283 remaining, 140 (49.5%) had pNENs classified as malignant and were included in this analysis.
There were 77 males (55%) and 63 females (45%). The mean age was 58.7 ± 14.4 years, with the largest proportion in the 50–59 years age range (37.1%). Clinicopathological features are reported in Table 1: only 16 patients (11.4%) had functioning pNENs, which were as a result of gastrin production (n = 8), excess insulin production (n = 3) or other hormones (glucagonoma, n = 2; somatostatinoma, n = 2; ACTH-producing tumour, n = 1). In symptomatic patients, the main symptoms reported were pain (n = 47, 37.9%), weight loss (n = 27, 21.8%) and jaundice (n = 21, 16.9%). The body tail of the pancreas was the most frequent site of the tumour (n = 81, 57.8%). According to the 2010 WHO classification,16 the majority of patients were classified as NET-G2 (n = 57, 40.7%), whereas according to TNM classification (UICC-WHO)16,17 more than half were classified as having stage IV disease (n = 80, 57.1%).
Table 1.
Demographic characteristics, clinicopathological features and treatments performed in 140 patients with malignant pancreatic neuroendocrine tumours, overall and according to the 2010 WHO classification16
| Characteristic | Overall (n = 140) | NET-G1 (n = 40) | NET-G2 (n = 57) | NEC-G3 (n = 34) | P-value |
|---|---|---|---|---|---|
| Male | 77 (55.0%) | 21 | 31 | 22 | 0.533 |
| Age (mean), years | 58.7 | 57.1 | 58.3 | 59.1 | 0.771 |
| Non-functioning | 124 (88.6%) | 33 | 49 | 33 | 0.124 |
| Symptomatic | 81 (57.8%) | 20 | 28 | 24 | 0.104 |
| Primary tumour diameter (mean), mm | 42.5 | 36.7 | 45.9 | 41.6 | 0.023 |
| Lymph node metastases | 89 (63.6%) | 30 | 28 | 28 | 0.002 |
| Hepatic metastases | 66 (47.1%) | 16 | 31 | 16 | 0.384 |
| Radical resection | 76 (54.3%) | 22 | 32 | 18 | 0.974 |
| Palliative resection | 22 (15.7%) | 6 | 11 | 5 | 0.871 |
| No resection | 40 (30.0%) | 12 | 14 | 11 | 0.922 |
| Liver resection | 22 (15.7%) | 5 | 10 | 7 | 0.683 |
| Somatostatin analogues | 63 (45.0%) | 20 | 32 | 11 | 0.091 |
| PRRT | 14 (10.0%) | 3 | 6 | 4 | 0.781 |
| Ablative treatments | 15 (10.7%) | 4 | 6 | 4 | 1.000 |
| Chemotherapy | 30 (21.4%) | 1 | 13 | 14 | 0.002 |
Nine patients were not classified because grading assessment was not available. Bold indicates significant P-value.
PRRT, peptide receptor radio therapy
A surgical resection represented the most frequent treatment: 76 patients (54.3%) underwent a radical resection and 22 underwent a palliative resection (15.7%). The type of radical resection was pancreatoduodenectomy in 27 patients (35.5%), distal pancreatectomy in 41 patients (53.9%), total pancreatectomy in 5 patients (6.6%) and middle pancreatectomy in 3 patients (3.9%). A palliative resection was done by pancreatoduodenectomy (n = 8, 36.4%) or distal pancreatectomy (n = 14, 63.6%). Nine patients (11.8%) undergoing a radical resection and 13 (59.1%) undergoing a palliative resection also underwent a liver resection. Forty-two patients (30%) had no resection of the tumour, although 12 of these underwent non-resective surgery (exploratory laparotomy in 11 patients and a by-pass procedure in one patient).
A total of 101 patients (72.1%) received other non-surgical treatments; some patients received more than one treatment (Table 1). The distribution of treatments according to the type of surgery (radical resection, palliative resection and no resection) is shown in Table 2.
