SUMMARY
The interaction between episodic retrieval and visual attention is relatively unexplored. Given that systems mediating attention and episodic memory appear to be segregated, and perhaps even in competition, it is unclear how visual attention is recruited during episodic retrieval. We investigated the recruitment of visual attention during the suppression of gist-based false recognition, the tendency to falsely recognize items that are similar to previously encountered items. Recruitment of visual attention was associated with activity in the dorsal attention network. The inferior parietal lobule, often implicated in episodic retrieval, tracked veridical retrieval of perceptual detail and showed reduced activity during the engagement of visual attention, consistent with a competitive relationship with the dorsal attention network. These findings suggest that the contribution of the parietal cortex to interactions between visual attention and episodic retrieval entails distinct systems that contribute to different components of the task while also suppressing each other.
INTRODUCTION
Episodic memory and visual attention have conventionally been studied independently. As a result, their interaction is poorly understood. Nonetheless, it is likely that these systems interact extensively and that these interactions are functionally significant (Chun and Turk-Browne, 2007; Chun and Johnson, 2011; Chun et al., 2011). Broadly, attention can be divided into two forms: external attention, which refers to the selective processing of sensory input, and internal attention, which refers to the selective processing of internal representations maintained in the absence of an available sensory input and includes processes such as working memory, cognitive control, and long-term memory retrieval (Chun et al., 2011; Chun and Johnson, 2011). In the present paper, we focus on the interaction between external visual attention and episodic memory.
Two types of interactions between visual attention and episodic memory have been previously studied. First, perceptual processing of the visual environment benefits from recent experiences. For instance, when searching for a car when exiting a shopping mall, people presumably rely on both episodic memory and visual search. Several experiments have demonstrated that both implicit and explicit long-term memory can facilitate visual search (Chun, 2000; Summerfield et al., 2006; Becker and Rasmussen, 2008; Chanon and Hopfinger, 2008). Summerfield and colleagues (2006) found that visual search of complex scenes guided by recent experience is associated with activity in the hippocampus, a region known to be critical to episodic memory. Second, we tend to remember information that is attended to during encoding and forget information that is ignored during encoding (Wolfe et al., 2007; Uncapher and Rugg, 2009). Recently, Uncapher and colleagues (2011) have shown that the effect of attention on encoding can depend on how attention is engaged: under certain conditions, top-down attention can result in more effective memory encoding than bottom-up attention (see also Uncapher and Wagner, 2009). These two points of contact between visual attention and episodic memory have been the focus of the handful of studies that have examined the interaction between these two systems.
Episodic memory depends not only on the ability to encode information during the original event, but also on the ability to retrieve and interpret relevant information when it is required to achieve current goals. Although it is well known that visual attention can modulate the encoding of information into memory, the critical question of how episodic memory and visual attention interact when people are attempting to retrieve episodic memories has not been thoroughly explored.
Cognitive-behavioral research on source monitoring and memory distortions suggests that visual attention should play an important role in episodic memory retrieval. The ability to emphasize the retrieval of specific perceptual details, while deemphasizing the retrieval of other components of a memory, such as conceptual information or emotional associations, is a critical feature of episodic memory retrieval (Johnson et al., 1993; Schacter et al., 1999). Focusing on specific perceptual details is important for avoiding memory distortions (Johnson, 1997; Schacter et al., 1999), such as reality monitoring errors, which involve confusing material that was thought about or imagined with material that actually happened (Johnson et al., 1993). Attention to perceptual detail is also important for avoiding gist-based false recognition, which occurs when one mistakenly recognizes an item that has a general similarity to a previously encountered item: focusing on perceptual details that are diagnostic of an item’s prior presentation can lead to significant reductions in false recognition (Schacter et al., 1999; Gallo et al., 2004). Given the functional importance of attending to specific, diagnostic perceptual details stored in episodic memory, it seems likely that episodic retrieval should draw upon visual attention by directing attention toward the visual details of a cue that are relevant to the retrieval demands.
Functional neuroimaging findings also speak to the role of visual attention in episodic retrieval. Although not conventionally associated with episodic memory, a large number of neuroimaging studies have indicated that the left lateral parietal cortex systematically tracks the retrieval of information from episodic memory (Wagner et al., 2005; Cabeza et al., 2008; Vilberg and Rugg, 2008; Shimamura, 2011). Given a well-established role for the parietal cortex in external attention, it has been proposed that the parietal cortex may also control orienting toward and maintaining attention on internal mnemonic representations (Wagner et al., 2005; Cabeza et al., 2008). These proposals have prompted a debate about the relationship between episodic retrieval, attention, and the parietal cortex. Some investigators have argued that the neural signatures of episodic retrieval and attention represent a common parietal attention system (Cabeza, 2008; Cabeza et al., 2008; Ciaramelli et al., 2008), whereas others have argued that memory and attention are anatomically segregated within parietal cortex (Hutchinson et al., 2009; Sestieri et al., 2010). However, despite recent interest in the relationship between visual attention and episodic retrieval, there is a paucity of data concerning their direct interaction and, in particular, which neural systems are involved when episodic memory draws on visual attention to meet retrieval demands.
In the perceptual domain, in tasks such as visual search of cluttered displays or visual detection, top-down visual attention has been associated with activity in a set of regions commonly referred to as the dorsal attention network (Kastner and Ungerleider, 2000; Corbetta and Shulman, 2002). Within the lateral parietal cortex, this network includes the anterior intraparietal sulcus (IPS), the medial bank of the mid-IPS, the posterior IPS, and the superior parietal lobule. However, the regions of the lateral parietal cortex most consistently implicated in episodic retrieval are the lateral bank of the IPS and the inferior parietal lobule (IPL; Wagner et al., 2005). Indeed, activity in the IPL has been associated with the attempt to retrieve specific details from memory (e.g., Dobbins and Wagner, 2005). Recent observations suggest a striking division of labor within the lateral parietal cortex, linking the dorsal attention network with perception and the IPL with memory (Sestieri et al., 2010). Consistent with this proposal, functional magnetic resonance imaging (fMRI) studies have found that activity in the angular gyrus is highly correlated with the hippocampus at low frequencies (i.e., resting state connectivity), suggesting that these regions are functionally related to one another (Vincent et al., 2006). The angular gyrus and the hippocampus are part of a larger set of coactive regions, often referred to as the default network, which has been associated with disengagement from the external environment and processing of internally generated representations, such as episodic memories (Buckner et al., 2008). In fact, it has been suggested that the dorsal attention network and the default network are in a competitive relationship to one another, such that activation of one network implies suppression of the other (Fox et al., 2005), although it has also been suggested that this “anticorrelation” may reflect a statistical artifact (Murphy et al., 2009; Anderson et al., 2011). Given the proposal that the neural systems mediating attention and memory are anatomically segregated, and perhaps even in opposition, it is unclear what neural systems are involved when visual attention is recruited during episodic retrieval. Does the recruitment of visual attention by episodic retrieval engage the same brain regions implicated in top-down visual attention in the perceptual domain (dorsal attention network), brain regions associated with episodic retrieval (default network), or both?
In the experiment described here, we directly investigated the recruitment of visual attention during episodic retrieval. Specifically, we dissociated attention to specific perceptual detail and successful retrieval of specific perceptual detail. We accomplished this goal using a paradigm we recently developed that shows that gist-based false recognition, which occurs when one mistakenly recognizes an item that is similar to an item that was previously encountered (Reyna and Brainerd, 1995; Koutstaal and Schacter, 1997), occurs primarily because of a failure to retrieve detailed information that is still stored in memory (Guerin et al., 2012). Critically, our data established that attention to the specific perceptual details relevant to the task is not sufficient to overcome this failure. Rather, reinstatement of the studied item, a potent cue that enables participants to retrieve diagnostic details from memory, is required to substantially reduce gist-based false recognition. Thus, attention to specific perceptual details can occur in the absence of successful retrieval of task-relevant perceptual details. In addition to shedding light on the mechanisms leading to memory distortion, this experimental paradigm also enables us to isolate and directly investigate the recruitment of visual attention during episodic retrieval.
