Abstract
We examined physical growth and behavioral outcomes in 226 10-year-old children who were participants in a longitudinal study of prenatal cocaine exposure (PCE), while controlling for other factors that affect development. During the first trimester, 42% of the women used cocaine, with use declining across pregnancy. At the 10-year follow-up, the caregivers were 37 years old, had 12.8 years of education, and 50% were African American. First trimester cocaine exposure predicted decreased weight, height, and head circumference at 10 years. First trimester cocaine use also predicted maternal ratings of less sociability on the EAS Temperament Survey and more withdrawn behavior problems on the Child Behavior Checklist, more anxious/depressed behaviors on the Teacher Report Form, and more self-reported depressive symptoms on the Children’s Depression Inventory. In addition, exposure to violence mediated the effect of PCE on child and teacher reports of depressive symptoms, but not of maternal reports of sociability and withdrawn behaviors. These behaviors may be precursors of later psychiatric problems.
Keywords: prenatal cocaine exposure, growth, behavior problems, depression
1. Introduction
The National Pregnancy and Health Survey in 1992 showed that 1% of pregnant women used cocaine (NIDA 1996). There have been no national estimates of cocaine use during pregnancy since that time. Research studies have shown higher rates of prenatal cocaine use ranging from 2.6% to 18%, which vary with sample location and demographics (e.g., Eyler et al., 1994; Zuckerman et al., 1989). Although most women who use cocaine decrease or quit their use during early pregnancy, the effects of this pattern of use have been largely unstudied.
Recent reviews of the effects of prenatal cocaine exposure (PCE) on child development have suggested that the most consistent effects are found on behavioral domains (Ackerman et al., 2010; Buckingham-Howes et al., 2013). PCE has been reported to be associated with higher rates of behavior problems on the mother-rated Child Behavior Checklist (CBCL) and/or on teacher-rated scales, particularly on externalizing behaviors such as aggression and delinquency (Bada et al., 2007, 2011, 2012; Bennett et al., 2007, 2013; Delaney-Black et al., 2000, 2004; Fisher et al., 2011; Kable et al., 2008; Lester et al., 2012; McLaughlin et al., 2011; Minnes et al., 2010; Morrow et al., 2009; Nordstrom Bailey et al., 2005; Sood et al., 2005; Whitaker et al., 2011). We have found that offspring exposed prenatally to cocaine had more problems with behavior regulation at 1 year (Richardson et al., 2008), and with externalizing and internalizing behaviors at 3 (Richardson et al., 2009) and 7 years (Richardson et al., 2011) than non-exposed offspring. However, other reports have not shown significant effects of PCE on child behaviors, including externalizing problems (Accornero et al., 2002, 2006; Bada et al., 2008, 2011; Bennett et al., 2002, 2013; Bridgett & Mayes, 2011; Delaney-Black et al., 2000; Gerteis et al., 2011; Greenwald et al., 2011; Linares et al., 2006; Minnes et al., 2010; Savage et al., 2005; Warner et al., 2006b). Thus, inconsistencies remain in the field, which could be a result of methodological differences among studies such as racial composition, level and pattern of PCE, type of informant and assessment, and varying control variables. Further, some effects of PCE have been reported to vary with the gender of the child (e.g., Bennett et al., 2013; Delaney-Black et al., 2000).
When evaluating the effects of PCE, it is also important to consider other predictors of child behavior problems. Research has shown that maternal depression (Hammen, 2009; Spence et al., 2002; Weissman et al, 1997), socioeconomic status (Bradley & Corwin, 2002; Duncan et al., 1994), home environment and parenting characteristics (McLeod et al., 2007), and parental substance use (Hanson et al., 2006) are associated with child behavior problems. These factors frequently co-occur (Bradley & Corwin, 2002) and thus must be considered in evaluating the effects of PCE.
Behavior problems are also significantly associated with exposure to family and community violence (Heim et al., 2008; Schwab-Stone et al., 1999, 2012). Violence exposure is prevalent: 60% of youth in a national survey experienced one or more types of violence in the family, school, or community (Finkelhor et al., 2009). While some reports of the effects of PCE have considered the effects of violence exposure (Bada et al., 2011; Delaney-Black et al., 2011; Frank et al., 2011; Gerteis et al., 2011; LaGasse et al., 2006; Warner et al., 2011), the findings are inconsistent in terms of the relations among PCE, violence exposure, and behavior problems. In our earlier analyses of the effects of PCE on behavior at 7 years, we were unable to examine the role of violence exposure because we did not have a measure of exposure to violence at that phase (Richardson et al., 2011). At 10 years, we included a measure of exposure to violence and are thus now able to explore whether exposure to violence will mediate the effects of PCE on the 10-year behavior outcomes.
We will investigate whether there are effects of PCE on behavior in middle childhood, as brain development continues to undergo changes during this developmental period (Lenroot & Giedd, 2006), while taking into account other factors that are associated with child behavior problems. Further, we will consider whether exposure to violence mediates the effects of PCE on behavior problems.
We will also investigate the effects of PCE on growth at 10 years as this is a domain known to be affected by teratogens (Vorhees, 1989). We have found PCE to be associated with growth deficits at each of our previous follow-up phases (Richardson et al., 1999, 2008, 2009, 2011). Relations between PCE and reduced growth in childhood have also been reported by several other researchers (Chasnoff et al., 1998; Covington et al., 2002; Minnes et al., 2006; Rivkin et al., 2008; Shankaran et al., 2011). However, in other studies, no detrimental effects of PCE were found on childhood growth (Arendt et al., 2004; Bada et al., 2012; Chasnoff et al., 1998; Frank et al., 2002; Kilbride et al., 2006; Lumeng et al., 2007; Warner et al., 2006a), the effects on growth were not reported, or an increase in BMI and obesity was found in certain subgroups of offspring with PCE (LaGasse et al., 2011; Shankaran et al., 2010).
2. Methods
2.1. Study Design
Women 18 years or older who attended the prenatal clinic at Magee-Womens Hospital (MWH) in Pittsburgh, PA from March 1988 through December 1992 were eligible to participate. Written consent was obtained according to the guidelines of the University of Pittsburgh’s Institutional Review Board and the Research Review and Human Experimentation Committee of MWH. A Certificate of Confidentiality was obtained from the Department of Health and Human Services to assure participants that their responses could not be subpoenaed.
Women were initially approached for interview during their fourth or fifth prenatal month by trained research staff. Women were not enrolled if they came in for their first prenatal visit after the fifth month or if they did not speak English. No information was obtained from the medical charts about a woman’s drug use before she was asked to participate in the study. Ninety percent of the women approached agreed to be interviewed. Medical chart reviews were conducted to assess whether refusal to participate was associated with drug use. A random sample of those women who were approached but refused to participate was selected: Only 5% had a history of drug use during the current pregnancy.
At the initial assessment, women were interviewed about their use of cocaine, crack, alcohol, marijuana, tobacco and other drug use for the year prior to pregnancy and for the first trimester. Information was also obtained regarding sociodemographic characteristics, life events (Dohrenwend et al., 1978), social support (Berkman & Syme, 1979), and psychiatric symptomatology (Center for Epidemiologic Studies – Depression Scale [CES-D], Radloff, 1977; Spielberger State-Trait Anxiety Inventory [STAI], Spielberger et al., 1970). These domains comprise the core data set.
Of the women initially interviewed, 320 (18%) met the inclusion criteria and were enrolled in the study. All women who reported using any cocaine or crack during the first trimester were enrolled. The next woman interviewed who reported no cocaine or crack use during pregnancy or the year before pregnancy was also enrolled. This method of sampling resulted in the inclusion of women with a range of cocaine and crack use, allowing an examination of dose-response relationships between PCE and outcome.