Table 2.
Demographic characteristics, clinicopathological features and treatments performed in 140 patients with malignant pancreatic neuroendocrine tumours undergoing a radical resection (group A), palliative resection (group B) or without resection (group C)
| Characteristic | Group A (n = 76) | Group B (n = 22) | Group C (n = 42) | P-value |
|---|---|---|---|---|
| Male | 40 | 12 | 25 | 0.797 |
| Age (mean), years | 56.9 | 62.7 | 60.6 | 0.840 |
| Non-functioning | 67 | 17 | 40 | 0.095 |
| Symptomatic | 34 | 12 | 35 | 0.000 |
| Primary tumour diameter (mean), mm | 43.4 | 45.0 | 42.1 | 0.018 |
| Tumour site: | ||||
| Pancreatic head | 25 | 8 | 19 | 0.048 |
| Pancreatic body-tail | 46 | 14 | 21 | 0.234 |
| Pancreatic diffuse | 5 | 0 | 2 | (*) |
| Lymph node metastases | 47 | 15 | 27 | 0.861 |
| Hepatic metastases | 9 | 21 | 36 | 0.000 |
| Liver resection | 9 | 13 | 0 | 0.000 |
| Somatostatin analogues | 19 | 18 | 26 | 0.408 |
| PRRT | 2 | 1 | 11 | (*) |
| Ablative treatments | 3 | 8 | 4 | (*) |
| Chemotherapy | 13 | 2 | 15 | 0.042 |
(*) not applicable due to the small numbers. Bold indicates significant P-value.
PRRT, peptide receptor radio therapy.
At 2-year follow-up, 62 patients (44.3%) were disease free, 25 had stable residual disease (17.8%) and 21 had progressive disease (15.0%). Thirty-two patients (22.8%) died from their disease during follow-up. The median follow-up was 20.9 months. Overall, 2-year progression-free survival (PFS) was 63.8% (Fig. 1).
Figure 1.
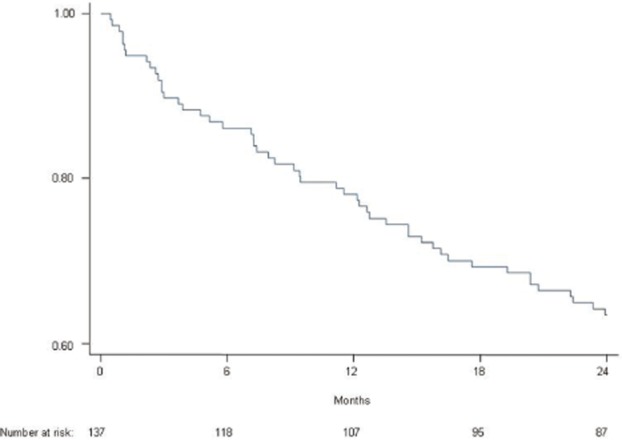
Overall progression-free survival curve of 140 patients with a malignant pancreatic neuroendocrine tumour
Patients were divided into three groups according to the 2010 WHO classification (Table 1). The type of surgical treatment was the same across the three groups, as were non-surgical treatments with the exception of more frequent use of chemotherapy in NEC-G3 patients. As expected, the prognosis was correlated with the stage of the neoplasm: in Fig. 2, PFS curves according to the 2010 WHO classification are reported, confirming a significant correlation between survival and stage of the neoplasm (P < 0.001).
Figure 2.
