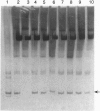
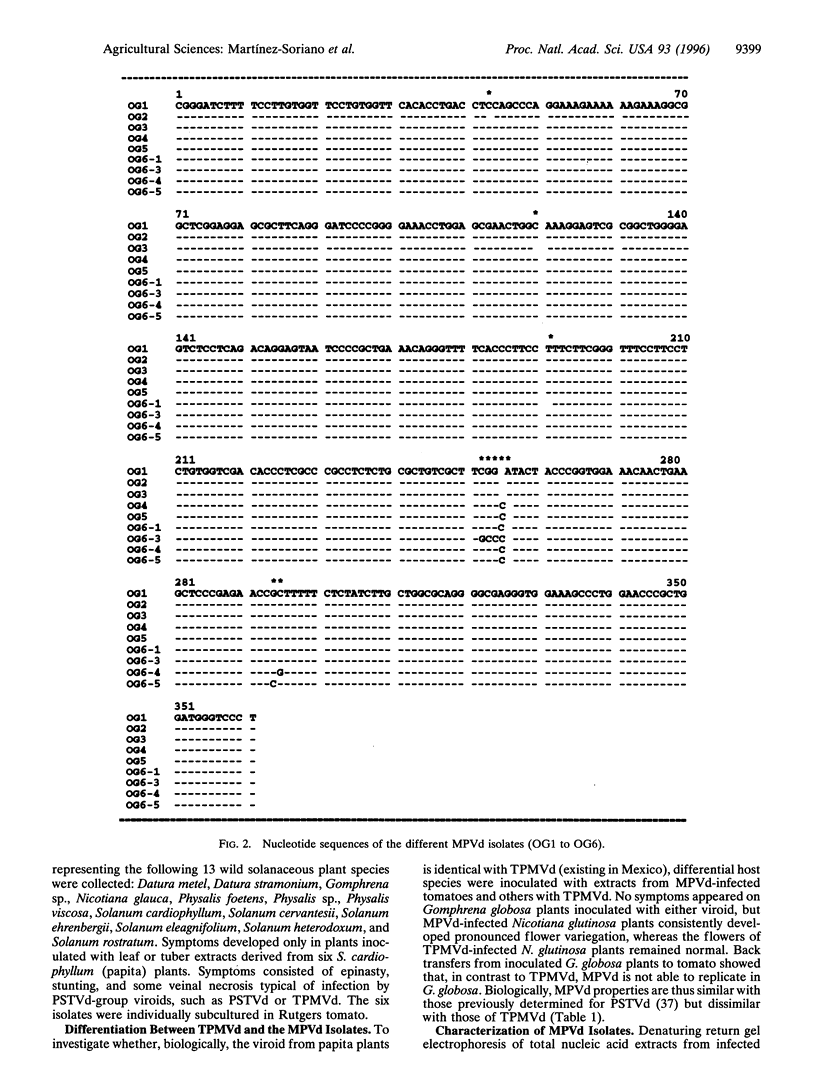
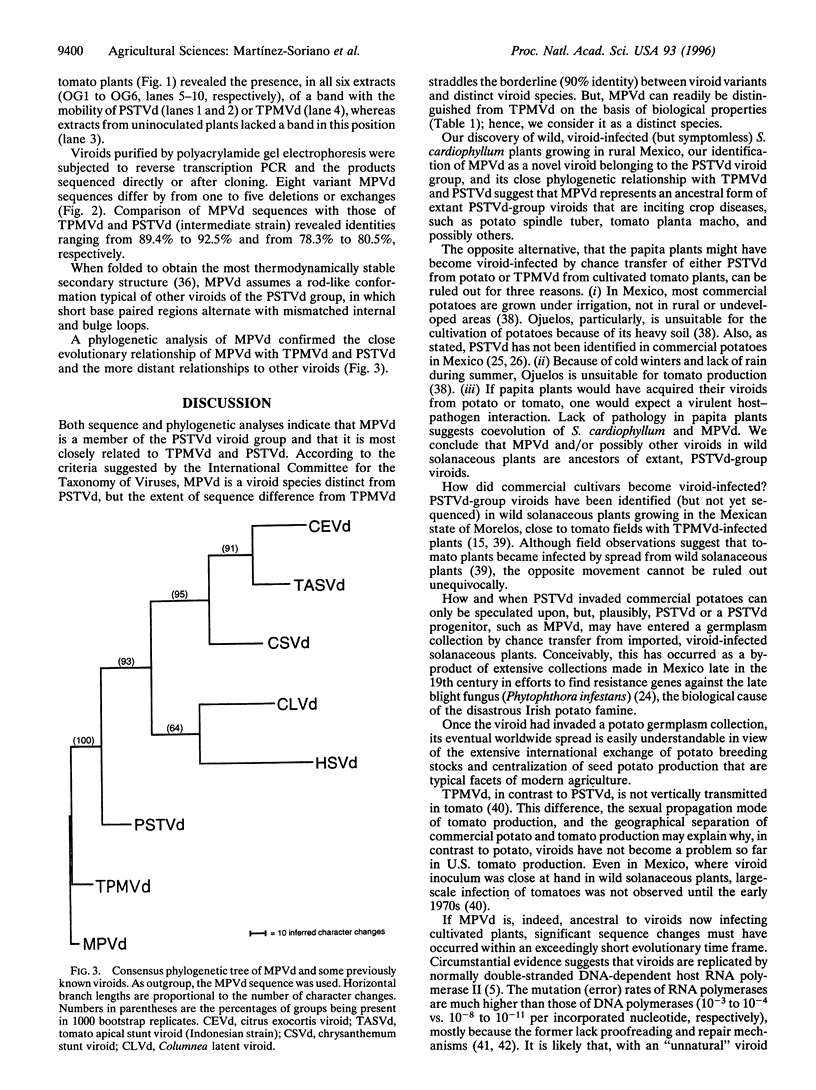
Abstract
The potato spindle tuber disease was first observed early in the 20th century in the northeastern United States and shown, in 1971, to be incited by a viroid, potato spindle tuber viroid (PSTVd). No wild-plant PSTVd reservoirs have been identified; thus, the initial source of PSTVd infecting potatoes has remained a mystery. Several variants of a novel viroid, designated Mexican papita viroid (MPVd), have now been isolated from Solanum cardiophyllum Lindl. (papita güera, cimantli) plants growing wild in the Mexican state of Aguascalientes. MPVd's nucleotide sequence is most closely related to those of the tomato planta macho viroid (TPMVd) and PSTVd. From TPMVd, MPVd may be distinguished on the basis of biological properties, such as replication and symptom formation in certain differential hosts. Phylogenetic and ecological data indicate that MPVd and certain viroids now affecting crop plants, such as TPMVd, PSTVd, and possibly others, have a common ancestor. We hypothesize that commercial potatoes grown in the United States have become viroid-infected by chance transfer of MPVd or a similar viroid from endemically infected wild solanaceous plants imported from Mexico as germplasm, conceivably from plants known to have been introduced from Mexico to the United States late in the 19th century in efforts to identify genetic resistance to the potato late blight fungus, Phytophthora infestans.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branch A. D., Robertson H. D. A replication cycle for viroids and other small infectious RNA's. Science. 1984 Feb 3;223(4635):450–455. doi: 10.1126/science.6197756. [DOI] [PubMed] [Google Scholar]
- Diener T. O. Potato spindle tuber "virus". IV. A replicating, low molecular weight RNA. Virology. 1971 Aug;45(2):411–428. doi: 10.1016/0042-6822(71)90342-4. [DOI] [PubMed] [Google Scholar]
- Diener T. O., Raymer W. B. Potato spindle tuber virus: a plant virus with properties of a free nucleic acid. Science. 1967 Oct 20;158(3799):378–381. doi: 10.1126/science.158.3799.378. [DOI] [PubMed] [Google Scholar]
- Diener T. O. Subviral pathogens of plants: viroids and viroidlike satellite RNAs. FASEB J. 1991 Oct;5(13):2808–2813. doi: 10.1096/fasebj.5.13.1717335. [DOI] [PubMed] [Google Scholar]
- Eigen M. Selforganization of matter and the evolution of biological macromolecules. Naturwissenschaften. 1971 Oct;58(10):465–523. doi: 10.1007/BF00623322. [DOI] [PubMed] [Google Scholar]
- Eigen M. Viral quasispecies. Sci Am. 1993 Jul;269(1):42–49. doi: 10.1038/scientificamerican0793-42. [DOI] [PubMed] [Google Scholar]