Women selected for the study cohort were interviewed at seven months about their substance use during the second trimester and data were collected on all of the variables in the core data set. The women were interviewed again at 24 to 48 hours postpartum, when they were asked about third trimester substance use and the measures in the core data set were repeated. All newborns received comprehensive physical examinations, generally within 24 to 48 hours of delivery, by study nurse clinicians who were unaware of prenatal exposure status.
The mothers and infants were assessed at 1, 3, 7, and 10 years postpartum. At all phases, the mothers were interviewed with the core data set, including questions about substance use over the past year, sociodemographic characteristics, life events, social support, household composition, and psychiatric symptoms.
2.2 Sample Characteristics
Of the 320 women selected for the study, 17 became ineligible for participation because of abortion/miscarriage/infant death (N=5), home delivery (N=1), or moving out of the area (N=11). Of the remaining 303 eligible women, 1 was lost to follow-up and 2 refused further participation. Thus, delivery assessments were completed on 300 mothers. Four pairs of twins and 1 child with Trisomy 21 were excluded from further follow-up, yielding a birth cohort of 295 mothers and infants.
At the 10-year follow-up, 229 subjects out of the 295 in the birth cohort were seen (78% of the birth cohort). Five women refused the 10-year phase only, 10 refused any further contact, 26 were lost to follow-up, 17 moved, 3 children were in foster care and could not be located, and 5 children had died. Subjects who participated in the 10-year phase (N = 229) did not differ from those who did not participate (N = 66) on the following: prenatal alcohol, tobacco, marijuana, or other illicit drug exposure; maternal race, age, education, income, marital status, work status; parity, pregnancy, labor, or delivery complications; or infant birth weight, length, head circumference, or gestational age. The two groups did differ on third trimester cocaine use with a higher percentage of users among the 10-year participants than among the non-participants (12% vs. 3%, p < .01). Three children were excluded from the analyses (one with Alagille Syndrome and two with Tourette’s Syndrome), resulting in a final analysis file of 226.
At the 10-year follow-up, 13% of the children were not in maternal custody, in which case the custodian was interviewed. The mean age of the caregivers was 37 years (range = 27 - 74), their mean level of education was 12.8 years (range = 9 – 18), 50% were African American, 39% were married, 51% had a man living in the household, the median family income was $1650/month (range = $0 – $8,400), and 66% worked and/or attended school.
The mean age of the children at the 10-year assessment was 10.7 years (median = 10.7; SD = 0.6; range = 10 - 12). Ninety percent were seen by 11.5 years of age. Fifty-three percent were males. The average weight was 97.6 pounds (SD = 31; range = 51 - 207), the average height was 57.4 inches (SD = 3.4; range = 48 - 65), and the average head circumference was 544 mm (SD = 19; range = 500 – 620). The mean Stanford-Binet Intelligence Scale (SBIS) (4th Edition) (Thorndike et al., 1986) composite score was 94.7 (SD = 14.7; range = 54 – 149). At 10 years, 21% of the children were receiving special education services at school, and the median grade in school was fifth grade (range = 2 – 7).
2.3. Variables
2.3.1. Maternal Cocaine and Other Substance Use
Maternal cocaine and crack, tobacco, alcohol, marijuana, and other illicit drug use were assessed during confidential interviewing by research staff at each phase. Cocaine and crack use were reported in lines, rocks, or grams, or in cost at 10 years if the woman could not report quantity. Reported use of lines or rocks was converted into gram equivalents as follows: one line was estimated to be 1/30th (0.03) of a gram; one rock of crack was estimated to be 0.2 grams. Estimates were based on information from toxicology laboratories and law enforcement officials in Pittsburgh. Usual, maximum, and minimum quantity and frequency of cocaine/crack use were converted into average number of grams per day. Cocaine/crack use was converted back to lines/day for descriptive purposes. For these analyses, first trimester cocaine use was used in three ways: 1) as a continuous variable (grams/day), 2) as any use/no use, and 3) as frequent use (≥ 1 line/day) versus non-frequent use (< 1 line/day). Cocaine use was dichotomized into use/no use for the second and third trimesters and for the 10-year phase because of the small number of women who used during those time periods.
The alcohol and marijuana variables were average number of drinks or joints per day, respectively. Both were log transformed to reduce skewness. Tobacco use was analyzed as number of cigarettes per day. Alcohol, marijuana, and tobacco use were ascertained for each trimester of pregnancy, as well as at 10 years postpartum. These measures were used as continuous variables in the analyses. See Day and Robles (1989) and Richardson et al. (2008) for detailed information about calculation of the first trimester substance use variables.
2.3.2. Child Measures
At 10 years, the child’s height, weight, and head circumference were measured by the child assessment staff who were blind to prenatal and current drug use. A history of the child’s illnesses, injuries, and hospitalizations since the previous phase was obtained from the mother.
The mothers completed the EAS temperament survey (Buss & Plomin, 1984), a widely used and reliable measure of temperament, rating the child in terms of emotionality or distress, degree of activity, sociability, and shyness. The Cronbach’s alphas were 0.78, 0.75, 0.711, and 0.66 for the four domains, respectively. The Child Behavior Checklist (CBCL) (Achenbach, 1991a) was completed by the mother about the child’s behavior over the last six months. The Teacher Report Form (TRF) (Achenbach, 1991b), which parallels the parent version, was completed by the 10-year-old’s teacher about the child’s behavior over the last two months. The teachers had no knowledge of the child’s prenatal exposure status. The following CBCL and TRF t-scores were analyzed: withdrawn, anxious/depressed, attention problems, delinquent behavior, aggressive behavior, total internalizing problems, total externalizing problems, and total behavior problems. Mothers also completed the Home Observation for Measurement of the Environment – Short Form (HOME-SF: Baker & Mott, 1989), which assesses the quality of cognitive stimulation and emotional support provided by the family.
The children completed the Children’s Depression Inventory (CDI), a measure of childhood depression that was adapted from the Beck Depression Inventory (Kovacs, 1992). The CDI was developed for use with 7- to 17-year-olds. It has a first to third grade reading level, and good internal consistency and validity have been reported (Kovacs, 1992; Sitarenios & Stein, 2004). The CDI measures distress and general psychopathology over the last two weeks, rather than clinically defined depression, and assesses symptoms such as negative mood, negative self-esteem, and anhedonia. The total raw score was used as the outcome measure.
The Screen for Adolescent Violence Exposure (SAVE) (Hastings & Kelly, 1997) is a 28-item self-report scale assessing lifetime exposure to violence in the neighborhood, at school, and at home. Two samples totaling 1,250 inner-city 11- to 18-year-olds were used to develop the SAVE: Excellent reliability (alpha coefficients ranged from .65 to .95) and validity were demonstrated (Hastings & Kelly, 1997). In addition, the items were at an appropriate reading level for our sample. Examples of items include: “I have heard about someone getting shot”, “I have seen someone get badly hurt”, “Grownups hit me”, “Someone has pulled a knife on me”, and “I have seen someone get killed”. We adapted the SAVE for use in our study, changing the Likert-scale ratings into dichotomous responses (yes = directly experienced or witnessed; no = no experience or only seen on TV or heard on radio).
The CDI and the SAVE were given to the children to read and complete during their break in order to afford them privacy, unless the cognitive assessment indicated that their reading level was too low for them to complete the forms on their own. In this case, the examiner read the items to the child from her form, while the child indicated the response on his/her form.