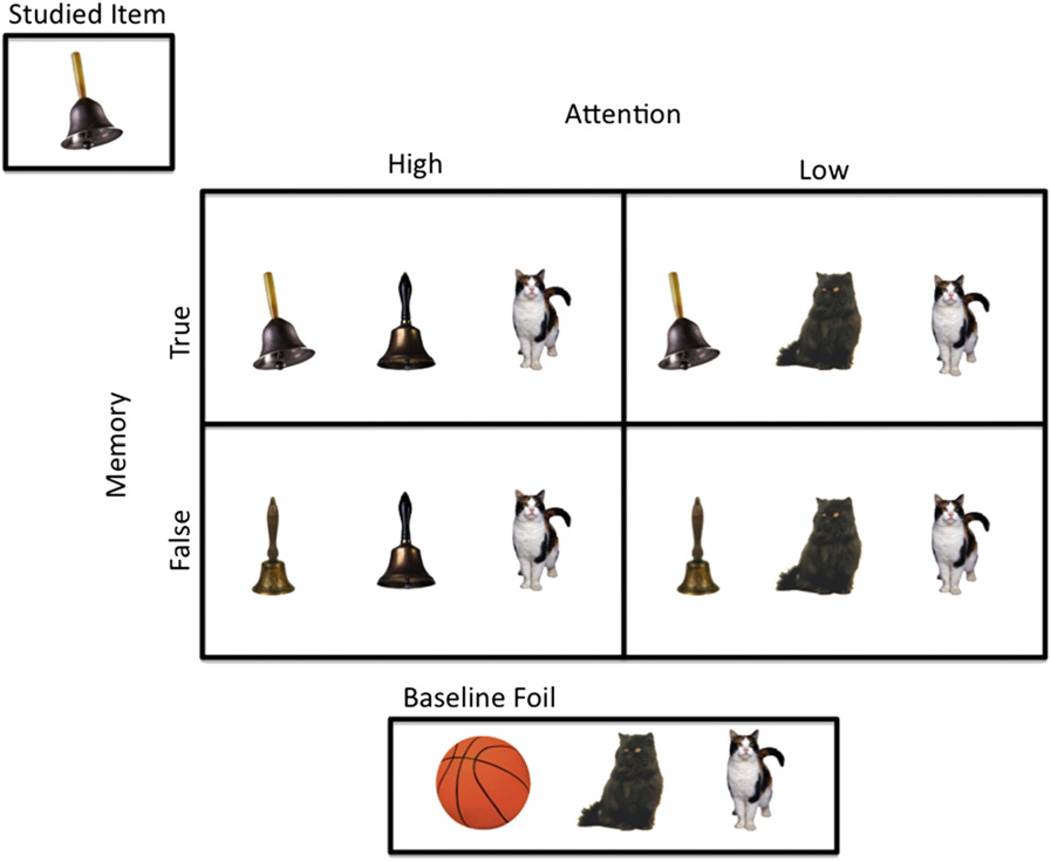
The experimental paradigm is illustrated in Figure 1. Participants study a series of pictures. Then, they undergo a memory test while brain activity is indirectly measured with fMRI. On each trial of the recognition test, participants are presented with three pictures. Their task is to select one of the pictures as a previously studied item or reject all three items as novel. Note that the task is not a forced-choice recognition task: on some trials, no target is presented and the correct response is to reject all three items as new. In contrast to standard yes/no recognition, in the present task participants are switching their attention between test items over the course of the trial. In the examples shown in Figure 1, the silver bell is the previously studied (target) item. Some of the pictures are conceptually related to previously studied items by virtue of the fact that they are drawn from the same semantic category and share a common verbal label (e.g., the brass bells). The participant is specifically warned about these items and instructed to classify them as “new” rather than “old.” When two related items are presented together, both items seem familiar to the participant and each is regarded as a candidate target. In order to decide whether one of the items was studied, participants visually scrutinize and systematically compare the two related items, as confirmed by eye tracking. Despite this increased attention to the perceptual details that are relevant to the task, participants persist in falsely recognizing the related items at a high rate, an instance of gist-based false recognition. This is referred to as the Attention-High/False Memory condition. When the target (studied) item is presented next to the related item, participants also visually scrutinize and systematically compare the target and the related item. In this case, however, they overwhelmingly select the target item in favor of the related distracter, clearly indicating that the specific perceptual details distinguishing the target and the related item are still stored in memory. We refer to this as the Attention-High/True Memory condition. When the related item is presented by itself, participants visually scrutinize the items less and falsely recognize the related item with high frequency. We refer to this as the Attention-Low/False Memory condition. When the target item is presented by itself, participants also scrutinize the items less. However, they correctly select the target item with high frequency. We refer to this as the Attention-Low/True Memory condition. These four conditions constitute a 2 × 2 factorial design that crosses attention to perceptual detail (High versus Low) and successful retrieval of perceptual detail (True versus False). To provide a measure of baseline false alarm rates and to assess nonspecific recognition memory, we also include a Baseline Foil condition in which all three items are unrelated to the study materials. Critically, all of the conditions in the experiment differed only in terms of the content of the participant’s memory. Differences in engagement of visual attention across conditions were driven by episodic retrieval processes, not the perceptual content of the display or explicit instructions, thus allowing us to investigate the recruitment of visual attention by ongoing episodic retrieval demands.
Figure 1. Experimental Paradigm.
See Introduction for further details. See also Figure S1.
RESULTS
Behavioral Data
Accuracy data are reported in Table 1 (reaction time data are reported in Table 2). In the Attention-Low/False Memory condition, false recognition of the related item was substantially larger than false recognition of single items in the Baseline Foil condition (e.g., the basketball in Figure 1; 0.38 versus 0.08; t(29) = 18.48, p < 0.001), representing a standard gist-based false recognition effect. High rates of false recognition persisted in the Attention-High/False Memory condition: false recognition of the related items was considerably larger than false recognition of paired items in the Baseline Foil condition (e.g., the kittens in Figure 1; 0.47 versus 0.13; t(29) = 19.69, p < 0.001). When the relevant baseline false recognition rates in the Baseline Foil condition are subtracted from the gist-based false recognition rates, Attention had no effect on rates of gist-based false recognition in the False Memory conditions (t(29) = 1.38, p = 0.18). However, in the Attention-High/True Memory condition, participants overwhelming selected the correct target item in favor of the related distracter (0.65 versus 0.10; t(29) = 17.61, p < 0.001), clearly indicating that information distinguishing the target and the related item was still stored in memory. The primary factor determining whether critical diagnostic perceptual details can be retrieved from memory and gist-based false recognition can be suppressed is whether the target item is made available as a cue on the recognition test. Attention to the perceptual details that are relevant to the discrimination, which does not result in retrieval of the target item, is not sufficient (see Guerin et al., 2012, for further discussion). These findings also complement Tulving’s observations of the effects of similarity in forced-choice recognition: in general, the similarity among test items on a recognition test is a less important determinant of performance than the similarity of the test items to information that is stored in memory (Tulving, 1981; see also Busey et al., 2000).
Table 1. Accuracy.
| Att. High/True Memory | Att. High/False Memory | Att. Low/True Memory | Att. Low/False Memory | Baseline Foil | |
|---|---|---|---|---|---|
| Target | 0.65 (0.03) | NA | 0.76 (0.02) | NA | NA |
| Related foil | 0.10 (0.01) | 0.47 (0.02) | NA | 0.38 (0.02) | NA |
| Paired unrelated foil | NA | NA | 0.06 (0.01) | 0.11 (0.01) | 0.13 (0.02) |
| Single unrelated foil | 0.03 (0.01) | 0.05 (0.01) | NA | NA | 0.08 (0.01) |
| New | 0.21 (0.02) | 0.46 (0.03) | 0.17 (0.02) | 0.49 (0.02) | 0.76 (0.03) |
Note: SEM in parentheses.
Table 2. Reaction Times (ms).
| Att. High/True Memory | Att. High/False Memory | Att. Low/True Memory | Att. Low/False Memory | Baseline Foil | |
|---|---|---|---|---|---|
| Target | 2,105 (79) | NA | 1,963 (65) | NA | NA |
| Related foil | Low N | 2,680 (86) | NA | 2,313 (74) | NA |
| New | Low N | 2,811 (99) | Low N | 2,761 (108) | 2,615 (102) |
Note: We exclude incorrect responses (with the exception of false alarms to related foils in the False Memory conditions) because these occurred infrequently and are associated with high estimation error and missing data values for certain participants. SEM in parentheses.
