Abstract
Objective
Here we evaluated whether neurocognitive disorders in HIV-infected individuals on effective antiretroviral therapy (ART) are associated with persistent monocyte activation as indexed by levels of soluble CD163 (sCD163), shed by monocyte/macrophages.
Design
Chronically, HIV-infected individuals were examined at two consecutive visits median [interquartile range (IQR)] 16 (7–32) months apart. All patients were on ART and durably virologically suppressed (plasma HIV RNA <50 copies/ml) at all visits. Thirty-four age-matched HIV-seronegative patients were used as controls.
Methods
A global deficit score (GDS) was calculated based on comprehensive neuropsychological assessment according to standard methods. Neuropsychological and medical data were used to assign neurocognitive status according to published guidelines for HIV-associated neurocognitive disorders (HAND) as follows: neuropsychologically normal (NP-nml), asymptomatic neuropsychological impairment (ANI) and minor neurocognitive disorder (MND). sCD163 in plasma and cerebrospinal fluid was measured using ELISA.
Results
GDS-impaired patients had higher plasma sCD163 than those who were not impaired [median (IQR) 1401 ng/ml (1057–2258) versus 955 ng/ml (586–1313); Wilcoxon P = 0.028]. Patients with MND (N = 6) had significantly higher plasma sCD163 than ANI (P = 0.04) or NP-nml (P = 0.02). Whereas plasma sCD163 levels dropped in patients who were stably GDS-unimpaired after the first visit (P < 0.032), levels remained elevated in those who remained GDS-impaired (P = 0.50).
Interpretation
These findings are consistent with persistent monocyte/macrophage activation in neurophysiologically impaired HIV-infected individuals despite virally suppressive ART. Overall, these observations underscore the significance of monocyte/macrophage immune responses in HIV, persistent monocyte activation in HAND and the value of sCD163, as a plasma marker of neurocognitive impairment.
Keywords: antiretroviral therapy, biomarker, CD163 protein, cellular immunity, macrophages, neuropsychological, plasma
Introduction
Despite antiretroviral therapy (ART) that successfully reduces plasma viral load to undetectable levels and restores immune function, there remains an excess burden of neurocognitive disability in HIV-infected individuals. Potential explanations for the persistence of HIV-associated neurocognitive disorders (HAND) despite virologic suppression include irreversible prior injury, low-level viral replication not detected by available assays, central nervous system (CNS) toxicities of antiretrovirals and persistent immune activation. Previous work has focused on activation of T lymphocytes – measured, for example, as CD8+HLA-DR+CD38+ T cells – in virally suppressed individuals [1,2]. Lymphocyte activation has been linked to depletion of CD4+ T cells in gut lymphoid tissue, resulting in microbial translocation [3–6]. However, activation specifically involving cells of the monocyte-macrophage lineage may be most relevant for CNS disease and is understudied.
We have recently described that monocyte expansion from bone marrow during SIV infection correlates with the rate of AIDS progression and severity of macrophage-mediated tissue pathogenesis [7,8].We examined sCD163 plasma levels in chronic and early HIV-infected patients and demonstrated elevated sCD163 in plasma of chronic (>1 year) HIV-infected individuals compared with HIV-seronegatives [9]. In the same study with effective antiretroviral therapy (ART), sCD163 declined in parallel to HIV-RNA, but did not return to HIV-seronegative levels, suggesting residual monocyte/macrophage activation even with undetectable virus [9]. Interestingly, with early HIV infection (<1 year), ART decreased sCD163 in plasma to levels similar to those in seronegative controls [9]. Thus, we showed that monocyte/macrophage-derived sCD163 is a novel marker of HIV activity that links viral replication with monocyte/macrophage activation. sCD163 is not only a marker of HIV activity, but correlates with macrophage inflammation in coronary atherosclerosis (noncalcified plaques) [10]. Moreover, sCD163 correlates with vascular inflammation in HIV patients demonstrated by FDG-PET, which directly reflects endothelial and macrophage activation and metabolic activity [11]. This increased inflammation is not related to traditional markers of coronary inflammation, such as CRP and D-dimer, but strongly correlated with sCD163.