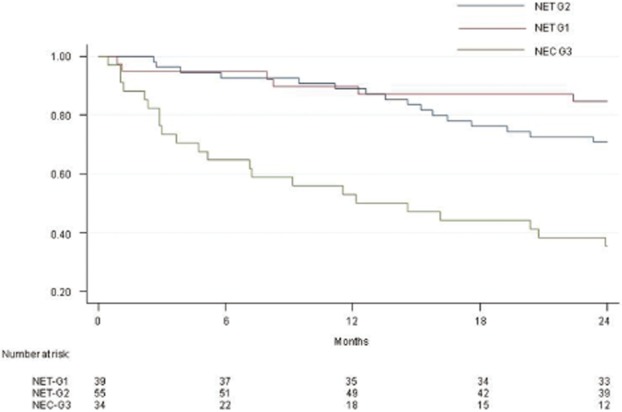
Progression-free survival curves of 131 patients with a malignant pancreatic neuroendocrine tumour, according to the 2010 WHO classification (40 NET G1, 57 NET G2, 34 NEC G3). (NET G1 versus NET G2: P = 0.155; NET G1 versus NEC G3: 0.000)
Patients were also divided according to the type of surgical treatment received: a radical resection (group A), a palliative resection (group B) or no surgical resection (group C); clinicopathological features and other treatments performed are reported in Table 2. Figure 3 shows PFS curves of patients divided by type of surgical procedure, confirming a significant better 2-year survival for patients undergoing a radical resection (P < 0.0001). Among patients undergoing a palliative resection, survival was significantly correlated with Ki67 values: in particular, 14 patients with a Ki67 value ≤5% had a 2-year PFS of 54% compared with 18% in 8 patients with Ki67 values > 5% (P < 0.001). Thirteen patients with concomitant liver resection had a slightly worse 2-year PFS than 9 patients without liver resection, although this was not significant (35% versus 58%, P = 0.168).
Figure 3.
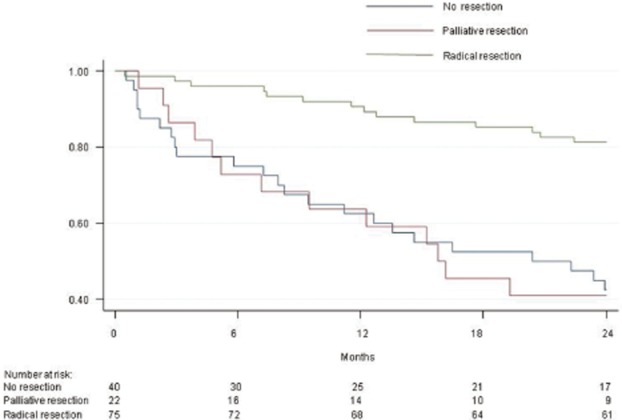
Progression-free survival curves of 140 patients with a malignant pancreatic neuroendocrine tumour undergoing a radical resection (76 patients), palliative resection (22 patients) or no resection (42 patients). Radical resection versus no resection: P = 0.000; palliative resection versus no resection: P = 0.868
In univariate analysis, poor histological differentiation, Ki67 values > 5%, Ki67 values > 10%, the presence of symptoms, liver or lymph node metastases and a non-radical resection were significantly correlated with poor 2-year PFS (Table 3). In multivariate analysis, a radical resection and Ki67 values > 5% or > 10% were significantly correlated with overall survival (OS), whereas a radical resection was the only parameter significantly correlated with PFS (Table 4).
Table 3.
Univariate analysis for 2-year progression-free survival
| Variables | Hazard ratio (95% CI) | P |
|---|---|---|
| Radical resection | ||
| No | 1.0 | |
| Yes | 0.23 (0.12–0.55) | 0.000 |
| Histology | ||
| Well differentiated | 1.0 | |
| Poorly differentiated | 6.3 (3.08–12.98) | 0.000 |
| Tumour site | ||
| Body-tail | 1.0 | |
| Head | 1.07 (2.08–12.98) | 0.864 |
| Hormonal syndrome | ||
| No | 1.0 | |
| Yes | 0.26 (0.03–1.78) | 0.186 |
| Presence of symptoms | ||
| No | 1.0 | |
| Yes | 3.4 (1.45–8.66) | 0.006 |
| Ki 67 values | ||
| <2% | 1.0 | |
| 3–5% | 5.1 (0.58–46.49) | 0.147 |
| 6–10% | 21.3 (2.69–177.86) | 0.004 |
| >10% | 28.5 (3.88–220.14) | 0.001 |
| Lymph node metastases | ||
| No | 1.0 | |
| Yes | 2.6 (1.06–6.35) | 0.033 |
| Hepatic metastases | ||
| No | 1.0 | |
| Yes | 3.8 (1.65–8.30) | 0.001 |
| Somatostatin analogues | ||
| No | 1.0 | |
| Yes | 1.0 (0.48–1.98) | 0.995 |
| Chemotherapy | ||
| No | 1.0 | |
| Yes | 1.8 (0.48–3.57) | 0.116 |
| PRRT | ||
| No | 1.0 | |
| Yes | 0.7 (0.27–1.86) | 0.540 |
| Ablative treatments | ||
| No | 1.0 | |
| Yes | 0.9 (0.32–2.64) | 0.974 |
PRRT, peptide receptor radio therapy. Bold indicates significant P value.