Eye Tracking Data
Eye tracking data were collected to confirm that participants systematically compared the candidate targets in the Attention-High conditions. The number of saccades between related pictures was used to measure this comparison process, restricted to trials associated with hits or gist-based false alarms. These data are presented in Figure S1 (available online). These data were analyzed using an analysis of variance (ANOVA) with factors for Attention (High versus Low) and Memory (True versus False), with participants modeled as a random effect. The main effect of Attention was significant (F(1,29) = 362.51, p < 0.001), indicating that the average number of saccades between related pictures was higher in the Attention-High conditions. The main effect of Memory was also significant (F(1,29) = 4.42, p < 0.05), indicating that the average number of saccades between related pictures was higher in the False Memory conditions. The interaction was not significant (F(1,29) = 2.08, p = 0.16). Similar results were obtained when using the total number of saccades as the dependent measure (Figure S1).
Effects of Eye Movements on fMRI Data
The differences in eye movements across conditions are consistent with the design of the task. However, many of the same regions that control eye movements also control top-down orienting of attention (Corbetta et al., 1998). We were interested in determining the neural correlates of the engagement of visual attention during episodic retrieval, above and beyond any activation differences that were due merely to eye movements. Our principal approach to dealing with this issue was to integrate measurements of eye movements into the fMRI analysis using hierarchical regression. Specifically, the number of between-picture saccades, the number of total saccades, and reaction time were regressed out of the data before evaluating differences between conditions. Because the relationship between these behavioral variables and the fMRI data is unlikely to be strictly linear, we used a series of fourth-order polynomials to model a potentially nonlinear response. All fMRI results reported here reflect findings that were obtained after regressing out these behavioral variables. Importantly, however, qualitatively similar results were obtained when no hierarchical regression was run (Figures S2 and S3). In addition to the hierarchical regression, further confirmatory analyses were conducted (see below).
Whole-Brain Analysis of Variance
To identify brain regions associated with attention to specific perceptual details and successful retrieval of specific perceptual details, we conducted a whole-brain (i.e., voxel-wise) ANOVA with factors for Attention (High versus Low) and Memory (True versus False), with participants modeled as a random effect.
Main Effect of Attention
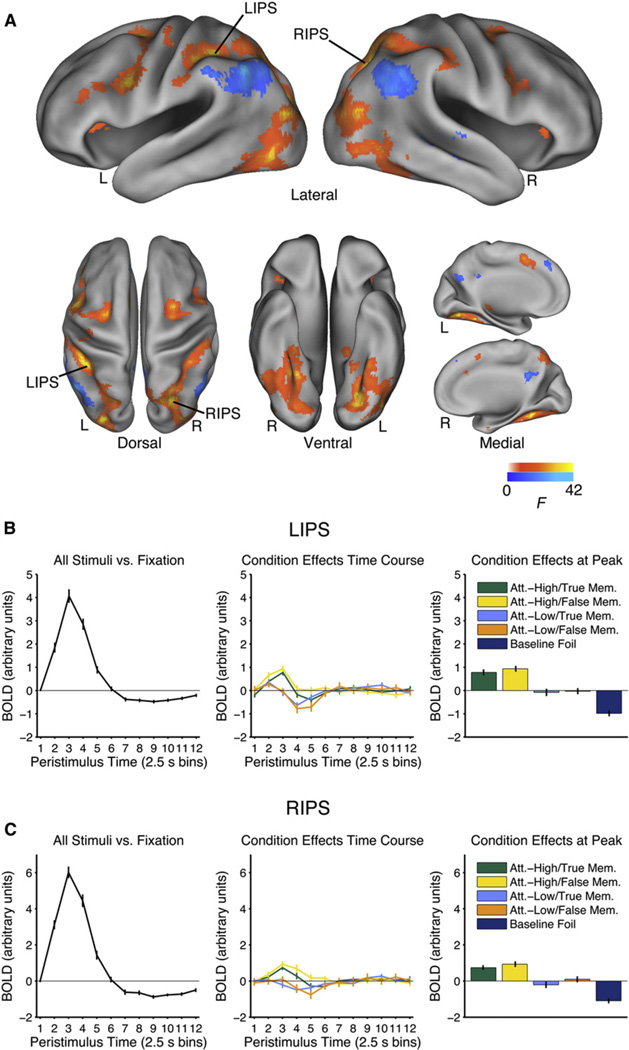
Regions associated with the engagement of visual attention during episodic retrieval were identified by isolating regions showing a significant main effect of Attention. Activation was observed in the anterior, medial, and posterior IPS bilaterally, the ventral temporal cortex bilaterally, the lateral occipital cortex bilaterally, the inferior frontal gyrus bilaterally, the medial frontal gyrus bilaterally, the left middle frontal gyrus, and the right anterior cingulate (Figure 2, warm colors), a pattern that is broadly consistent with previous studies of top-down visual attention (Kastner and Ungerleider, 2000; Corbetta and Shulman, 2002).
Figure 2. Main Effect of Attention.
(A) Regions associated with the recruitment of visual attention during episodic retrieval (main effect of Attention). Regions in which average activation in the Attention-High conditions is greater than average activation in the Attention-Low conditions are shown in warm colors (effects in the opposite direction are shown in cool colors). Time courses of the event-related response are shown for (B) the left IPS (LIPS; −38, −42, 46) and (C) the right IPS (RIPS; 28, −66, 46). The time course on the left shows the mean event-related time course estimated in level 2. The time course in the middle shows the condition effects estimated in level 15 for the conditions of interest. These time courses reflect deviation of each condition from the mean event-related response after correcting for trial-by-trial differences in eye movements and reaction time. The panel on the right also shows the data from level 15, restricting attention to the peak response (third time point) to facilitate comparisons across conditions. See Figure S2. True Memory conditions are restricted to hits and False Memory conditions are restricted to gist-based false alarms. Error bars show SEM. L = left; R = right.
Additionally, engagement of visual attention during episodic retrieval was associated with less activity in the IPL and other regions likely overlapping with the default network: right posterior cingulate, left precuneus, left medial frontal gyrus, and right lateral temporal cortex (Figure 2, cool colors). This finding is consistent with previous investigations of visual attention (e.g., Sestieri et al., 2010) and previous observations that the dorsal attention network is negatively correlated with the default network at low frequencies, which could imply a competitive relationship between these systems (Fox et al., 2005; cf. Murphy et al., 2009; Anderson et al., 2011).