In this study, we sought to determine the role of monocyte activation in persistent neurocognitive impairment in HIV-infected individuals on ART with durable virologic suppression. Monocyte activation was estimated via sCD163 plasma and CSF levels, as CD163 is cleaved from the surface of macrophages and shed as sCD163 following activation and differentiation of monocyte and macrophages [12]. We hypothesized that virologically suppressed HIV-infected patients would show persistent activation of monocytes in blood that correlates with HAND and so this would be the first monocyte/macrophage-specific marker in plasma that correlated with neuropsychological impairment in HIV-infected patients on effective ART. Additionally, we re-evaluated sCD163 levels and neurocognitive status at a follow-up visit where virologic suppression was maintained in order to determine both the stability of neurocognitive status and its relation to persistent monocyte activation as indexed by sCD163. The utility of sCD163 was assessed by comparing it to other markers that have shown promise in previous studies including sCD14 [13–16], MCP-1 [17–19], and neopterin [20–22].
Material and methods
Ethics statement
The University of California San Diego (UCSD) Human Research Protection Program approved the current study and all study participants provided written informed consent to participate.
Participants
All study volunteers were enrolled at the UCSD site of the NIH-funded CNS HIV Antiretroviral Therapy Effects Research (CHARTER) study or the UCSD HIV Neurobehavioral Research Program. Exclusion criteria included: neurologic disorder not related to HIV infection (e.g. stroke, uncontrolled seizure disorder, head injury with loss of consciousness for longer than 30 min); current or past psychotic disorder (e.g. schizophrenia) not related to stimulant or other substance use; conditions that prevent use of consistent neurocognitive testing (e.g. severe visual or hearing impairment); and participants less than 18 years of age. At the time of neurocognitive testing, participants reported no use of illicit substances in the past week (excluding marijuana) and were required to have a negative toxicology screen. Plasma from EDTA-anticoagulated blood and CSF were obtained from HIV-infected individuals on ART at two time points [median interquartile range (IQR) interval between visits 16 (7–32) months] and from 34 similarly age-matched HIV-seronegative individuals (HIV negative). All HIV-infected patients were on ART and durably virologically suppressed (plasma HIV RNA <50 copies/ml at all visits) at visits A and B and all visits in between.
HIV RNA quantitation
An ultrasensitive [lower limit of detection = 50 (1.7 log10) copies RNA/ml] was used for plasma and CSF viral load quantification as previously described [9].
Neuropsychological assessment
All participants completed a comprehensive neurocognitive test battery covering seven cognitive domains, which are commonly affected by HIV-associated CNS dysfunction. The battery has been described in detail previously [23]. The seven domains assessed were speed of information processing, learning, memory, executive functioning, verbal fluency, attention/working memory, and motor skills. Raw test scores were converted to demographically adjusted normative standard scores (T scores), which correct for effects of age, education, sex and ethnicity, as appropriate [24]. Overall neurocognitive performance was summarized as Global Deficit Scores (GDS), which range from 0 (T-score >39) to 5 (T-score <20), with higher scores indicating greater levels of impairment [25]. Patients with GDS greater than or equal to 0.5 were classified as neurocognitively impaired.
To further classify presence and severity of neurocognitive impairment, we applied a published objective algorithm that has been shown to yield excellent interrater reliability in previous multisite studies [26]. This algorithm conforms to the Frascati criteria for diagnosing HAND [27], which requires at least mild impairment in at least two of the seven ability domains. HIV-associated neurocognitive disorders (HAND) were diagnosed according to criteria outlined in Antinori et al. [27]. Briefly, asymptomatic neurocognitive impairment (ANI) and mild neurocognitive disorder (MND) both require the presence of at least mild neuropsychological impairment that involves two or more ability domains, and is not readily attributable to comorbid conditions. ANI is asymptomatic in the sense that specified criteria for establishing at least mild negative effects on everyday functioning have not been met. MND requires additional functional decline, established by at least two types of evidence regarding decreased everyday functioning. HIV-associated dementia (HAD), requires overall neuropsychological impairment of at least moderate severity that is not readily attributable to comorbid conditions. In addition, ‘major’ functional decline must be established by evidence of at least two types of everyday functioning problems that are of greater severity than with MND. Functional impairment was measured both by self-report and performance-based measures as described in Blackstone et al. [28].