2.4. Statistical Analyses
The relations between PCE and the outcome variables were first examined descriptively by comparing the means and box plots of the outcome variables between exposed and non-exposed children. Significant covariates for the final model were selected using stepwise multiple regression analyses. To explore the stability of the variables entering the model, the initial significance level for entry and removal was 0.10. Variables that were significant at an alpha of 0.05 were retained for the final models. Maternal/caregiver sociodemographic and psychosocial characteristics, child characteristics, and current and other prenatal substance use were considered as covariates because of their associations with the outcomes and/or PCE in initial bivariate analyses and based on the literature and prior analyses of these data. Table 1 shows the covariates that were considered for inclusion in the analyses. All regressions were run separately by trimester to assess the effects of exposure during each time period. Because weight at age 10 was positively skewed, it was log transformed for the regression analyses to reduce skewness.
Table 1.
Sample characteristics of variables considered for inclusion in the analyses
| No cocaine use 1st trimester |
Cocaine use 1st trimester |
p valuea | |
|---|---|---|---|
| First Trimester Maternal Characteristics | n = 126 | n = 100 | |
| African American (%) | 43 | 59 | < .05 |
| Age (yrs) (mean, SD) | 24.0 (4.5) | 26.4 (5.3) | < .001 |
| Education (yrs) (mean, SD) | 12.1 (1.4) | 11.9 (1.2) | ns |
| Family Income ($/mo) (mean, SD) | 734 (600) | 592 (690) | < .05 |
| Single (%) | 73 | 87 | < .05 |
| Work/Attend School (%) | 48 | 37 | ns |
| Cigarettes/Day (mean, SD) | 5.9 (8.5) | 10.2 (9.3) | < .001 |
| Drinks/Day (mean, SD) | 0.3 (0.6) | 1.9 (2.7) | < .001 |
| Joints/Day (mean, SD) | 0.07 (0.3) | 0.4 (1.0) | < .01 |
| Other Illicit Drugs (excluding cocaine) (%) | 4 | 11 | ns |
| 10-Year Caregiver Characteristics | |||
| Age (yrs) (mean, SD) | 35.7 (6.1) | 38.9 (7.1) | < .001 |
| Education (yrs) (mean, SD) | 12.8 (1.5) | 12.7 (1.6) | ns |
| Family income ($/mo) (mean, SD) | 2196 (1533) | 1704 (1207) | < .01 |
| Single (%) | 50 | 74 | < .001 |
| Work/Attend school (%) | 65 | 68 | ns |
| Cigarettes/Day (mean, SD) | 7.1 (11.0) | 8.4 (9.3) | < .05 |
| Drinks/Day (mean, SD) | 0.5 (1.0) | 1.2 (1.7) | < .001 |
| Joints/Day (mean, SD) | 0.04 (0.3) | 0.10 (0.4) | ns |
| Cocaine (% use) | 0.8 | 21.0 | < .001 |
| Other Drugs (excluding cocaine) (%) | 2 | 6 | ns |
| Depression (CES-D) (mean, SD) | 39.9 (10.3) | 40.6 (9.0) | ns |
| Hostility (STAI) (mean, SD) | 15.6 (3.9) | 16.8 (4.8) | < .05 |
| Home environment (HOME) (mean, SD) | 12.8 (2.7) | 12.6 (2.7) | ns |
| Household size (#) (mean, SD) | 4.3 (1.4) | 4.2 (1.6) | ns |
| Life events (mean, SD) | 4.2 (0.3) | 4.6 (0.3) | ns |
| Maternal height (ins) (mean, SD) | 64.3 (2.1) | 64.6 (2.8) | ns |
| 10-Year Child Characteristics | |||
| Age (yrs) (mean, SD) | 10.8 (0.6) | 10.6 (0.6) | ns |
| Custody status (% non-maternal) | 6 | 22 | < .001 |
| Exposure to violence (SAVE) (mean, SD) | 6.2 (4.0) | 8.2 (4.5) | < .001 |
| Gender (% male) | 53 | 53 | ns |
| Grade in school (mean, SD) | 5.3 (0.8) | 5.1 (0.8) | ns |
| Number of hospitalizations (mean, SD) | 0.2 (0.6) | 0.2 (0.4) | ns |
| Number of illnesses (mean, SD) | 0.6 (0.8) | 0.6 (0.9) | ns |
| Number of siblings (mean, SD) | 1.6 (1.2) | 1.4 (1.2) | ns |
| Special education (% yes) | 21 | 20 | ns |
| Stanford-Binet Composite Score (mean, SD) | 94.4 (14.3) | 95.2 (15.1) | ns |
Based on t-test or Mann-Whitney for continuous variables and on Chi-square test for dichotomous variables.
The TRF was missing for 55 subjects. We first examined whether the missing cases were associated with PCE. The missing rates among non-exposed and exposed offspring were not significantly different (27 vs. 21%, respectively; p = 0.3). We then imputed 43 cases based on the TRF from the previous phase to increase the statistical testing power. We were not able to impute the remaining 12 cases because of insufficient data. Imputation did not change the relation between the TRF anxiety scale and PCE. The means for the non-exposed and exposed subjects prior to imputation were 53.2 and 54.7; after imputation they were 53.3 and 54.8, respectively.
Residuals and the modified Cook’s statistic (Cook & Weisberg, 1982) were used to identify possible outliers and influential points. There were no outliers or influential points for weight, height, or the CBCL, one outlier and one influential case for head circumference, and one influential point each for the CDI, EAS, and TRF. The tolerance of each predictor was examined to assure that the estimated regression slopes were not unstable due to multicollinearity. Only those relations with PCE that remained stable after removal of the outliers or influential cases are included in this report.
Mediating analyses were also conducted to determine the effects of exposure to violence and of birth weight. Mediation was evaluated through path analysis using the product of coefficients (Sobel, 1987) and by examining the significance of the direct effect after inclusion of the mediating effect in the model. If the direct effect remained significant, we considered the mediation to be partial. Parameters were estimated using Lisrel 8.5 (Jöreskog & Sörbom, 2001).
3. Results
3.1. Descriptive Analyses
During the first, second, and third trimesters, 42.4%, 7.5%, and 10.3% of the women used cocaine, respectively, and 18.6%, 4.5%, and 6.2% used ≥ 1 line/day, respectively. The mean level of cocaine use for the women who used during the first trimester was approximately 8 lines/day. Of these women, 50% reported snorting powder cocaine only, while the rest smoked crack. During the second and third trimesters, the mean level of use among the users was approximately 6 lines/day and 5 lines/day, respectively. Of the women who used during the third trimester, 20% reported snorting powder cocaine only and the rest smoked crack. All women who used second and third trimester had used first trimester. Cocaine use increased during the early postpartum years (14.9% and 12.9% at 1 and 3 years), but decreased to 8.6% and 9.7% at 7 and 10 years, respectively. At 10 years, 3.5% used ≥ 1 line/day and the mean level of use for the users was approximately 4 lines/day; 67% used powder cocaine only and 33% used crack.
At enrollment, women who used cocaine during the first trimester were more likely to be African-American, older, had lower family incomes, and were more likely to be single than were non-users during the first trimester (Table 1). First trimester users were also significantly more likely to use more tobacco cigarettes, alcohol, and marijuana during the first trimester than were the first trimester non-users. At 10 years, these same characteristics continued to differ; family income was lower, more caregivers were single, and current use of tobacco cigarettes, alcohol, and cocaine was greater among first trimester users compared to non-users. In addition, at 10 years, caregiver hostility, non-maternal custody, and exposure to violence were higher among the offspring of first trimester users compared to those of non-users (Table 1).