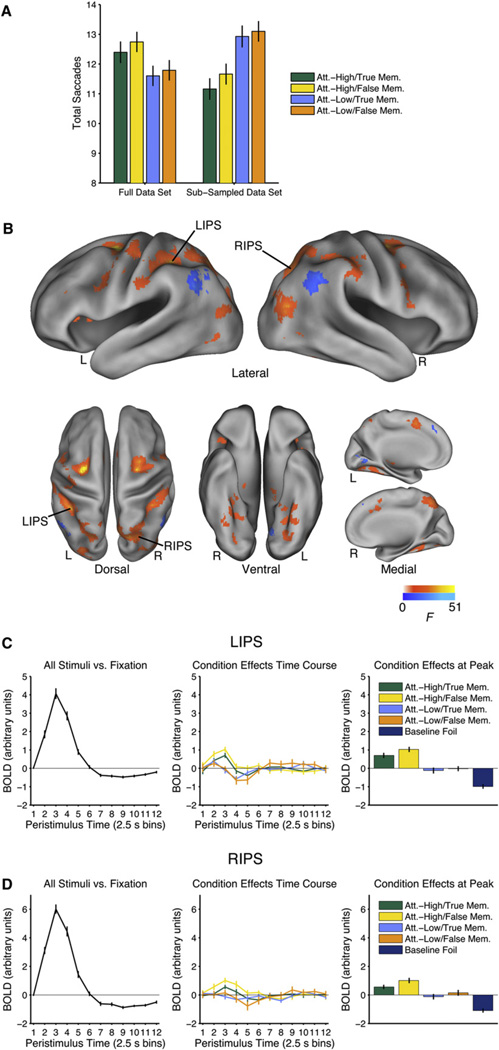
Given that the brain regions involved in top-down visual attention overlap with regions involved in the control of eye movements (Corbetta et al., 1998), it could still be argued that the activation shown in Figure 2 reflects neural activity associated with eye movements that was not adequately corrected for by the hierarchical regression. We conducted further confirmatory analyses to ensure that the hierarchical regression was robust. Specifically, we subsampled the data in order to reverse the direction of eye movement differences across the conditions. In the original data set, there are more saccades in the Attention-High conditions than the Attention-Low conditions. In order to reverse the direction of this effect on a participant-wise basis, we sorted the trials within each condition according to the number of saccades that occurred on that trial. In each Attention-High condition, we took all scores below the 60th percentile. In each Attention-Low condition, we took all scores above the 40th percentile. As shown in Figure 3A, in the subsampled data, the number of saccades was much larger in the Attention-Low conditions than the Attention-High conditions (F(1,29) = 148.97, p < 0.001). In fact, the absolute value of the difference between conditions was much larger in the subsampled data than in the original data. As in the original data, the main effect of Memory was significant (F(1,29) = 4.44, p < 0.05) and the interaction was not significant (F(1,29) = 2.47, p = 0.13).
Figure 3. Controlling for Eye Movements.
The same data presented in Figure 2, except that the direction of eye movement differences across conditions has been artificially reversed by selectively subsampling the data.
(A) The number of saccades in the Attention-High conditions and the Attention-Low conditions in the original data set (left) and after the subsampling procedure (right).
(B) Regions associated with the recruitment of visual attention during episodic retrieval (main effect of Attention) after the subsampling procedure reversed the direction of eye movement effects across conditions. Time courses of the event-related response are shown for (C) the left IPS (LIPS) and (D) the right IPS (RIPS). The time course on the left shows the mean event-related time course estimated in level 2 (numerically identical to Figure 2). The time course in the middle shows the condition effects estimated in level 15 for the conditions of interest. These time courses reflect deviation of each condition from the mean event-related response after correcting for trial-by-trial differences in eye movements and reaction time and after the subsampling procedure reversed the direction of eye movement effects across conditions. The panel on the right also shows the data from level 15, restricting attention to the peak response (third time point) to facilitate comparisons across conditions. Regions of interest are based on the main analysis (Figure 2).
See Figure S4. True Memory conditions are restricted to hits and False Memory conditions are restricted to gist-based false alarms. Error bars show SEM. L = left; R = right.
The subsampled data were then subjected to the same analysis as the original data set. If the hierarchical regression is robust, the subsampled data should lead to similar conclusions: the effects of eye movements have already been satisfactorily modeled, so any further classification of the data on the basis of eye movements should have no effect. Alternatively, if the activation presented in Figure 2 reflects the effects of eye movements, there should be a substantial reversal of these effects when the sub-sampled data are subjected to the same analysis.
The same basic pattern of activation seen in the main analysis (Figure 2) is also seen in the subsampled data (Figure 3). Although there is an expected slight reduction in the overall magnitude and extent of activation, which results from a reduction in power, the peak activations in parietal cortex are still clearly apparent. Time courses from the subsampled data (Figures 3C and 3D) closely resemble those obtained from the original data set. Similar conclusions were obtained when using the number of saccades between pictures as the measure of interest (Figure S4). There is a hint of residual effects of eye movements in early visual cortex (Figure 3, cool colors). Critically, however, activation of the dorsal attention network persisted despite these modest residual effects. These confirmatory analyses indicate that the hierarchical regression was robust and that the findings reported in Figure 2 cannot be attributed to the effects of eye movements.
Main Effect of Memory
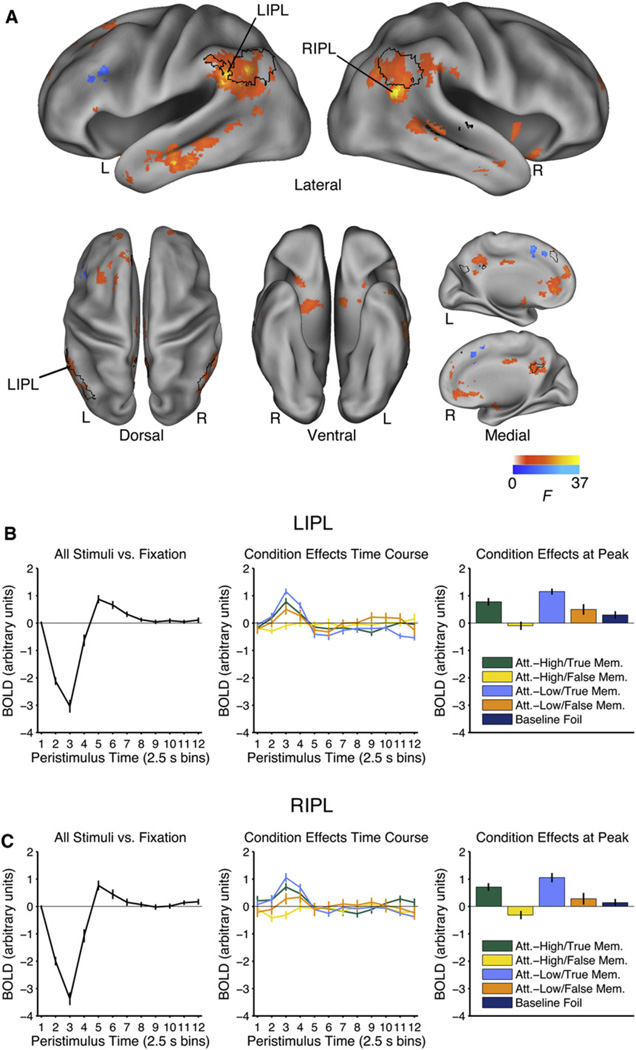
To identify regions associated with the retrieval of specific perceptual detail, we identified regions showing a significant main effect of Memory. Greater activity for true recognition than false recognition was observed in the IPL bilaterally, medial parietal cortex bilaterally, medial prefrontal cortex bilaterally, lateral temporal cortex bilaterally, superior frontal gyrus bilaterally, left inferior frontal gyrus, right insula, and right parahippocampal gyrus (Figure 4, warm colors). This pattern of activity is broadly consistent with previous observations of the neural correlates of the successful recovery of information from episodic memory (Wagner et al., 2005; Spaniol et al., 2009). To aid comparison to Figure 2, regions that were less active in the Attention-High conditions than the Attention-Low conditions have been demarcated by a black border. Note the considerable overlap between regions less active during engagement of visual attention and regions associated with the successful retrieval of specific perceptual details. IPL was less active during stimulus trials than fixation trials (Figures 4B and 4C, plots on the left), a trademark feature of default network regions (Buckner et al., 2008). Greater activity for false recognition was observed in the left lateral and medial frontal gyrus (Figure 4, cool colors).
Figure 4. Main Effect of Memory.
(A) Regions associated with successful retrieval of perceptual detail (main effect of Memory). Regions in which average activity in the True Memory conditions is greater than average activity in the False Memory conditions are shown in warm colors (effects in the opposite direction are shown in cool colors). To aid comparison to Figure 2, regions that were less active in the Attention-High conditions than the Attention-Low conditions have been demarcated by a black border. Time courses of the event-related response are shown for (B) the left IPL (LIPL; −58, −50, 32) and (C) the right IPL (RIPL; 52, −58, 20). The time course on the left shows the mean event-related time course estimated in level 2. The time course in the middle shows the condition effects estimated in Level 15 for the conditions of interest. These time courses reflect deviation of each condition from the mean event-related response after correcting for trial-by-trial differences in eye movements and reaction time. The panel on the right also shows the data from level 15, restricting attention to the peak response (third time point) to facilitate comparisons across conditions. True Memory conditions are restricted to hits and False Memory conditions are restricted to gist-based false alarms. See Figure S3. Error bars show SEM. L = left; R = right.