Table 4.
Cox multivariate analysis for overall and progression-free survival
| Variable | Hazard risk | 95% CI | P |
|---|---|---|---|
| Overall survival | |||
| Radical resection | 0.04 | 0.004;0.4 | 0.0060 |
| Ki67 > 5% | 7.4 | 1.95;28.2 | 0.0020 |
| Ki67 > 10% | 50.3 | 4.4;573.7 | 0.0020 |
| Progression-free survival | |||
| Radical resection | 0.2 | 0.056;0.71 | 0.0130 |
Variables included in the regression model: gender (male versus female), age (<40 versus 41–50 versus 51–60 versus 61–70 versus > 70 years), resection (radical versus palliative versus no resection), histology (well differentiated versus poorly differentiated), tumour site (head versus body-tail), hormonal syndrome (yes versus no), presence of symptoms (yes versus no), Ki67 values (<2% versus 3–5% versus 6–10% versus > 10%), lymph node metastases (yes versus no), hepatic metastases (yes versus no), treatment with somatostatin analogues (yes versus no), treatment with chemotherapy (yes versus no), PRRT (yes versus no) and ablative treatments (yes versus no).
Discussion
Information on pNENs is mostly derived from small, retrospective, uncontrolled studies conducted on highly selected patients. The present data offer the advantage of including a non-selected group of patients diagnosed with malignant pNENs, and represents a true picture of current therapeutic practice in Italy, especially considering that the study enrolled about one-quarter of cases expected in the entire country.13 This prospective, multicentre analysis confirms that a surgical resection, when feasible, should be the cornerstone of treatment in this cohort. In addition, a radical resection represents the most significant prognostic factor for both increased PFS and OS.
The present analysis was limited to patients with malignant disease, although the distinction between benign and malignant pNENs is quite complex and controversial as shown by the several classification systems proposed in recent years.16–18 For clinically benign pNENs, surgery without the requirement for additional treatments is standard practice with the choice of surgical procedure usually the main clinical decision.19 In comparison, the clinical management of malignant pNENs is much more complex, with a range of different surgical and non-surgical therapies that may be used in combination and/or at different disease stages.20 At present, there are no clear guidelines or recommendations on the best treatment strategy to be used. The choice of treatment often reflects the specific clinical experience and competence of the clinician and/or centre, rather than being based on the best clinical evidence.
The present study provides information on the type of treatments performed in patients with malignant pNENs in the first 2 years after diagnosis and show that a surgical resection remains by far the most frequently performed treatment. General agreement exists that complete removal of the tumour should be the first-line therapeutic approach whenever technically feasible21 and the present data confirm this attitude.