ELISA assays
sCD163 was detected by ELISA (Trillium Diagnostics) in the plasma and CSF according to the manufacturer’s instructions. Plasma was diluted 1 : 500 and CSF was diluted 1 : 250 for sCD163 quantitation. sCD14 was detected by ELISA (R&D Systems) in the plasma and CSF according to the manufacturer’s instructions. Plasma and CSF samples were diluted 1 : 200 for sCD14 quantitation. In addition, ELISAs were utilized to quantify CSF levels of monocyte chemotactic protein-1 (MCP-1) and neopterin according to the manufacturer’s instructions (R&D Systems and Immuno-Biological Laboratories, respectively). CSF samples were diluted 1 : 2 for the MCP-1 quantitation, and were undiluted for the neopterin ELISA.
Statistical analysis
Prism version 5.0a (GraphPad Software, Inc.) software or Microsoft Excel version 12.2.4 were used for statistical analysis. An ANOVA was first performed for analysis of variation among groups of data. If the ANOVA was significant (P < 0.05), then post-hoc t-tests were performed. Paired t-tests were used for all matched samples. A Spearman rank test was used for all correlations.
Results
Table 1 provides demographic and clinical information at baseline for the 68 study participants, including 34 healthy HIV seronegative controls and 34 HIV-infected individuals on effective ART with undetectable viral loads. Overall, HIV-infected patients were mostly middle-aged white men (mean age of 43, 91% male, 68% white) with 50% patients having AIDS (CDC category C and/or CD4 cell count <200 cells/µl). The HIV-positive and HIV-negative groups were well matched with respect to demographics and education. The HIV-positive group had experienced significant immune recovery on their ART, from a median nadir CD4 cell count of 218 cells/µl to a current CD4 cell count of 628 cells/µl. Despite virologic suppression on antiretroviral therapy, HIV-positive patients had higher plasma sCD163 levels than HIV negative (P = 0.01; Table 3). Plasma sCD14 and CSF neopterin were also significantly elevated in HIV-positive patients (0.03 and P < 0.0001, respectively, Table 3). sCD163, sCD14, and MCP-1 in CSF were not significantly different between HIV-negative and HIV-positive patients (Table 3).
Table 1.
Demographic and clinical characteristics of HIV-negative participants and neurophysiologically normal and HAND HIV-positive participants.
| HIV-positive | ||||
|---|---|---|---|---|
| HIV− | NP-nml | HAND (ANI– MND) | *P | |
| N | 34 | 19 | 15 (9 ANI–6 MND) | |
| Age year (mean, SD) | 44 (13) | 42.8 (8.8) | 44.0 (12.2) | 0.75 |
| Sex Male – N (%) | 30 (88%) | 17 (89%) | 14 (93%) | 0.69 |
| Race/ethnicity white – N (%) | 21 (62%) | 12 (63%) | 11 (73%) | 0.28 |
| Education-year mean (SD) | 14 (1.9) | 13 (2.6) | 14 (2.6) | 0.37 |
| Months between visits – median (IQR) | NA | 17.8 (7.0–34.3) | 14.5 (7.0–25.3) | 0.29 |
| Current CD4 – median (IQR) | ND | 628 (462–796) | 658 (442–876) | 0.85 |
| Nadir CD4 – median (IQR) | NA | 215 (180–300) | 240 (197–356) | 0.46 |
| Duration HIV mos – median (IQR) | NA | 123 (40.6–198) | 72.3 (24.3–172) | 0.37 |
| Current ART mos– median (IQR) | NA | 16.5 (7.8–24.3) | 12.6 (6.9–35.7) | 0.74 |
ANI, asymptomatic neurocognitive impairment; ART, antiretroviral therapy; HAND, HIV-associated neurocognitive disorders; MND, mild neurocognitive disorder; NA, not applicable; ND, not determined; NP-nml, neuropsychologically normal.
P value is for NP-nml versus HAND.
Table 3.