Attention × Memory Interaction
The Attention × Memory interaction was significant in five relatively small clusters within prefrontal cortex. Four of these clusters were not significant in the control analysis in which the hierarchical regression was omitted; we do not consider these clusters further. In the remaining cluster, in left anterior prefrontal cortex (−20, 56, 2), a region of interest (ROI) analysis was conducted (restricting attention to the peak at the fourth time point). Activity was greater in the Attention-High/False Memory condition than the Attention-High/True Memory condition (F(1,29) = 4.71, p < 0.05). In contrast, there was a trend for lower activity in the Attention-Low/False Memory condition than the Attention-Low/True Memory condition (F(1,29) = 3.40, p = 0.08).
Direct Comparison of Dissociable Parietal Regions
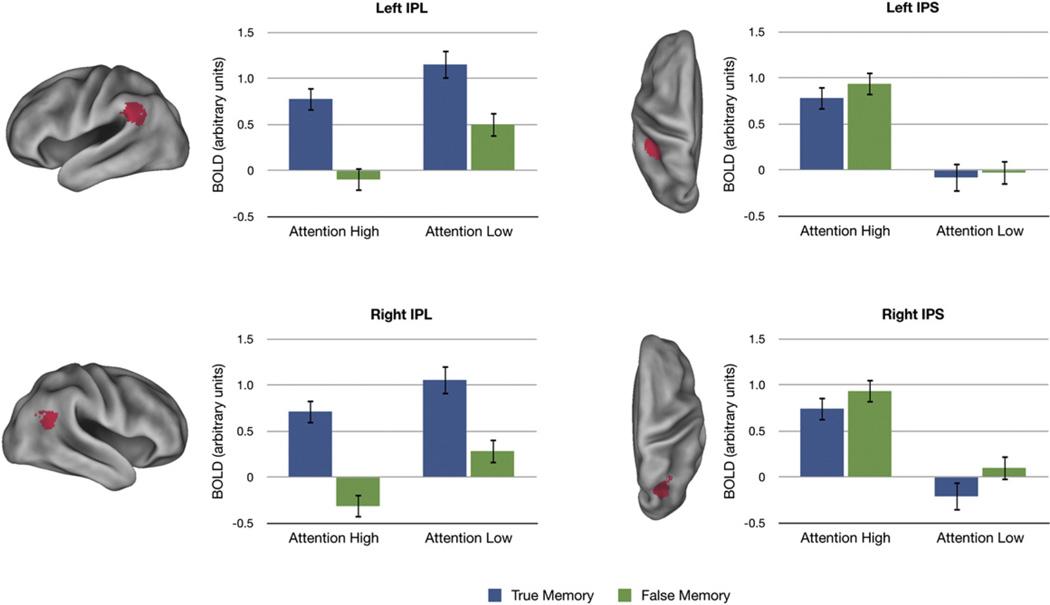
We directly compared regions implicated in attention and memory to ensure that the apparent dissociation across parietal cortex is independent of the whole-brain threshold employed. ROIs were defined based on the maxima indicated in Figures 2 and 4 (LIPS, RIPS, LIPL, RIPL; third time point only; Figure 5) and entered into an ANOVA (separately for each hemisphere) with factors for Attention (High versus Low), Memory (True versus False), and Region (IPS versus IPL), with participants modeled as a random effect. Critically, the Attention × Region interaction was significant (left: F(1,29) = 107.38, p < 0.001; right: F(1,29) = 57.81, p < 0.001), indicating that the effect of Attention significantly differed across regions. We then analyzed each region separately. Of course, there was a significant main effect of Attention in IPS (left: F(1,29) = 68.95, p < 0.001; right: F(1,29) = 43.62, p < 0.001). The main effect of Attention in IPL is more informative (left: F(1,29) = 11.26, p < 0.01; right: F(1,29) = 9.54, p < 0.01). These effects were in the opposite direction than was observed in the IPS. Thus, the Attention × Region interaction is a crossover interaction, constituting a double dissociation between these regions. Critically, the Memory × Region interaction was also significant (left: F(1,29) = 39.20, p < 0.001; right: F(1,29) = 36.6, p < 0.001), indicating that the effect of Memory significantly differed across regions. We then analyzed each region separately. Of course, there was a significant main effect of Memory in IPL (left: F(1,29) = 47.88, p < 0.001; right: F(1,29) = 34.97, p < 0.001). The main effect of Memory in IPS was not significant (left: F(1,29) = .98, p = .33; right: F(1,29) = 2.56, p = 0.12). The Region × Attention × Memory interaction was not significant (both hemispheres: F ≤ 1). These analyses indicate that the dissociation between the IPS and the IPL does not depend on the threshold employed in the whole-brain analysis.
Figure 5. Dissociable Effects of Memory and Attention across Parietal Regions.
Means are shown for ROIs defined by the maxima in Figures 2 and 4 (third time point only). Note that zero does not correspond to fixation baseline. Error bars show SEM. The volumetric ROIs (see Supplemental Information) have been projected onto the cortical surface (shown in red) to aid visualization (left: lateral views of IPL; right: dorsal views of IPS).
DISCUSSION
The interaction between visual attention and episodic retrieval is poorly understood. Given that the neural systems mediating attention and episodic memory appear to be anatomically segregated, and perhaps even in competition, it is unclear which neural systems are engaged when visual attention is recruited during episodic retrieval. We investigated the recruitment of visual attention by episodic retrieval during the suppression of gist-based false recognition. When two similar candidate targets were presented next to each other, participants had to systematically compare the two items and attend to the details that distinguished them in order to decide whether one of the items was old (Attention-High conditions). This process was associated with increased activity in regions previously associated with top-down visual attention (Kastner and Ungerleider, 2000; Corbetta and Shulman, 2002), including the IPS (Figure 2). These results suggest that systems for top-down visual attention, although not typically associated with episodic retrieval, can play an important role when retrieval of specific visual details is required. Although activity in the IPS was associated with the attempt to retrieve perceptual detail, it was not associated with successful retrieval of perceptual detail. In contrast, activity in the IPL, and other regions likely overlapping with the default network, was associated with the successful retrieval of perceptual detail from memory (Figure 4). Thus, the IPS and the IPL make dissociable contributions to the retrieval of perceptual detail. Below, we discuss the implications of these findings for models of the role of the parietal cortex in episodic retrieval and visual attention.
Episodic Retrieval Recruits the Dorsal Attention Network during Attempts to Retrieve Perceptual Detail
When two candidate targets were presented adjacent to one another (Attention-High conditions), participants had to systematically compare the two candidate targets and attend to the details that distinguished them in order to decide which item was old. Activity in these conditions was assessed relative to conditions in which a candidate target was presented next to two unrelated items (Attention-Low conditions) and participants scrutinized the visual details of the pictures less, as confirmed by eye tracking (Figure S1). The conditions of the experiment did not differ in terms of the perceptual display; only the content of the participant’s memory differed across conditions. Therefore, any engagement of visual attention occurred as a result of episodic retrieval processes. The attempt to retrieve perceptual detail from memory was associated with engagement of regions previously implicated in top-down attention, including the IPS, collectively referred to as the dorsal attention network (Kastner and Ungerleider, 2000; Corbetta and Shulman, 2002). These findings indicate that the attempt to retrieve specific perceptual details from episodic memory in order to suppress false recognition is associated with engagement of the same neural systems for top-down visual attention that are utilized in other domains, such as visual detection or visual search of cluttered displays (Kastner and Ungerleider, 2000; Corbetta and Shulman, 2002). This observation contrasts sharply with the finding that episodic retrieval in general—and the attempt to retrieve specific details in particular—is associated with activity within components of the default network (Dobbins and Wagner, 2005; Wagner et al., 2005), that likely reflects, at least in part, a disengagement from processing of external stimuli and increased processing of internally generated representations (Buckner et al., 2008). Rather, the results suggest that the dorsal attention network makes an important contribution to episodic retrieval when the retrieval of specific perceptual details is required.