The role of palliative surgery is under debate,11,22–25 with recent data failing to show any survival advantage after a primary tumour resection in the presence of liver metastases26 and the European NeuroEndocrine Tumor Society's (ENETS) guidelines do not recommend debulking for unresectable primary NF-pNENs.4 In the present series, the main reason for a non-radical resection was the presence of hepatic metastases. Results after a palliative resection in this study are unsatisfactory, with a 2-year PFS rate significantly worse than that observed after a radical resection and similar to that of patients without a resection. Moreover, no survival advantage was observed in the subgroup of palliative resection patients with concomitant liver resection. This confirms the limited therapeutic benefit of palliative surgery in malignant pNENs. However, it should be noted that the 2-year prognosis of the subgroup of patients with a Ki67 proliferative index < 5% was considered satisfactory, suggesting that palliative surgery can be considered a possible option in selected patients who have pre-operative assessment of disease grade.26
It is interesting to observe that the decision to perform surgery or not and the possibility of achieving a radical resection did not correlate with the degree of differentiation of the neoplasm according to 2010 WHO classification. To some degree this finding is not surprising, as very limited information on tumour differentiation is available before surgery and the decision to operate is often still based on morphological rather than biological findings. In the present series, neoplasms not treated by resection were more frequently non-functioning and located in the head of the pancreas: the greater complexity of a pancreatoduodenectomy compared with a distal pancreatectomy probably accounts for the lower number of resections performed when the tumour was located in the head of the gland. Other than the rate of hepatic metastases, no different clinicopathological characteristics were observed between patients undergoing radical or palliative surgery.
The majority of patients undergoing surgery also received other treatments. This included almost all of those undergoing palliative surgery, but also about 40% of those who underwent a radical resection. In some patients these treatments were to treat disease recurrence after resection, but in other patients were administered with adjuvant intent, an indication currently not recommended.5,21 The most frequent additional treatment was the use of somatostatin analogues; these were administered irrespective of the tumour proliferation index and confirms the widespread use of these agents, consistent with their good safety profile and recent demonstration of efficacy.5,27 Ablative treatments were performed in just over one-third of patients undergoing palliative surgery, highlighting the greater importance attributed to liver-directed treatments for disease control after primary removal. PRRT had a limited application, probably reflecting the lack of comparative studies and definite application criteria for this option.28
Whereas the degree of differentiation of the tumour was not correlated with the surgical choice, it correlates with the choice of medical treatments, in particular chemotherapy. This is in accordance with recent guidelines29 and confirms the key role of Ki67 assessment in planning the multidisciplinary treatment of pNENs.
The overall prognosis of patients with a malignant pNEN was confirmed to be quite good, with a 64% 2-year PFS that is in accordance with other reports.1,30,31 As recently underlined,32 it is important to consider PFS instead of survival in the prognostic evaluation of pNENs, because of the observed long survival period after progression in many patients. Radical resection was confirmed to be the most powerful prognostic determinant, not only for OS but also for PFS: this finding is well known and has been previously reported.3,4,12,21 One possible criticism of this finding is that radical surgery was simply a surrogate for less advanced disease, as patients who did not undergo a radical resection were typically those with liver metastases. As such, the presence of liver metastases may be the determining factor in the duration of PFS or OS, rather than whether patients underwent a radical resection or not. To investigate this more fully, it would be necessary for long-term follow-up radical surgery patients to see how many develop liver or distant metastases and the impact of this on survival. The 2010 WHO staging classification proved to be a significant tool for predicting survival between well and poorly differentiated pNENs, but was less useful in differentiating between NET G1 and NET G2 patients, as has been previously reported.14 In this regard, it is important to observe that a Ki67 cut-off value of 5% is confirmed as a significant prognostic determinant in both univariate and multivariate analysis.33 The possible introduction of a cut-off of 5%, either instead of or together with the 2% cut-off value currently used, could usefully be included in the ongoing considerations to improve TNM staging systems for pNENs.31 This especially so given that the current grading system is more general and intended for NENs at all anatomical sites of the gastroenteropancreatic tract.14 Non-surgical treatments were not prognostic determinants in our series, although this is not surprising given the small numbers of patients receiving these treatments and the short period of follow-up.