3.2. Regression Analyses
First trimester cocaine use was a significant predictor of reduced weight, height, and head circumference at 10 years of age (Table 2). In addition, second trimester cocaine use significantly predicted head circumference at 10 years. The adjusted mean growth parameters were significantly different between the offspring of the women who did and did not use cocaine during the first trimester: Children exposed to cocaine during the first trimester of pregnancy weighed 10 pounds less (92 vs. 102 lbs, p < .01) and were an inch shorter (57 vs. 58 in, p < .04); their head circumference was six millimeters smaller (540 vs. 546 mm, p < .05), they had a lower body mass index (19.4 vs. 21.4, p < .05), and they were less likely to be obese (12 vs. 33% > 95th percentile, p < .001) than the unexposed children, after adjusting for significant covariates.
Table 2.
10-year outcomes that were significantly predicted by PCEa
| Outcome Variable | Total R2 | Significant Predictors | Raw Beta | Standardized regression coefficient | p value |
|---|---|---|---|---|---|
| Weight | 0.15 | Child age | 0.2 | 0.32 | < .001 |
| First trimester cocaine | -0.1 | -0.14 | < .05 | ||
| Current illicit drug useb | -0.2 | -0.12 | < .05 | ||
| Height | 0.36 | Child age | 2.7 | 0.50 | < .001 |
| Maternal height | 0.2 | 0.18 | < .001 | ||
| Genderc | -1.0 | -0.15 | < .01 | ||
| Raced | -1.0 | -0.15 | < .01 | ||
| First trimester cocaine | -0.7 | -0.10 | < .05 | ||
| Current illicit drug useb | -1.8 | -0.10 | < .05 | ||
| Head circumference | 0.11 | Child age | 7.5 | 0.27 | < .001 |
| First trimester cocaine | -6.1 | -0.18 | < .01 | ||
| Second trimester cocaine | -8.1 | -0.12 | < .05 | ||
| EAS sociability | 0.13 | Maternal depression | -0.02 | -0.24 | < .01 |
| Raced | 0.2 | 0.15 | < .01 | ||
| First trimester cocaine | -0.4 | -0.15 | < .05 | ||
| Current maternal alcohol | 0.2 | 0.13 | < .05 | ||
| Genderc | -0.2 | -0.12 | < .05 | ||
| CBCL withdrawn | 0.18 | Maternal depression | 0.2 | 0.35 | < .001 |
| Current maternal alcohol | -2.1 | -0.16 | < .01 | ||
| Home environment | -0.3 | -0.13 | < .05 | ||
| First trimester cocaine | 3.5 | 0.12 | < .05 | ||
| TRF anx/depressed | 0.01 | First trimester cocaine | 1.3 | 0.12 | < .05 |
| CDI total | 0.11 | SBIS composite score | -0.1 | -0.27 | < .001 |
| Maternal depression | 0.1 | 0.15 | < .01 | ||
| First trimester cocaine | 1.2 | 0.11 | < .05 |
Results are presented only for those child outcomes where prenatal cocaine was significant. Predictors are listed in order of standardized regression coefficient, an indication of the magnitude of the effect. Analyses were run separately by trimester but are shown together for ease of presentation.
Excludes marijuana and cocaine
0 = Female, 1 = Male
0 = African-American, 1 = Caucasian
First trimester cocaine use was a significant predictor of the child being rated by the mother as less sociable on the EAS and as more withdrawn on the CBCL (Table 2). In addition, the teacher’s report of the child’s anxious/depressed symptoms was predicted by first trimester cocaine use. There were no detrimental effects of first trimester cocaine exposure on the other scales of the CBCL or the TRF. First trimester cocaine use was also a significant predictor of more self-reported depressive symptoms on the Children’s Depression Inventory at 10 years (Table 2). The children who were exposed to cocaine during the first trimester had adjusted CDI raw scores that were higher than those children who were not exposed during the first trimester (7.3 vs. 6.1, p < .05). There were no effects of second or third trimester use on any of the behavior outcomes.
3.3. Mediating Analyses
As can be seen in Figure 1, exposure to violence mediated the effect of first trimester exposure on the CDI. That is, the effect of first trimester use on 10-year child depressive symptoms was due to the effect of first trimester use on child exposure to violence. The indirect effect, measured as the product of coefficients, was significant (Sobel z = 2.7, p < .01). The standardized total effect of PCE on the CDI was equal to 0.13. Almost half of the effect of PCE on the CDI was due to mediation through exposure to violence. Exposure to violence also mediated the significant effects of PCE on the TRF anxious/depressed scale. Exposure to violence was not significantly related to the EAS sociability or CBCL withdrawn subscales and therefore did not mediate the effects of PCE on these variables: The direct effect of first trimester exposure remained significant.
Figure 1.
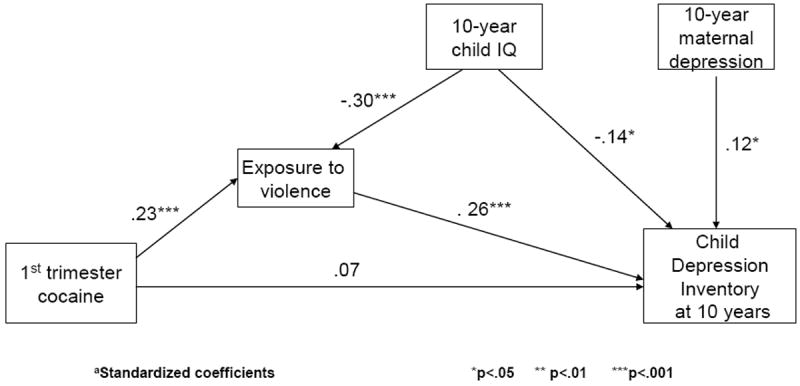
Exposure to violence mediates the relation between first trimester cocaine exposure and child depressive symptoms at 10 yearsa
We also tested whether the effect of PCE on birth weight (Richardson et al., 1999) mediated the effect of PCE on 10-year weight. We found that there was both an indirect effect of first trimester use and birth weight on 10-year weight (Sobel z = -1.9, p = .05) and a direct effect of first trimester exposure on 10-year weight (t = -1.8, p < .05) (Figure 2).
Figure 2.
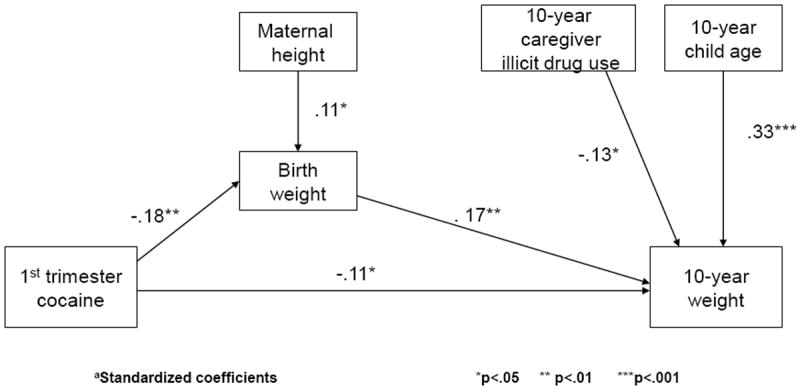
Birth weight partially mediates the relation between first trimester cocaine exposure and weight at 10 yearsa
4. Discussion
In this 10-year follow-up of a prospective, longitudinal study, we investigated whether PCE was related to growth and behavior, as was found at previous phases. In the growth domain, first trimester cocaine use predicted reduced 10-year weight, height, and head circumference. There was a direct effect of PCE on 10-year weight that was not solely due to the effects of PCE on birth weight (Richardson et al., 1999). Most researchers have either not reported whether there are effects of PCE on child growth in this age group or have not found effects (Rivkin et al., 2008; Warner et al., 2006a). We found that both 10-year weight and height were affected by first trimester use, which is consistent with symmetric effects on growth that result from exposure early in pregnancy (Bayer et al., 1993; Villar & Belizan, 1982). These findings are an indication that PCE has a long-lasting effect on growth, consistent with teratologic principles.