The recruitment of regions associated with top-down visual attention during the attempt to retrieve perceptual detail likely reflects perceptual processing of the cues themselves. Indeed, the pattern of eye movements clearly suggests that participants visually scrutinized the pictures to a greater degree in the Attention-High conditions. However, there is evidence that regions of the parietal cortex associated with top-down visual attention can be engaged during recall of a picture even in the absence of any visual stimulus (Wheeler et al., 2006), suggesting that systems for top-down visual attention can also be recruited during processing of internally generated mnemonic representations. Future experiments should directly compare processing of internally generated mnemonic representations and externally perceived retrieval cues.
Effects of Eye Movements on fMRI Data
There is a close relationship between the deployment of visual attention and the control of eye movements: the dorsal attention network is associated with both functions (Corbetta et al., 1998). In the current experiment, recruitment of visual attention during episodic retrieval was reflected in the pattern of eye movements. The differences in eye movements across conditions are a natural consequence of the engagement of visual attention during episodic retrieval. However, it is important to ask whether the dorsal attention network activity reported here is due merely to eye movements or whether it reflects the engagement of attention above and beyond any “low-level” or “bottom-up” influence of eye movements. To address this issue, it is tempting to simply instruct participants to maintain fixation. However, saccade suppression would likely become more difficult when participants are attempting to retrieve specific perceptual details, which is important because the dorsal attention network is also associated with the suppression of saccades (Brown et al., 2008). Whereas differences in saccade suppression across conditions cannot be measured directly, differences in eye movements across conditions can be measured very accurately. Our approach was thus to allow participants to move their eyes freely and to integrate the resulting measurements into the analysis of the fMRI data. We used a hierarchical regression approach to control for the effects of eye movements on the fMRI data prior to analyzing differences between conditions. In order to ensure that the model was sufficiently flexible to accurately model the effects of eye movements on the data, a series of fourth-order polynomials were used to model a potentially nonlinear relationship. Multiple eye tracking measures (saccades between related pictures and total number of saccades) were regressed out, as well as reaction time. Engagement of the dorsal attention network during episodic retrieval was minimally affected by these statistical controls, strongly suggesting that activation of the dorsal attention network in the present task is dominated by top-down, volitional attention rather than eye movements per se. A control analysis in which the hierarchical regression was not performed produced very similar results, indicating that our findings do not hinge on the method of analysis and that critical attention or memory related activity was not inadvertently removed from the data. Of course, any statistical correction can only be as good as the statistical model and the measurements obtained. To evaluate whether the findings reflect measurement error or an inadequately modeled residual effect of eye movements, we subjected the data to a strong test: we subsampled the data to substantially reverse the direction of eye movement effects across conditions. We found some evidence for a residual effect of eye movements in early visual cortex. However, activation of the dorsal attention network was still clearly present despite these modest residual effects (Figure 3), once again suggesting that activation of the dorsal attention network in the present task is dominated by top-down, volitional attention. Although we cannot unequivocally rule out that there are any residual effects of eye movements in the present findings, it is clear that the dorsal attention network activation is robust against even very aggressive statistical controls for eye movements. The weight of the evidence therefore favors the hypothesis that dorsal attention network activation in the present task reflects top-down, volitional orienting of attention in response to episodic retrieval demands.
The IPL Tracks Retrieval of Perceptual Detail
Activity in the IPS was associated with the recruitment of visual attention during attempts to retrieve perceptual detail. However, it was not associated with the actual retrieval of visual detail (although it is possible that IPS supported retrieval of visual information unrelated to accurate responding). In contrast, the IPL and other regions likely overlapping with the default network were associated with the successful retrieval of visual detail, assessed by comparing hits (True Memory) to gist-based false alarms (False Memory; Figure 4). Some previous studies of gist-based false recognition have observed greater activation for true recognition than gist-based false recognition in lateral parietal cortex (Slotnick and Schacter, 2004; Kensinger and Schacter, 2007; Kim and Cabeza, 2007). The IPL has been associated with the successful retrieval of information from memory in a large number of studies (Wagner et al., 2005; Spaniol et al., 2009). Although damage to the parietal cortex is not conventionally associated with memory impairment, recent findings suggest that patients with parietal damage may experience reduced confidence in their memories (Simons et al., 2010). These findings have led to an active debate in the literature on the role of this region in episodic memory. It has been proposed that the IPL facilitates a working memory buffer for retrieved information (Wagner et al., 2005; Vilberg and Rugg, 2008), accumulates mnemonic information until a decision bound is reached (Wagner et al., 2005; cf. Guerin and Miller, 2011), facilitates bottom-up attention to retrieved information (Wagner et al., 2005; Cabeza, 2008; Cabeza et al., 2008; Ciaramelli et al., 2008; cf. Hutchinson et al., 2009; Sestieri et al., 2010), or enables the binding of features stored in separate cortical regions (Shimamura, 2011).
It is currently unclear whether activity in the IPL is sensitive to the retrieval of perceptual detail per se or whether it is sensitive to the retrieval of detailed information from episodic memory regardless of its content. There is some reason to suspect that successful retrieval effects obtained in the IPL are not specific to perceptual detail per se. Successful retrieval effects in the lateral parietal cortex are obtained in multiple modalities (Shannon and Buckner, 2004) with a wide variety of stimuli and tasks, some of which (e.g., recognition of printed words) probably rely much more on the retrieval of conceptual information and an internally experienced “cognitive context” than perceptual details (Craik and Tulving, 1975). Support for this hypothesis comes from a study by Dobbins and Wagner (2005) (see Wagner et al., 2005, Figure 4, to aid comparison). They compared a conceptual source memory task to a perceptual source memory task. Relative to a simple novelty detection task, both tasks activated the IPL. They also found that the perceptual source memory task was associated with greater activity than the conceptual source memory task in a variety of regions, including parietal regions likely overlapping with those shown in Figure 2. Although they did not distinguish between the attempt to retrieve conceptual or perceptual information and successful retrieval of this information—which the present results suggest can be critical—their findings are broadly consistent with the foregoing argument. Future experiments should directly test whether activity in the IPL is sensitive to the type of information being retrieved.
The IPL tracks successful retrieval across a wide range of conditions. However, successful retrieval is not the only factor that affects IPL activity. For instance, violations of retrieval expectations also modulate IPL activity (O’Connor et al., 2010), but this finding does not exclude the possibility that IPL plays a role in episodic memory. O’Connor et al. observed similar expectation violation effects in the hippocampus, which clearly plays a role in episodic memory. However, the pattern of activity in IPL is complex and cannot be naively interpreted as a proxy for successful retrieval. Indeed, our observation that IPL activity is reduced when visual attention is engaged is further evidence that IPL activity is affected by factors other than successful retrieval.
Implications for the Attention to Memory Model
Our observations of functional dissociations between dorsal and ventral regions of the lateral parietal cortex are consistent with recent formulations of the “attention to memory” model. According to this model, parietal systems associated with attention are not limited to the processing of perceptual information; these systems also play a role in orienting attention toward and maintaining attention on mnemonic representations (Wagner et al., 2005; Cabeza, 2008; Cabeza et al., 2008; Ciaramelli et al., 2008). Building on the dual system model of Corbetta and Shulman (2002), it has been proposed that the dorsal parietal cortex, including the IPS and superior parietal lobule, facilitates top-down attention toward perceptions and memories. The ventral parietal cortex (i.e., IPL) facilitates bottom-up attention toward perceptions and memories. According to the model, this ventral region serves as a “circuit breaker” that redirects attention toward new information that is task relevant or urgent (Cabeza, 2008; Cabeza et al., 2008; Ciaramelli et al., 2008). The attention to memory model can account for the finding that the dorsal parietal cortex was more active during attempts to retrieve specific perceptual details because it proposes that the dorsal parietal cortex facilitates top-down, volitional orienting of visual attention as well as volitional attention toward specific mnemonic representations, such as stored visual details. The model can also account for the finding that the ventral parietal cortex was more active during successful retrieval of perceptual details because the recovery of task-relevant details from memory should engage the “circuit breaker.” Thus, our findings are broadly consistent with the attention to memory model.