Limitations of this study include the heterogeneity of treatments performed and the absence of defined inclusion criteria to decide therapeutic choices. In addition, imaging evaluation during the follow-up period was not standardized across centres. However, these study weaknesses are frequently seen in other clinical series assessing the outcome of malignant pNENs; the rarity and clinical heterogeneity of these tumours has prevented large, randomized, controlled trials aimed at evaluating the specific role of single treatment modalities.
In conclusion, the present study highlights that a radical surgical resection, when feasible, represents the first-choice treatment for malignant pNENs, offering the best chance for extended PFS. As such, it should be considered for every patient with a pNEN. Somatostatin analogues are the most frequently administered non-surgical treatment, with liver-directed therapies often performed in patients with liver metastases. Staging of the tumour, according to the 2010 WHO classification, is significantly correlated with PFS, whereas the Ki67 proliferation index is a powerful prognostic indicator, especially if 5% or 10% cut-off values are chosen.
Acknowledgments
The authors would like to thank Monica Simonetti of Centro Consulenze, Firenze, Italy, for her technical and statistical support.
This study was partially supported by an unrestricted grant from Ipsen, Milan, Italy.
Appendix A1
Members of the AISP-Network Study Group
| Investigator | Center | City | |
|---|---|---|---|
| Di Carlo | Valerio | OSPEDALE S. RAFFAELE | Milano |
| Pederzoli | Paolo | AZIENDA OSPEDALIERA VERONA (POLICLINICO) | Verona |
| Delle Fave | Gianfranco | AZIENDA OSPEDALE POLICLINICO S. ANDREA | Roma |
| Pedrazzoli | Sergio | AZIENDA OSPEDALIERA DI PADOVA | Padova |
| Tomassetti | Paola | AZIENDA OSPEDALIERA POLICLINICO S. ORSOLA – MALPIGHI | Bologna |
| Garcea | Domenico | OSPEDALE PIERANTONI | Forlì |
| Uomo | Generoso | AZIENDA OSPEDALIERA ‘A. CARDARELLI’ | Napoli |
| Colangelo | Ettore | OSPEDALE CIVILE | Pescara |
| Mosca | Franco | OSPEDALE CISANELLO | Pisa |
| Fronda | Gian Ruggero | OSPEDALE S. GIOVANNI BATTISTA MOLINETTE | Torino |
| Bresadola | Fabrizio | POLICLINICO UNIVERSITARIO A GESTIONE DIRETTA | Udine |
| Cantore | Maurizio | OSPEDALE CIVILE | Carrara |
| Leone | Biagio Eugenio | OSPEDALE S. GERARDO | Monza |
| Farinati | Fabio | AZIENDA OSPEDALIERA DI PADOVA | Padova |
| Toma | Sandro Salvatore | OSPEDALE CASA SOLLIEVO DELLA SOFFERENZA | San Giovanni Rotondo |
| Luppi | Gabriele | AZIENDA OSPEDALIERA POLICLINICO | Modena |
| Bene | Anna | AZIENDA POLICLINICO UNIVERSITARIO | Messina |
| Bajetta | Emilio | ISTITUTO NAZIONALE PER CURA TUMORI | Milano |
| Ruffini | Livia | AZIENDA OSPEDALIERA G. BROTZU | Cagliari |
| Gebbia | Vittorio | CLINICA MADDALENA | Palermo |
| Liguori | Luciano | OSPEDALE BELLARIA | Bologna |
| De Toma | Giorgio | AZIENDA UNIVERSITARIA POLICLINICO UMBERTO I | Roma |
| Dogliotti | Luigi | AZIENDA SANITARIA OSPEDALIERA ‘S. LUIGI’ | Orbassano |
| Massidda | Bruno | POLICLINICO UNIVERSITARIO | Cagliari |
Conflicts of interest
None declared.