- Elena S. F., Dopazo J., Flores R., Diener T. O., Moya A. Phylogeny of viroids, viroidlike satellite RNAs, and the viroidlike domain of hepatitis delta virus RNA. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5631–5634. doi: 10.1073/pnas.88.13.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose C. The synthesis of glycoproteins in human melanoma cells infected with varicella-zoster virus. Virology. 1980 Feb;101(1):1–9. doi: 10.1016/0042-6822(80)90478-x. [DOI] [PubMed] [Google Scholar]
- Hadidi A., Cress D. E., Diener T. O. Nuclear DNA from uninfected or potato spindle tuber viroid-infected tomato plants contains no detectable sequences complementary to cloned double-stranded viroid cDNA. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6932–6935. doi: 10.1073/pnas.78.11.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Keen N. T., Tamaki S., Kobayashi D., Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988 Oct 15;70(1):191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- Levy L., Lee I. M., Hadidi A. Simple and rapid preparation of infected plant tissue extracts for PCR amplification of virus, viroid, and MLO nucleic acids. J Virol Methods. 1994 Oct;49(3):295–304. doi: 10.1016/0166-0934(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Owens R. A., Chen W., Hu Y., Hsu Y. H. Suppression of potato spindle tuber viroid replication and symptom expression by mutations which stabilize the pathogenicity domain. Virology. 1995 Apr 20;208(2):554–564. doi: 10.1006/viro.1995.1186. [DOI] [PubMed] [Google Scholar]
- Owens R. A., Diener T. O. RNA intermediates in potato spindle tuber viroid replication. Proc Natl Acad Sci U S A. 1982 Jan;79(1):113–117. doi: 10.1073/pnas.79.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchta H., Herold T., Verhoeven K., Roenhorst A., Ramm K., Schmidt-Puchta W., Sänger H. L. A new strain of potato spindle tuber viroid (PSTVd-N) exhibits major sequence differences as compared to all other PSTVd strains sequenced so far. Plant Mol Biol. 1990 Sep;15(3):509–511. doi: 10.1007/BF00019169. [DOI] [PubMed] [Google Scholar]
- Qu F., Heinrich C., Loss P., Steger G., Tien P., Riesner D. Multiple pathways of reversion in viroids for conservation of structural elements. EMBO J. 1993 May;12(5):2129–2139. doi: 10.1002/j.1460-2075.1993.tb05861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semancik J. S., Morris T. J., Weathers L. G., Rodorf B. F., Kearns D. R. Physical properties of a minimal infectious RNA(viroik) associated with the exocortis disease. Virology. 1975 Jan;63(1):160–167. doi: 10.1016/0042-6822(75)90381-5. [DOI] [PubMed] [Google Scholar]
- Woodgett J. R. cDNA cloning and properties of glycogen synthase kinase-3. Methods Enzymol. 1991;200:564–577. doi: 10.1016/0076-6879(91)00172-s. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]