However, this model has been the subject of debate. The principal criticism is that the parietal regions associated with visual attention are not the same regions associated with the successful retrieval of information from episodic memory. In a recent meta-analysis, Hutchinson et al. (2009) concluded that, within the IPL, activations associated with bottom-up attention are anterior to activations associated with episodic retrieval. Further, within more dorsal regions of the parietal cortex, activations associated with top-down attention are more medial than activations associated with episodic memory (see also Nelson et al., 2010).
On the other hand, some overlap between visual attention and episodic memory can be observed within the parietal cortex (Cabeza et al., 2011). In our own experiment, in IPS (Figure 2), a region that was defined by attention-related activity, the Baseline Foil condition is far less active than any other condition (all p < 0.001), representing a standard parietal “old/new” effect thought to reflect memory retrieval or related processes (Wagner et al., 2005). Although it has become clear that there is not a one-to-one correspondence between parietal memory and attention systems, any complete account of the lateral parietal cortex must explain observed overlap between the neural correlates of attention and memory. A full resolution of this issue will likely hinge on further developments in our understanding of the extensive functional heterogeneity within lateral parietal cortex, which appears to include several functional subdivisions (Nelson et al., 2010). It will also be important to investigate the relationship between attention and memory at the level of an individual’s anatomy (e.g., Sestieri et al., 2010), since normalization tends to blur boundaries between adjacent but functionally distinct regions.
Dynamic Interactions between Attention and Memory
We have found that the dorsal attention network, although not typically associated with episodic retrieval, can make important contributions to episodic retrieval when the retrieval of perceptual details is required. We also found that the IPL—a region that has been consistently associated with the retrieval of information from episodic memory—actually shows reduced activity when visual attention is engaged during episodic retrieval (Figure 2). This result was obtained even within a region of the IPL defined explicitly as tracking the retrieval of specific perceptual details (Figures 4 and 5). A general finding in the perceptual domain is that attention-demanding tasks that activate the dorsal attention network also produce deactivation in the IPL, particularly the angular gyrus (e.g., Sestieri et al., 2010). This pattern dovetails with the finding that the dorsal attention network and the default network are negatively correlated at low frequencies (i.e., resting state functional connectivity), which may suggest that these two networks have a competitive relationship to one another (Fox et al., 2005; cf. Murphy et al., 2009; Anderson et al., 2011).
The notion that parietal systems mediating visual attention and episodic retrieval may actually suppress one another has gained further support from the recent findings of Sestieri et al. (2010). They compared a visual search task and a memory task. The visual task engaged regions of the IPS overlapping those seen in Figure 2, as well as regions of the superior parietal lobule. In contrast, the memory task engaged the IPL, overlapping with the regions shown in Figure 4. Critically, the visual task was also associated with reduced activity in the IPL, consistent with our own results (Figure 2) and the foregoing discussion. Conversely, the memory task was associated with reduced activity in the posterior IPS. This finding could imply that engaging in perceptual processing leads to suppression of regions associated with memory retrieval; conversely, engaging in memory retrieval leads to suppression of regions associated with perceptual processing. Imaging data alone cannot demonstrate that one region is actively inhibiting another. Nonetheless, considering recent findings in light of this hypothesis provides an interesting and potentially fruitful path forward for future research.
The possibility that visual attention and episodic memory neurally compete with one another presents an apparent paradox: how can visual attention simultaneously contribute to the retrieval of perceptual detail and suppress regions associated with the successful retrieval of perceptual detail? It is possible, for instance, that successful retrieval effects in IPL actually reflect, at least in part, suppression of IPL during sustained attention to memory, which is presumably greater when retrieval is failing. However, the conspicuous absence of an inverse effect in the dorsal attention network is difficult to reconcile with this hypothesis. Another interesting possibility is that deactivation of the IPL actually reflects a finer tuning of activity rather than general suppression (Sestieri et al., 2010). These considerations underscore the need for further research investigating interactions between the dorsal attention network and the default network in contexts where both networks make significant contributions to the task, such as when episodic retrieval recruits visual attention (see Spreng et al., 2010, for a related discussion).
Conclusion
Visual attention is integral to episodic retrieval when the recovery of specific perceptual details is required, such as during attempts to suppress false recognition. The contribution of the parietal cortex to this interaction is complex, with distinct systems contributing to different components of the task while also suppressing each other. The dorsal parietal cortex is associated with the attempt to retrieve perceptual detail, which likely reflects the recruitment of top-down visual attention during episodic retrieval. In contrast, the ventral parietal cortex is associated with the successful retrieval of perceptual detail, which is consistent with previous findings that this region tracks the retrieval of specific details from memory (Vilberg and Rugg, 2008). Interestingly, activity in the ventral parietal cortex was reduced when visual attention was recruited during episodic retrieval. This finding is in agreement with previous proposals that the dorsal attention network and the default network oppose one another (Fox et al., 2005; Sestieri et al., 2010; cf. Murphy et al., 2009; Anderson et al., 2011). This pattern of results suggests a clear need to study in greater detail how two apparently opposed brain networks can simultaneously contribute to the retrieval of perceptual detail from episodic memory.
EXPERIMENTAL PROCEDURES
Participants
Participants were 30 college students (17 male) recruited from the Boston metropolitan area and were paid $70 in compensation. All participants provided informed consent as approved by the Institutional Review Board at Harvard University. (See Supplemental Information.) Behavioral results from a partially overlapping sample have been described previously (Guerin et al., 2012).
Stimuli
Four hundred triplets of object photographs were used as stimuli. Triplets of related pictures were drawn from the same semantic category and had a common verbal label. Pictures in a triplet were selected to be perceptually distinct members of a category and, at a minimum, differed in terms of color or orientation. Examples of stimuli are shown in Figure 1. Stimuli were counter-balanced across conditions (see Supplemental Information).
Procedure
During the study session, participants were presented with a series of 160 objects (500 ms duration; 1,500 ms ISI). The participant’s task was to indicate by a button press whether the pictured object could fit into a 13-inch box in the real world. Participants were then placed in an MRI scanner. Following approximately 10 min of anatomical scanning, the recognition memory test began. The various conditions of the recognition test are shown in Figure 1 (see Introduction for further detail). The occurrence of similar foils was clearly explained to all participants. Each trial lasted 5 s. (See Supplemental Information.)
Magnetic Resonance Imaging
A high-resolution T1-weighted anatomical image and T2*-weighted functional images sensitive to blood oxygenation level-dependent (BOLD) signal were collected using standard procedures with a Siemens TIM Trio 3 Tesla MRI scanner. Standard preprocessing using SPM8 was conducted. Subsequent analysis was implemented using customized programs. The participant-level fMRI time series was modeled using a standard least-squares voxel-wise linear model. A hierarchical regression approach was used (i.e., the residuals at level i are the data of interest at level i+1). The number of saccades between pictures within a trial, the number of total saccades in a trial, and reaction time were regressed out of the data prior to inspecting differences across conditions. For each predictor, a series of fourth-order polynomials were used to model a potentially nonlinear response between the predictors and the BOLD signal. Importantly, the reported findings do not depend heavily on this particular analysis approach. A more conventional nonhierarchical voxel-wise linear model produced qualitatively similar results. (Figures S2 and S3). All reported stereotaxic coordinates refer to the MNI template and are reported as (x, y, z). Throughout, statistical maps have been thresholded voxel-wise at p < 0.01. An additional cluster extent threshold of 38 or more contiguous voxels enforced a whole-brain correction for multiple comparisons at p < 0.05 (see Supplemental Information).
Supplementary Material
ACKNOWLEDGMENTS
Thanks to Ross Mair, Tammy Moran, Caroline West, Miguel Cutiongco, David Dornblaser, Rebecca Hersher, and Allison Hyland for assistance, Marcus Johnson for assistance with the eye tracker, Elissa Aminoff and Wilma Koutstaal for providing stimuli, and Scott Slotnick for providing software for the Monte Carlo simulation. This work was supported by NRSA AG034699 to S.A.G. and National Institute of Health MH060941 to D.L.S.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes four figures and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.neuron.2012.08.020.