Immune markers in the CSF and plasma of HIV-infected humans on ART with long term viral suppression.
| Immune markers | HIV negative |
HIV positive Visit A |
HIV positive Visit B |
*P value (HIV negative versus Visit A) |
#P value (Visit A versus B) |
|---|---|---|---|---|---|
| Plasma sCD163 ng/ml | 871.3 ± 55.6 | 1343.0 ± 161.4 | 1122.9 ± 123.7 | 0.01 | 0.01 |
| Plasma sCD14 ng/ml | 1353 ± 251.6 | 1983.5 ± 251.6 | 2070.7 ± 248.3 | 0.03 | NS |
| CSF MCP-1 ng/ml | 467.7 ± 25.5 | 548.7 ± 52.3 | 500.0 ± 27.0 | NS | NS |
| CSF Neopterin nmol/l | 17.5 ± 3.8 | 181.8 ± 15.7 | 123.2 ± 17.8 | <0.0001 | 0.05 |
| CSF sCD163 ng/ml | 71.1 ± 9.0 | 65.2 ± 7.7 | 73.6 ± 10.5 | NS | NS |
| CSF sCD14 ng/ml | 132.4 ± 12.4 | 105.5 ± 10.5 | 110.0 ± 7.6 | NS | NS |
Mean ± SEM of immune markers measured in HIV-negative individuals and HIV-positive individuals at visit A and B.
Student t-tests were performed between HIV-negative and HIV-positive patients at visit A.
Paired t-tests were performed between visits A and B to determine if there was statistical significance between the groups. NS, not significant, P value more than 0.05.
At the initial visit, 19 HIV-positive patients were neurocognitively normal (NP-nml) and 15 met criteria for HAND, including six (40%) MND and nine (60%) ANI (Table 1). Those with and without HAND were well matched demographically and on HIV disease and treatment variables (Table 1). Patients with MND had significantly higher plasma sCD163 than ANI (P = 0.04) or NP-nml (P = 0.02) (Fig. 1a). Plasma or CSF sCD14, CSF neopterin, CSF sCD163 or CSF MCP-1 levels were not significantly different between neuropsychological-nml, ANI and MND (data not shown).
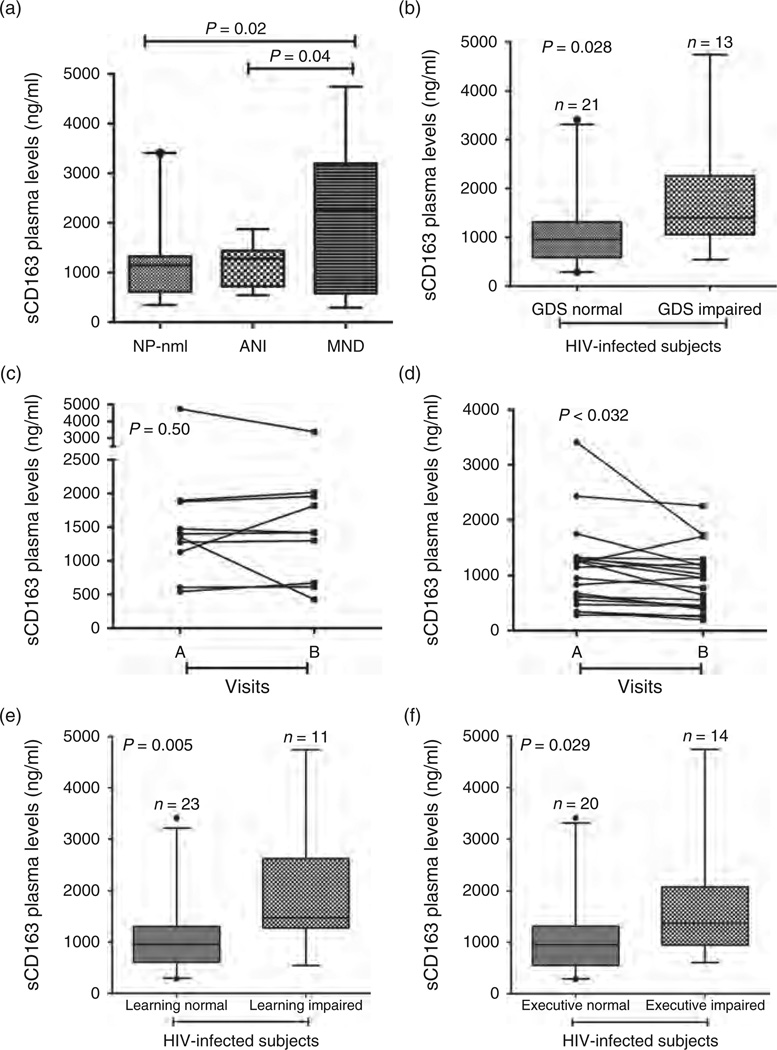
Fig. 1. sCD163 in the plasma is elevated in neurocognitively impaired HIV-infected patients with long-term viral suppression.