We also found that first trimester cocaine exposure predicted maternal ratings of less sociability and more withdrawn behaviors in the offspring, teacher ratings of more anxious/depressed behaviors, and child self-report of more depressive symptoms. These effects of PCE remained significant after controlling for sociodemographic characteristics and for other prenatal and current substance use. Thus, the domain of withdrawn/depressive behavior is consistently affected, regardless of the source of the report. We also found that current maternal depression was a predictor of child withdrawn/depressed behaviors. This finding is consistent with other literature: Offspring of depressed mothers have an increased risk of psychopathology, including internalizing symptoms and depression (Hammen, 2009; Weissman et al, 1997).
At 7 years, we found that PCE predicted both internalizing and externalizing behaviors. Thus, our findings of continued effects on internalizing behaviors at 10 years are consistent with our previous phases, as well as with other literature (Bada et al., 2007; Buckingham-Howes et al., 2012). We did not find any effects of PCE on externalizing behaviors at 10 years. As we showed in the introduction, there are inconsistencies in the literature in terms of the effects of PCE on externalizing behaviors, with findings varying by type of assessment and observer, and by gender. This conclusion was also reached by Ackerman et al. (2010) and Buckingham-Howes et al. (2013). At age 15, we did find that PCE was predictive of adolescent substance use, independent of these earlier 10-year effects (Richardson et al., 2013). Future analyses will investigate whether the effects of PCE on internalizing symptoms continue into adolescence, a neglected area of investigation (Buckingham-Howes et al., 2013).
A unique contribution of this report is that we investigated the mediating effect of exposure to violence. Childhood exposure to violence was correlated with both PCE and with 10-year behavior. The effect of violence exposure on internalizing behaviors in children is consistent with a recent meta-analysis (Fowler et al., 2009). In our analyses, when violence exposure was modeled as a mediator between PCE and 10-year behavior, PCE no longer predicted self-reported depressive symptoms or teacher reports of anxious/depressed behaviors. However, there continued to be direct effects of PCE on mother-reported child temperament characteristics of lower sociability and increased withdrawn behaviors. Childhood exposure to violence may not have an effect on temperament, in contrast to the effects of violence exposure on mood states such as depressive symptoms, which are more variable across development. Temperament is a personality characteristic that is relatively stable across development (Buss & Plomin, 1984; Mathiesen & Tambs, 1999; Novosad & Thoman, 1999). PCE may exert a biological effect on development that is observed in this measure of temperament.
In this and previous reports from our study, first trimester cocaine use has been a significant predictor of maternal and teacher reports of temperament and behavior (Richardson et al., 2008, 2009, 2011). PCE has been shown in laboratory studies to alter neural development, affecting brain function and behavior, and to have a direct effect on the development of monoaminergic systems (Meyer et al., 1993; Ronnekleiv et al., 1998; Seidler & Slotkin, 1992; Whitaker-Azmitia 1998), which regulate neuronal proliferation and migration (Buznikov et al., 2001; Levitt 1998; Lidow & Wang, 1995) and disrupt cortical architecture (McCarthy & Bhide, 2012; Seidler et al., 1995; Zachor et al., 1994). These direct effects of PCE on the developing nervous system may cause changes in brain structure and function that are an underlying mechanism for the behavioral outcomes reported here.
Strengths of this study include our assessment of the spectrum of exposure and the timing of PCE on development. Most other studies aggregate cocaine use across pregnancy and do not evaluate the timing of exposure. This sample represents the typical pattern of substance use in a general population of pregnant women and allows us to study the effects of exposure to cocaine early in pregnancy. One drawback of the lower prevalence of second and third trimester cocaine use is a potential lack of power to detect effects during these trimesters: We may have detected more effects with a higher prevalence of use. Additional strengths of the study include the prospective design, good follow-up rates, and statistical control for confounding factors, including other drugs and environmental influences such as exposure to violence. In addition, we have three different reporters of child behavior, the child, mother, and teacher, which strengthens confidence in the results.
A potential limitation of this study is that we do not have biological measures of prenatal drug use. This could lead to misclassification of women who denied use. However, this would reduce differences between groups, making the significant findings conservative estimates of the effects of PCE. Moreover, the short time period for detection with biological screening fails to detect many cocaine users (Julien, 1995, Verebey, 1987). We (Richardson et al., 1999) and others (Lester et al., 2001; Zuckerman et al., 1989) have reported that well-conducted interviews identify more users than does urine screening, and are an effective way to characterize the quantity, timing, and pattern of use (Richardson et al., 2006).
In summary, in this continued follow-up of a prospective, longitudinal study, we found that PCE was associated with child, maternal, and teacher reports of internalizing behaviors, consistent with our findings at previous phases. We also found that environmental characteristics such as exposure to violence play an important role in evaluating the effects of PCE. Exposure to violence mediated the effect of PCE on child and teacher reports of depressive symptoms, but not of maternal reports of sociability and withdrawn behaviors. These temperament characteristics may have implications for later psychiatric sequelae (Windle & Windle, 2006).
Highlights.
-
■
We examined child growth and behavior in a longitudinal study of prenatal cocaine use.
-
■
First trimester cocaine use predicted decreased growth at 10 years.
-
■
First trimester cocaine use predicted mother, teacher, and child ratings of behavior.
-
■
Child exposure to violence influenced the effect of prenatal cocaine use on some behaviors.
-
■
These behaviors may have implications for later psychiatric problems.
Acknowledgments
This research was supported by the National Institute on Drug Abuse grants DA05460 and DA12401 (G. Richardson, Principal Investigator).