REFERENCES
- Anderson JS, Druzgal TJ, Lopez-Larson M, Jeong E-K, Desai K, Yurgelun-Todd D. Network anticorrelations, global regression, and phase-shifted soft tissue correction. Hum. Brain Mapp. 2011;32:919–934. doi: 10.1002/hbm.21079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker MW, Rasmussen IP. Guidance of attention to objects and locations by long-term memory of natural scenes. J. Exp. Psychol. Learn. Mem. Cogn. 2008;34:1325–1338. doi: 10.1037/a0013650. [DOI] [PubMed] [Google Scholar]
- Brown MRG, Vilis T, Everling S. Isolation of saccade inhibition processes: rapid event-related fMRI of saccades and nogo trials. Neuroimage. 2008;39:793–804. doi: 10.1016/j.neuroimage.2007.09.035. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann. N Y Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Busey TA, Tunnicliff J, Loftus GR, Loftus EF. Accounts of the confidence-accuracy relation in recognition memory. Psychon. Bull. Rev. 2000;7:26–48. doi: 10.3758/bf03210724. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Role of parietal regions in episodic memory retrieval: the dual attentional processes hypothesis. Neuropsychologia. 2008;46:1813–1827. doi: 10.1016/j.neuropsychologia.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat. Rev. Neurosci. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Mazuz YS, Stokes J, Kragel JE, Woldorff MG, Ciaramelli E, Olson IR, Moscovitch M. Overlapping parietal activity in memory and perception: evidence for the attention to memory model. J. Cogn. Neurosci. 2011;23:3209–3217. doi: 10.1162/jocn_a_00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanon VW, Hopfinger JB. Memory’s grip on attention: the influence of item memory on the allocation of attention. Vis. Cogn. 2008;16:325–340. [Google Scholar]
- Chun MM. Contextual cueing of visual attention. Trends Cogn. Sci. 2000;4:170–178. doi: 10.1016/s1364-6613(00)01476-5. [DOI] [PubMed] [Google Scholar]
- Chun MM, Johnson MK. Memory: enduring traces of perceptual and reflective attention. Neuron. 2011;72:520–535. doi: 10.1016/j.neuron.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun MM, Turk-Browne NB. Interactions between attention and memory. Curr. Opin. Neurobiol. 2007;17:177–184. doi: 10.1016/j.conb.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Chun MM, Golomb JD, Turk-Browne NB. A taxonomy of external and internal attention. Annu. Rev. Psychol. 2011;62:73–101. doi: 10.1146/annurev.psych.093008.100427. [DOI] [PubMed] [Google Scholar]
- Ciaramelli E, Grady CL, Moscovitch M. Top-down and bottom-up attention to memory: a hypothesis (AtoM) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia. 2008;46:1828–1851. doi: 10.1016/j.neuropsychologia.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Tulving E. Depth of processing and retention of words in episodic memory. J. Exp. Psychol. Gen. 1975;104:268–294. [Google Scholar]
- Dobbins IG, Wagner AD. Domain-general and domain-sensitive prefrontal mechanisms for recollecting events and detecting novelty. Cereb. Cortex. 2005;15:1768–1778. doi: 10.1093/cercor/bhi054. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo DA, Weiss JA, Schacter DL. Reducing false recognition with criterial recollection tests: distinctiveness heuristic versus criterion shifts. J. Mem. Lang. 2004;51:473–493. [Google Scholar]
- Guerin SA, Miller MB. Parietal cortex tracks the amount of information retrieved even when it is not the basis of a memory decision. Neuroimage. 2011;55:801–807. doi: 10.1016/j.neuroimage.2010.11.066. [DOI] [PubMed] [Google Scholar]
- Guerin SA, Robbins CA, Gilmore AW, Schacter DL. Retrieval failure contributes to gist-based false recognition. J. Mem. Lang. 2012;66:68–78. doi: 10.1016/j.jml.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson JB, Uncapher MR, Wagner AD. Posterior parietal cortex and episodic retrieval: convergent and divergent effects of attention and memory. Learn. Mem. 2009;16:343–356. doi: 10.1101/lm.919109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK. Source monitoring and memory distortion. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1997;352:1733–1745. doi: 10.1098/rstb.1997.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Hashtroudi S, Lindsay DS. Source monitoring. Psychol. Bull. 1993;114:3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu. Rev. Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Remembering the specific visual details of presented objects: neuroimaging evidence for effects of emotion. Neuropsychologia. 2007;45:2951–2962. doi: 10.1016/j.neuropsychologia.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Kim H, Cabeza R. Trusting our memories: dissociating the neural correlates of confidence in veridical versus illusory memories. J. Neurosci. 2007;27:12190–12197. doi: 10.1523/JNEUROSCI.3408-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutstaal W, Schacter DL. Gist-based false recognition of pictures in older and younger adults. J. Mem. Lang. 1997;37:555–583. [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Cohen AL, Power JD, Wig GS, Miezin FM, Wheeler ME, Velanova K, Donaldson DI, Phillips JS, Schlaggar BL, Petersen SE. A parcellation scheme for human left lateral parietal cortex. Neuron. 2010;67:156–170. doi: 10.1016/j.neuron.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor AR, Han S, Dobbins IG. The inferior parietal lobule and recognition memory: expectancy violation or successful retrieval? J. Neurosci. 2010;30:2924–2934. doi: 10.1523/JNEUROSCI.4225-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyna V, Brainerd C. Fuzzy-trace theory: an interim synthesis. Learn. Individ. Differ. 1995;7:1–75. [Google Scholar]
- Schacter DL, Israel L, Racine C. Suppressing false recognition in younger and older adults: the distinctiveness heuristic. J. Mem. Lang. 1999;40:1–24. [Google Scholar]
- Sestieri C, Shulman GL, Corbetta M. Attention to memory and the environment: functional specialization and dynamic competition in human posterior parietal cortex. J. Neurosci. 2010;30:8445–8456. doi: 10.1523/JNEUROSCI.4719-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon BJ, Buckner RL. Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. J. Neurosci. 2004;24:10084–10092. doi: 10.1523/JNEUROSCI.2625-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura AP. Episodic retrieval and the cortical binding of relational activity. Cogn. Affect. Behav. Neurosci. 2011;11:277–291. doi: 10.3758/s13415-011-0031-4. [DOI] [PubMed] [Google Scholar]
- Simons JS, Peers PV, Mazuz YS, Berryhill ME, Olson IR. Dissociation between memory accuracy and memory confidence following bilateral parietal lesions. Cereb. Cortex. 2010;20:479–485. doi: 10.1093/cercor/bhp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick SD, Schacter DL. A sensory signature that distinguishes true from false memories. Nat. Neurosci. 2004;7:664–672. doi: 10.1038/nn1252. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Davidson PSR, Kim ASN, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield JJ, Lepsien J, Gitelman DR, Mesulam MM, Nobre AC. Orienting attention based on long-term memory experience. Neuron. 2006;49:905–916. doi: 10.1016/j.neuron.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Tulving E. Similarity relations in recognition. J. Verbal Learn. Verbal Behav. 1981;20:479–496. [Google Scholar]
- Uncapher MR, Rugg MD. Selecting for memory? The influence of selective attention on the mnemonic binding of contextual information. J. Neurosci. 2009;29:8270–8279. doi: 10.1523/JNEUROSCI.1043-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncapher MR, Wagner AD. Posterior parietal cortex and episodic encoding: insights from fMRI subsequent memory effects and dual-attention theory. Neurobiol. Learn. Mem. 2009;91:139–154. doi: 10.1016/j.nlm.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncapher MR, Hutchinson JB, Wagner AD. Dissociable effects of top-down and bottom-up attention during episodic encoding. J. Neurosci. 2011;31:12613–12628. doi: 10.1523/JNEUROSCI.0152-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: a review of evidence from a dual-process perspective. Neuropsychologia. 2008;46:1787–1799. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J. Neurophysiol. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn. Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Shulman GL, Buckner RL, Miezin FM, Velanova K, Petersen SE. Evidence for separate perceptual reactivation and search processes during remembering. Cereb. Cortex. 2006;16:949–959. doi: 10.1093/cercor/bhj037. [DOI] [PubMed] [Google Scholar]
- Wolfe JM, Horowitz TS, Michod KO. Is visual attention required for robust picture memory? Vision Res. 2007;47:955–964. doi: 10.1016/j.visres.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.