(a) sCD163 levels in plasma are increased in patients with mild neurocognitive disorder (MND) compared with asymptomatic neurocognitive impairment (ANI) (P = 0.04) and neuropsychologically normal (NP-nml) (P = 0.02). (b–f) At the initial visit HIV-infected patients were classified as impaired (n = 13) or normal (n = 21) for the comprehensive global deficit score (GDS). sCD163 was elevated in the plasma of HIV-infected patients with GDS impairment (b, P = 0.028). Of the 13 patients who were GDS-impaired at the first visit, 10 remained impaired at the second visit, and all but one of the 21 GDS-unimpaired patients remained normal. Whereas plasma sCD163 levels dropped in patients who were stably GDS-unimpaired across visits (c, P < 0.032, paired t-test), levels remained elevated in those who remained GDS-impaired (d, P = 0.50, paired t-test). Patients were also classified as impaired or normal in individual cognitive tests. sCD163 was elevated in patients with executive impairment (e, P = 0.029), and learning impairment (f, P = 0.005).
When overall neurocognitive performance was summarized using Global Deficit Scores (GDS), 21 HIV-positive patients were identified, as GDS normal and 13 (38%) were GDS impaired at the initial visit (Table 2). GDS-impaired patients had higher plasma sCD163 than those who were not impaired [median (IQR) 1401 ng/ml (1057–2258) versus 955 ng/ml (586–1313); Wilcoxon P = 0.028] (Fig. 1b).
Table 2.
Domain performance of impaired HIV-infected patients.
| Neurocognitive exams | Visit A | Visit B |
|---|---|---|
| aGlobal deficit score >0.5 = GDS impaired | 13 (38) | 11 (32) |
| Motor | 6 (17.6) | 7 (20.6) |
| Working memory | 9 (26.5) | 6 (17.6) |
| Verbal | 4 (12.1) | 5 (14.7) |
| aExecutive | 14 (41.2) | 8 (23.5) |
| SIP | 4 (11.8) | 5 (14.7) |
| aLearning | 11 (32.4) | 10 (29.4) |
| Global | 15 (44.1) | 15 (44.1) |
| Memory | 11 (32.4) | 12 (35.3) |
GDS, global deficit score; HAND, HIV associated neurological disorders; SIP, speeded information processing. Number (percentage) of patients with cognitive impairment at visit A and B (n = 34). GDS, motor, working memory, verbal, executive, SIP, learning, global, and memory neurocognitive exams were performed at visits A and B.
Indicates neurocognitive exams that correlated with sCD163 and/orneopterin in the plasma.
HIV-positive participants were examined at two visits at least 6 months apart [median 16.1 (IQR 6.8–31.9)]. Of the 13 patients who were GDS-impaired at the first visit, 10 remained impaired at the second visit, and all but one of the 21 GDS-unimpaired patients remained normal. All patients remained HIV-undetectable on their ART regimens. Whereas plasma sCD163 levels dropped in patients who were stably GDS-unimpaired across visits (P < 0.032) (Fig. 1c), levels remained elevated in those who remained GDS-impaired (P = 0.50) (Fig. 1d).
When examining individual neuropsychological domains as read-outs we found elevated sCD163 in patients with impaired executive functions and learning (Fig. 1e and f). Patients who were impaired in the learning domain had significantly higher sCD163 in plasma than learning normal HIV-infected patients (Fig. 1e, P = 0.005). Furthermore, patients with executive dysfunction had significantly higher sCD163 in plasma than executive normal HIV-infected patients (Fig. 1f, P = 0.029). These results are consistent with observations by others that executive and learning functions are impaired in HIV-infected patients with effective ART [23].
Following the observations above, we sought to determine if these markers changed or stabilized with long-term viral suppression [at least 6 months median 16.1 (IQR 6.8–31.9)]. Plasma sCD163 at visit A (1343.0 ± 161.4 ng/ml) significantly decreased when compared with plasma sCD163 at visit B (1122.9 ± 123.7 ng/ml) (Table 3, P = 0.01, paired t-test). CSF neopterin at visit A (181.8 ± 15.7 nmol/ml) was significantly lower when compared with CSF neopterin at visit B (123.2 ± 17.8 nmol/ml) (Table 3, P = 0.05, paired t-test). Plasma and CSF sCD14 and sCD163 and MCP-1 in CSF did not significantly change between visits (Table 3).