References
- 1.Halfdanarson TR, Rubin J, Farnell MB, Grant CS, Petersen GM. Pancreatic endocrine neoplasms: epidemiology and prognosis of pancreatic endocrine tumors. Endocr Relat Cancer. 2008;15:409–427. doi: 10.1677/ERC-07-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology. 2008;135:1469–1492. doi: 10.1053/j.gastro.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vagefi PA, Razo O, Deshpande V, McGrath DJ, Lauwers GY, Thayer SP, et al. Evolving patterns in the detection and outcomes of pancreatic neuroendocrine neoplasms. Arch Surg. 2007;142:347–354. doi: 10.1001/archsurg.142.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falconi M, Bartsch DK, Eriksson B, Klöppel G, Lopes JM, O'Connor JM, et al. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms of the digestive system: well-differentiated pancreatic non-functioning tumors. Neuroendocrinology. 2012;95:120–134. doi: 10.1159/000335587. [DOI] [PubMed] [Google Scholar]
- 5.Strosberg J, Kvols L. Antiproliferative effect of somatostatin analogs in gastroenteropancreatic neuroendocrine tumors. World J Gastroenterol. 2010;16:2963–2970. doi: 10.3748/wjg.v16.i24.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner NC, Strauss SJ, Sarker D, Gillmore R, Kirkwood A, Hackshaw A, et al. Chemotherapy with 5-fluorouracil, cisplatin and streptozocin for neuroendocrine tumours. Br J Cancer. 2010;102:1106–1112. doi: 10.1038/sj.bjc.6605618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 9.Imhof A, Brunner P, Marincek N, Briel M, Schindler C, Rasch H, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol. 2011;29:2416–2423. doi: 10.1200/JCO.2010.33.7873. [DOI] [PubMed] [Google Scholar]
- 10.Harring TR, Nguyen NT, Goss JA, O'Mahony CA. Treatment of liver metastases in patients with neuroendocrine tumors: a comprehensive review. Int J Hepatol. 2011;2011:154541. doi: 10.4061/2011/154541. doi: 10.4061/2011/154541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodul PJ, Strosberg JR, Kvols LK. Aggressive surgical resection in the management of pancreatic neuroendocrine tumors: when it is indicated. Cancer Control. 2008;15:314–321. doi: 10.1177/107327480801500406. [DOI] [PubMed] [Google Scholar]
- 12.Fischer L, Kleeff J, Esposito I, Hinz U, Zimmermann A, Friess H, et al. Clinical outcome and long-term survival in 118 consecutive patients with neuroendocrine tumours of the pancreas. Br J Surg. 2008;95:627–635. doi: 10.1002/bjs.6051. [DOI] [PubMed] [Google Scholar]
- 13.Zerbi A, Falconi M, Rindi G, Delle Fave G, Tomassetti P, Pasquali C, et al. Clinicopathological features of pancreatic endocrine tumors: a prospective multi center study in Italy of 297 sporadic cases. Am J Gastroenterol. 2010;105:1421–1429. doi: 10.1038/ajg.2009.747. [DOI] [PubMed] [Google Scholar]
- 14.Scarpa A, Mantovani W, Capelli P, Beghelli S, Boninsegna L, Bettini R, et al. Pancreatic endocrine tumors: improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol. 2010;23:824–833. doi: 10.1038/modpathol.2010.58. [DOI] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Bosman FT, Carneiro F. WHO Classification of Tumours, Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press; 2010. [Google Scholar]
- 17.Rindi G, Klöppel G, Couvelard A, Komminoth P, Körner M, Lopes JM, et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449:395–401. doi: 10.1007/s00428-006-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobin L, Gospodarowicz M, Wittekind C. TNM Classification of Malignant Tumours. 7th edn. Bognor Regis: Wiley Blackwell; 2009. [Google Scholar]