Footnotes
One item in the sociability subscale (“when s/he is alone, my child feels isolated”) did not correlate with any of the other items in that subscale. It was excluded, thus increasing the internal consistency of the subscale.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accornero V, Anthony JC, Morrow CE, Xue L, Bandstra ES. Prenatal cocaine exposure: an examination of childhood externalizing and internalizing behavior problems at age 7 years. Epidemiol Psichiatr Soc. 2006;15:20–9. [PMC free article] [PubMed] [Google Scholar]
- Accornero V, Morrow C, Bandstra E, Johnson A, Anthony J. Behavioral outcome of preschoolers exposed prenatally to cocaine: role of maternal behavioral health. J Pediatr Psychol. 2002;27:259–69. doi: 10.1093/jpepsy/27.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achenbach T. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. Burlington, VT: University of Vermont Department of Psychiatry; 1991a. [Google Scholar]
- Achenbach T. Manual for the Teacher’s Report Form and 1991 Profile. Burlington, VT: University of Vermont Department of Psychiatry; 1991b. [Google Scholar]
- Ackerman JP, Riggins T, Black MM. A review of the effects of prenatal cocaine exposure among school-aged children. Pediatrics. 2010;125:554–65. doi: 10.1542/peds.2009-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt RE, Short EJ, Singer LT, Minnes S, Hewitt J, Flynn S, et al. Children prenatally exposed to cocaine: developmental outcomes and environmental risks at seven years of age. J Dev Behav Pediatr. 2004;25:83–90. doi: 10.1097/00004703-200404000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bada HS, Bann CM, Bauer CR, Shankaran S, Lester B, LaGasse L, et al. Preadolescent behavior problems after prenatal cocaine exposure: relationship between teacher and caretaker ratings (Maternal Lifestyle Study) Neurotoxicol Teratol. 2011;33:78–87. doi: 10.1016/j.ntt.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bada H, Bann C, Whitaker T, Bauer C, Shankaran S, LaGasse L, et al. Protective factors can mitigate behavior problems after prenatal cocaine and other drug exposures. Pediatrics. 2012;130:1479–88. doi: 10.1542/peds.2011-3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bada H, Das A, Bauer CR, Shankaran S, Lester B, LaGasse L, et al. Impact of prenatal cocaine exposure on child behavior problems through school age. Pediatrics. 2007;119:348–59. doi: 10.1542/peds.2006-1404. [DOI] [PubMed] [Google Scholar]
- Bada H, Langer J, Twomey J, Bursi C, LaGasse L, Bauer C, et al. Importance of stability of early living arrangements on behavior outcomes of children with and without prenatal drug exposure. J Dev Behav Pediatr. 2008;29:173–82. doi: 10.1097/DBP.0b013e3181644a79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P, Mott F. National Longitudinal Study of Youth Child Handbook. Columbus, OH: Center for Human Resource Research, Ohio State University; 1989. [Google Scholar]
- Bayer S, Altman J, Russo R, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- Bennett D, Bendersky M, Lewis M. Children’s intellectual and emotional-behavioral adjustment at 4 years as a function of cocaine exposure, maternal characteristics, and environmental risk. Dev Psychol. 2002;38:648–58. doi: 10.1037//0012-1649.38.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D, Bendersky M, Lewis M. Preadolescent health risk behavior as a function of prenatal cocaine exposure and gender. J Dev Behav Pediatr. 2007;28:467–72. doi: 10.1097/DBP.0b013e31811320d8. [DOI] [PubMed] [Google Scholar]
- Bennett D, Marini V, Berzenski S, Carmody D, Lewis M. Externalizing problems in late childhood as a function of prenatal cocaine exposure and environmental risk. J Pediatr Psychol. 2013;38:296–308. doi: 10.1093/jpepsy/jss117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman L, Syme L. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol. 1979;109:186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF. Socioeconomic status and child development. Annu Rev Psychol. 2002;53:371–99. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- Bridgett DJ, Mayes LC. Development of inhibitory control among prenatally cocaine exposed and non-cocaine exposed youths from late childhood to early adolescence: the effects of gender and risk and subsequent aggressive behavior. Neurotoxicol Teratol. 2011;33:47–60. doi: 10.1016/j.ntt.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham-Howes S, Berger SS, Scaletti LA, Black MM. Systematic review of prenatal cocaine exposure and adolescent development. Pediatrics. 2013;131:e1917–36. doi: 10.1542/peds.2012-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham-Howes S, Oberlander SE, Kim EM, Black MM. Prenatal drug exposure and peer victimization in early adolescence: testing childhood anxiety/depression and aggression as possible mediators. J Dev Behav Pediatr. 2012;33:416–22. doi: 10.1097/DBP.0b013e31825609f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss A, Plomin R. Temperament: early developing personality traits. Hillsdale, NJ: L. Erlbaum, Publishers; 1984. [Google Scholar]
- Buznikov G, Lambert H, Lauder J. Serotonin and serotonin-like substances as regulators of early embryogenesis and morphogenesis. Cell Tissue Res. 2001;305:177–86. doi: 10.1007/s004410100408. [DOI] [PubMed] [Google Scholar]
- Chasnoff IJ, Anson A, Hatcher R, Stenson H, Laukea K, Randolph LA. Prenatal exposure to cocaine and other drugs: outcome at four to six years. Ann N Y Acad Sci. 1998;846:314–28. [PubMed] [Google Scholar]
- Cook R, Weisberg S. Residuals and influence in regression. New York: Chapman and Hall; 1982. [Google Scholar]
- Covington C, Nordstrom-Klee B, Ager J, Sokol R, Delaney-Black V. Birth to age 7 growth of children prenatally exposed to drugs. A prospective study. Neurotoxicol Teratol. 2002;24:489–96. doi: 10.1016/s0892-0362(02)00233-7. [DOI] [PubMed] [Google Scholar]
- Day N, Robles N. Methodological issues in the measurement of substance use. Ann N Y Acad Sci. 1989;562:8–13. doi: 10.1111/j.1749-6632.1989.tb21002.x. [DOI] [PubMed] [Google Scholar]
- Delaney-Black V, Chiodo LM, Hannigan JH, Greenwald MK, Janisse J, Patterson G, et al. Prenatal and postnatal cocaine expsoure predict teen cocaine use. Neurotoxicol Teratol. 2011;33:110–9. doi: 10.1016/j.ntt.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney-Black V, Covington C, Nordstrom B, Ager J, Janisse J, Hannigan JH, et al. Prenatal cocaine: quantity of exposure and gender moderation. J Dev Behav Pediatr. 2004;25:254–63. doi: 10.1097/00004703-200408000-00005. [DOI] [PubMed] [Google Scholar]
- Delaney-Black V, Covington C, Templin T, Ager J, Nordstrom-Klee B, Martier S, et al. Teacher-assessed behavior of children prenatally exposed to cocaine. Pediatrics. 2000;106:782–91. doi: 10.1542/peds.106.4.782. [DOI] [PubMed] [Google Scholar]
- Dohrenwend BS, Krasnoff K, Askenasy AR, Dohrenwend P. Exemplification of a method for scaling life events: the PERI life events scale. J Health Soc Behavior. 1978;19:205–29. [PubMed] [Google Scholar]
- Duncan GJ, Brooks-Gunn J, Klebanov P. Economic deprivation and early childhood development. Child Dev. 1994;65:296–318. [PubMed] [Google Scholar]
- Eyler F, Behnke M, Conlon M, Woods N, Frentzen B. Prenatal cocaine use: a comparison of neonates matched on maternal risk factors. Neurotoxicol Teratol. 1994;16:81–7. doi: 10.1016/0892-0362(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Finkelhor D, Turner H, Ormrod R, Hamby SL. Violence, abuse, and crime exposure in a national sample of children and youth. Pediatrics. 2009;124:1411–24. doi: 10.1542/peds.2009-0467. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Lester BM, Dgarmo DS, LaGasse LL, Lin H, Shankaran S, et al. The combined effects of prenatal drug exposure and early adversity on neurobehavioral disinhibition in childhood and adolescence. Dev Psychopathol. 2011;23:777–88. doi: 10.1017/S0954579411000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler PJ, Tompsett CJ, Braciszewski JM, Jacques-Tiura AJ, Baltes BB. Community violence: a meta-analysis on the effect of exposure and mental health outcome of children and adolescents. Dev Psychopathol. 2009;21:227–59. doi: 10.1017/S0954579409000145. [DOI] [PubMed] [Google Scholar]
- Frank DA, Rose-Jacobs R, Beeghly M, Augustyn M, Bellinger D, Cabral H, et al. Level of prenatal cocaine exposure and scores on the Bayley Scales of Infant Development: modifying effects of caregiver, early intervention, and birth weight. Pediatrics. 2002;110:1143–52. doi: 10.1542/peds.110.6.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DA, Rose-Jacobs R, Crooks D, Cabral HJ, Gerteis J, Hacker KA, et al. Adolescent initiation of licit and illicit substance use: impact of intrauterine exposures and post-natal exposure to violence. Neurotoxicol Teratol. 2011;33:100–9. doi: 10.1016/j.ntt.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerteis J, Chartrand M, Martin B, Cabral JH, Rose-Jacobs R, Crooks D, et al. Are there effects of intrauterine cocaine exposure on delinquency during early adolescence? A preliminary report. J Dev Behav Pediatr. 2011;32:393–401. doi: 10.1097/DBP.0b013e318218d9f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald M, Chiodo L, Hannigan J, Sokol R, Janisse J, Delaney-Black V. Teens with heavy prenatal cocaine exposure respond to experimental social provocation with escape not aggression. Neurotoxicol Teratol. 2011;33:198–204. doi: 10.1016/j.ntt.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen CL. Children of depressed parents. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 2. New York: The Guilford Press; 2009. pp. 275–97. [Google Scholar]
- Hanson RF, Self-Brown S, Fricker-Elhai A, Kilpatrick DG, Saunders BE, Resnick H. Relations among parental substance use, violence exposure and mental health: the national survey of adolescents. Addict Behav. 2006;31:1988–2001. doi: 10.1016/j.addbeh.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Hastings T, Kelly M. Development and validation of the Screen for Adolescent Violence Exposure (SAVE) J Abnorm Child Psychol. 1997;25:511–20. doi: 10.1023/a:1022641916705. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Jöreskog K, Sörbom D. LISREL 8.5 for Windows [Computer software] Lincolnwood, IL: Scientific Software International, Inc; 2001. [Google Scholar]
- Julien R. A primer of drug action. 7. New York: W.H. Freeman Company; 1995. [Google Scholar]
- Kable JA, Coles CD, Lynch ME, Platzman K. Physiological responses to social and cognitive challenges in 8-year olds with a history of prenatal cocaine exposure. Dev Psychobiol. 2008;50:251–65. doi: 10.1002/dev.20285. [DOI] [PubMed] [Google Scholar]
- Kilbride HW, Castor CA, Fuger KL. School-age outcome of children with prenatal cocaine exposure following early case management. J Dev Behav Pediatr. 2006;27:181–7. doi: 10.1097/00004703-200606000-00001. [DOI] [PubMed] [Google Scholar]
- Kovacs M. Children’s Depression Inventory. N. Tonawanda, NY: Multi-Health Systems, Inc; 1992. [Google Scholar]
- LaGasse L, Gaskins R, Bada H, Shankaran S, Liu J, Lester B, et al. Prenatal cocaine exposure and childhood obesity at nine years. Neurotoxicol Teratol. 2011;33:188–97. doi: 10.1016/j.ntt.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGasse LL, Hammond J, Liu J, Lester BM, Shankaran S, Bada H, et al. Violence and delinquency, early onset drug use, and psychopathology in drug-exposed youth at 11 years. Ann N Y Acad Sci. 2006;1094:313–8. doi: 10.1196/annals.1376.041. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–29. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lester B, ElSohly M, Wright L, Smeriglio V, Verter J, Bauer C, et al. The Maternal Lifestyle Study: drug use by meconium toxicology and maternal self-report. Pediatrics. 2001;107:309–17. doi: 10.1542/peds.107.2.309. [DOI] [PubMed] [Google Scholar]
- Lester B, Lin H, DeGarmo D, Fisher P, LaGasse L, Levine T, et al. Neurobehavioral disinhibition predicts initiation of substance use in children with prenatal cocaine exposure. Drug Alcohol Depend. 2012;126:80–6. doi: 10.1016/j.drugalcdep.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt P. Prenatal effects of drugs of abuse on brain development. Drug Alcohol Depend. 1998;51:109–25. doi: 10.1016/s0376-8716(98)00070-2. [DOI] [PubMed] [Google Scholar]
- Lidow M, Wang F. Neurotransmitter receptors in the developing cerebral cortex. Crit Rev Neurobiol. 1995;9:395–418. [PubMed] [Google Scholar]
- Linares TJ, Singer LT, Kirchner L, Short EJ, Min MO, Hussey P, et al. Mental health outcomes of cocaine-exposed children at 6 years of age. J Pediatr Psychol. 2006;31:85–97. doi: 10.1093/jpepsy/jsj020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng JC, Cabral HJ, Gannon K, Heeren T, Frank DA. Prenatal exposures to cocaine and alcohol and physical growth patterns to age 8 years. Neurotoxicol Teratol. 2007;29:446–57. doi: 10.1016/j.ntt.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiesen KS, Tambs K. The EAS temperament questionnaire – factor structure, age trends, reliability, and stability in a Norwegian sample. J Child Psychol Psychiatr. 1999;40:431–9. [PubMed] [Google Scholar]
- McCarthy DM, Bhide PG. Prenatal cocaine exposure decreases parvalbumin-immunoreactive neurons and GABA-to-projection neuron ration in the medial prefrontal cortex. Dev Neurosci. 2012;34:174–83. doi: 10.1159/000337172. [DOI] [PubMed] [Google Scholar]
- McLaughlin AA, Minnes S, Singer LT, Min M, Short EJ, Scott TL, et al. Caregiver and self-report of mental health symptoms in 9-year old children with prenatal cocaine exposure. Neurotoxicol Teratol. 2011;33:582–91. doi: 10.1016/j.ntt.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod BD, Weisz JR, Wood JJ. Examining the association between parenting and childhood depression: a meta-analysis. Clin Psychol Rev. 2007;27:986–1003. doi: 10.1016/j.cpr.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Meyer J, Shearman L, Collins L, Maguire R. Cocaine binding sites in fetal rat brain: implications for prenatal cocaine action. Psychopharmacology. 1993;112:445–51. doi: 10.1007/BF02244892. [DOI] [PubMed] [Google Scholar]
- Minnes S, Robin NH, Alt AA, Kirchner HL, Satayathum S, Salbert BA, et al. Dysmorphic and anthropometric outcomes in 6-year-old prenatally cocaine-exposed children. Neurotoxicol Teratol. 2006;28:28–38. doi: 10.1016/j.ntt.2005.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnes S, Singer LT, Kirchner HL, Short E, Lewis B, Satayathum S, et al. The effects of prenatal cocaine exposure on problem behavior in children 4-10 years. Neurotoxicol Teratol. 2010;32:443–51. doi: 10.1016/j.ntt.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow C, Accornero V, Xue L, Manjunath S, Culbertson J, Anthony J, et al. Estimated risk of developing selected DSM-IV disorders among 5-year-old children with prenatal cocaine exposure. J Child Fam Stud. 2009;18:356–64. doi: 10.1007/s10826-008-9238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Drug Abuse. Drug use among women delivering livebirths: 1992. Rockville, MD: US Department of Health and Human Services; 1996. National Pregnancy and Health Survey. [Google Scholar]
- Nordstrom Bailey B, Sood BG, Sokol RJ, Ager J, Janisse J, Hannigan JH, et al. Gender and alcohol moderate prenatal cocaine effects on teacher-report of child behavior. Neurotoxicol Teratol. 2005;27:181–9. doi: 10.1016/j.ntt.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Novosad C, Thoman EB. Stability of temperament over the childhood years. Am J Orthopsychiatr. 1999;69:457–64. doi: 10.1037/h0080394. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:385–401. [Google Scholar]