- 19.Akerstrom G, Hellman P. Surgery on neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab. 2007;21:87–109. doi: 10.1016/j.beem.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Oberg K. Neuroendocrine tumors of the digestive tract: impact of new classifications and new agents on therapeutic approaches. Curr Opin Oncol. 2012;24:433–440. doi: 10.1097/CCO.0b013e328353d7ba. [DOI] [PubMed] [Google Scholar]
- 21.Öberg KE. Gastrointestinal neuroendocrine tumors. Ann Oncol. 2010;21(Suppl. 7):vii72–vii80. doi: 10.1093/annonc/mdq290. [DOI] [PubMed] [Google Scholar]
- 22.Gurusamy KS, Pamecha V, Sharma D, Davidson BR. Palliative cytoreductive surgery versus other palliative treatments in patients with unresectable liver metastases from gastro-entero-pancreatic neuroendocrine tumours. Cochrane Database Syst Rev. 2009;(1) doi: 10.1002/14651858.CD007118.pub2. CD007118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solorzano CC, Lee JE, Pisters PW, Vauthey JN, Ayers GD, Jean ME, et al. Non-functioning islet cell carcinoma of the pancreas: survival results in a contemporary series of 163 patients. Surgery. 2001;130:1078–1085. doi: 10.1067/msy.2001.118367. [DOI] [PubMed] [Google Scholar]
- 24.Sarmiento JM, Heywood G, Rubin J, Ilstrup DM, Nagorney DM, Que FG. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg. 2003;197:29–37. doi: 10.1016/S1072-7515(03)00230-8. [DOI] [PubMed] [Google Scholar]
- 25.Capurso G, Bettini R, Rinzivillo M, Boninsegna L, Delle Fave G, Falconi M. Role of resection of the primary pancreatic neuroendocrine tumour only in patients with unresectable metastatic liver disease: a systematic review. Neuroendocrinology. 2011;93:223–229. doi: 10.1159/000324770. [DOI] [PubMed] [Google Scholar]
- 26.Bettini R, Mantovani W, Boninsegna L, Crippa S, Capelli P, Bassi C, et al. Primary tumour resection in metastatic nonfunctioning pancreatic endocrine carcinomas. Dig Liver Dis. 2009;41:49–55. doi: 10.1016/j.dld.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 28.Walter T, Brixi-Benmansour H, Lombard-Bohas C, Cadiot G. New treatment strategies in advanced neuroendocrine tumours. Dig Liver Dis. 2012;44:95–105. doi: 10.1016/j.dld.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson O, Van Cutsem E, Delle Fave G, Yao JC, Pavel ME, McNicol AM, et al. European Neuroendocrine Tumor Society. Poorly differentiated carcinomas of the foregut (gastric, duodenal and pancreatic) Neuroendocrinology. 2006;84:212–215. doi: 10.1159/000098013. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Carbonero R, Capdevila J, Crespo-Herrero G, Díaz-Pérez JA, Martínez Del Prado MP, Alonso Orduña V, et al. Incidence, patterns of care and prognostic factors for outcome of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): results from the National Cancer Registry of Spain (RGETNE) Ann Oncol. 2010;21:1794–1803. doi: 10.1093/annonc/mdq022. [DOI] [PubMed] [Google Scholar]
- 31.Rindi G, Falconi M, Klersy C, Albarello L, Boninsegna L, Buchler MW, et al. TNM staging of neoplasms of the endocrine pancreas: results from a large international cohort study. J Natl Cancer Inst. 2012;104:764–777. doi: 10.1093/jnci/djs208. [DOI] [PubMed] [Google Scholar]
- 32.Kulke MH, Siu LL, Tepper JE, Fisher G, Jaffe D, Haller DG, et al. Future directions in the treatment of neuroendocrine tumors: consensus report of the National Cancer Institute Neuroendocrine Tumor clinical trials planning meeting. J Clin Oncol. 2011;29:934–943. doi: 10.1200/JCO.2010.33.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boninsegna L, Panzuto F, Partelli S, Capelli P, Delle Fave G, Bettini R, et al. Malignant pancreatic neuroendocrine tumour: lymph node ratio and Ki67 are predictors of recurrence after curative resections. Eur J Cancer. 2012;48:1608–1615. doi: 10.1016/j.ejca.2011.10.030. [DOI] [PubMed] [Google Scholar]