- Richardson GA, Goldschmidt L, Leech S, Willford J. Prenatal cocaine exposure: effects on mother- and teacher-rated behavior problems and growth in school-age children. Neurotoxicol Teratol. 2011;33:69–77. doi: 10.1016/j.ntt.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Goldschmidt L, Willford J. The effects of prenatal cocaine use on infant development. Neurotoxicol Teratol. 2008;30:96–106. doi: 10.1016/j.ntt.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Goldschmidt L, Willford J. Continued effects of prenatal cocaine use: preschool development. Neurotoxicol Teratol. 2009;31:325–33. doi: 10.1016/j.ntt.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Hamel S, Goldschmidt L, Day N. Growth of infants prenatally exposed to cocaine/crack: a comparison of a prenatal care and a no prenatal care sample. Pediatrics. 1999;104:e18. doi: 10.1542/peds.104.2.e18. [DOI] [PubMed] [Google Scholar]
- Richardson GA, Huestis M, Day N. Assessing in utero exposure to cannabis and cocaine. In: Bellinger DC, editor. Human developmental neurotoxicology. New York: Taylor & Francis Group; 2006. pp. 287–302. [Google Scholar]
- Richardson GA, Larkby C, Goldschmidt L, Day NL. Adolescent initiation of drug use: effects of prenatal cocaine exposure. J Am Acad Child Adolesc Psychiatry. 2013;52:37–46. doi: 10.1016/j.jaac.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivkin MJ, Davis PE, Lemaster JL, Cabral HJ, Warfield SK, Mulkern RV, et al. Volumetric MRI study of brain in children with intrauterine exposure to cocaine, alcohol, tobacco, and marijuana. Pediatrics. 2008;121:741–50. doi: 10.1542/peds.2007-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnekleiv O, Fang Y, Choi W, Chai L. Changes in the midbrain-rostral forebrain dopamine circuitry in the cocaine-exposed primate fetal brain. Ann N Y Acad Sci. 1998;846:165–81. [PubMed] [Google Scholar]
- Savage J, Brodsky N, Malmud E, Giannetta J, Hurt H. Attentional functioning and impulse control in cocaine-exposed and control children at age ten years. J Dev Behav Pediatr. 2005;26:42–7. [PubMed] [Google Scholar]
- Schwab-Stone M, Chen C, Greenberger E, Silver D, Lichtman J, Voyce C. No safe haven. II: the effects of violence exposure on urban youth. J Am Acad Child Adolesc Psychiatry. 1999;38:359–67. doi: 10.1097/00004583-199904000-00007. [DOI] [PubMed] [Google Scholar]
- Schwab-Stone M, Koposov R, Vermeiren R, Ruchkin V. Cross-cultural findings on community violence exposure and internalizing psychopathology: comparing adolescents in the United States, Russia, and Belgium. Child Psychiatry Hum Dev. 2012 doi: 10.1007/s10578-012-0344-8. published online November. [DOI] [PubMed] [Google Scholar]
- Seidler F, Slotkin T. Fetal cocaine exposure causes persistent noradrenergic hyperactivity in rat brain regions: effects on neurotransmitter turnover and receptors. J Pharmacol Exp Ther. 1992;263:413–21. [PubMed] [Google Scholar]
- Seidler F, Temple S, McCook E, Slotkin T. Cocaine inhibits central noradrenergic and dopaminergic activity during the critical developmental period in which catecholamines influence cell development. Brain Res. 1995;85:48–53. doi: 10.1016/0165-3806(94)00186-4. [DOI] [PubMed] [Google Scholar]
- Shankaran S, Bann C, Bauer C, Lester B, Bada H, Das A, et al. Prenatal cocaine exposure and BMI and blood pressure at 9 years of age. J Hypertens. 2010;28:1166–75. [PMC free article] [PubMed] [Google Scholar]
- Shankaran S, Das A, Bauer C, Bada H, Lester B, Wright L, et al. Prenatal cocaine exposure and small-for-gestational-age status: effects on growth at 6 years of age. Neurotoxicol Teratol. 2011;33:575–81. doi: 10.1016/j.ntt.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitarenios G, Stein S. Use of the Children’s Depression Inventory. In: Maruish ME, editor. The use of psychological testing for treatment planning and outcomes assessment. 3. Mahwah, NJ: L. Erlbaum Associates, Publishers; 2004. pp. 1–37. [Google Scholar]
- Sobel M. Direct and indirect effects in linear structural equation models. Sociol Method Res. 1987;16:155–76. [Google Scholar]
- Sood BG, Nordstrom Bailey B, Covington C, Sokol RJ, Ager J, Janisse J, et al. Gender and alcohol moderate caregiver reported child behavior after prenatal cocaine. Neurotoxicol Teratol. 2005;27:191–201. doi: 10.1016/j.ntt.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Spence SH, Najman JM, Bor W, O’Callaghan MJ, Williams GM. Maternal anxiety and depression, poverty and marital relationship factors during early childhood as predictors of anxiety and depressive symptoms in adolescence. J Child Psychol Psychiat. 2002;43:457–69. doi: 10.1111/1469-7610.00037. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press, Inc.; 1970. [Google Scholar]
- Thorndike R, Hagen E, Sattler J. The Stanford-Binet Intelligence Scale. Fourth Edition. Chicago, IL: Riverside Publishing Company; 1986. [Google Scholar]
- Verebey K. Cocaine abuse detection by laboratory methods. In: Washton AM, Gold MS, editors. Cocaine - A clinician’s handbook. New York: Guilford Press; 1987. pp. 214–28. [Google Scholar]
- Villar J, Belizan J. The timing factor in the pathophysiology of the intrauterine growth retardation syndrome. Obstet Gynecol Surv. 1982;37:499–506. doi: 10.1097/00006254-198208000-00001. [DOI] [PubMed] [Google Scholar]
- Vorhees C. Concepts in teratology and developmental toxicology derived from animal research. In: Hutchings D, editor. Ann NY Acad Sci. Vol. 562. 1989. pp. 31–41. Prenatal abuse of licit and illicit drugs. [DOI] [PubMed] [Google Scholar]
- Warner TD, Behnke M, Eyler FD, Padgett K, Leonard C, Hou W, et al. Diffusion tensor imaging of frontal white matter and executive functioning in cocaine-exposed children. Pediatrics. 2006a;118:2014–24. doi: 10.1542/peds.2006-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner TD, Behnke M, Eyler FD, Szabo NJ. Early adolescent cocaine use as determined by hair analysis in a prenatal cocaine exposure cohort. Neurotoxicol Teratol. 2011;33:88–99. doi: 10.1016/j.ntt.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner TD, Behnke M, Hou W, Garvan CW, Wobie K, Eyler FD. Predicting caregiver-reported behavior problems in cocaine-exposed children at 3 years. J Dev Behav Pediatr. 2006b;27:83–92. doi: 10.1097/00004703-200604000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Warner V, Wickramaratne P, Moreau D, Olfson M. Offspring of depressed parents. 10 Years later. Arch Gen Psychiatry. 1997;54:932–40. doi: 10.1001/archpsyc.1997.01830220054009. [DOI] [PubMed] [Google Scholar]
- Whitaker T, Bada H, Bann C, Shankaran S, LaGasse L, Lester B, et al. Serial pediatric symptom checklist screening in children with prenatal drug exposure. J Dev Behav Pediatr. 2011;32:206–15. doi: 10.1097/DBP.0b013e318208ee3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker-Azmitia P. Role of the neurotrophic properties of serotonin in the delay of brain maturation induced by cocaine. Ann N Y Acad Sci. 1998;846:158–64. [PubMed] [Google Scholar]
- Windle M, Windle RC. Adolescent temperament and lifetime psychiatric and substance abuse disorders assessed in young adulthood. Pers Indiv Differ. 2006;41:15–25. [Google Scholar]
- Zachor D, Cherkes J, Fay C, Ocrant I. Cocaine differentially inhibits neuronal differentiation and proliferation in vitro. J Clin Invest. 1994;93:1179–85. doi: 10.1172/JCI117071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman B, Frank D, Hingson R, Amaro H, Levenson S, Kayne H, et al. Effects of maternal marijuana and cocaine use on fetal growth. N Engl J Med. 1989;320:762–8. doi: 10.1056/NEJM198903233201203. [DOI] [PubMed] [Google Scholar]