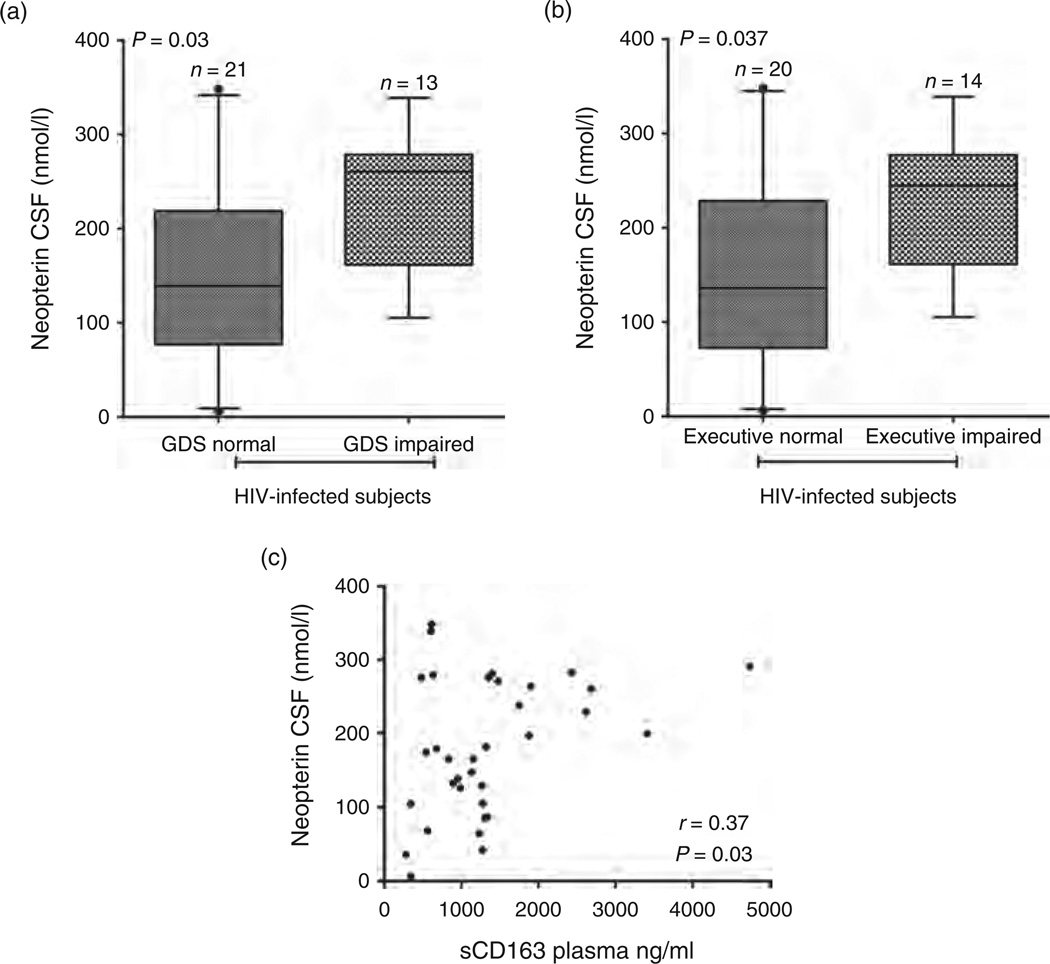
In addition to sCD163 in plasma we found that neopterin in CSF is elevated in GDS impaired HIV-infected patients compared with GDS normal HIV-infected patients (P = 0.03) (Fig. 2a). Patients who were impaired in the executive domain had significantly higher neopterin in CSF than executive normal HIV-infected patients (P = 0.037) (Fig. 2b). We did not detect a significant difference between plasma or CSF sCD14, CSF MCP-1, or CSF sCD163 between neurophysiologically impaired and neurophysiologically normal HIV-infected patients (data not shown). In HIV-infected patients, plasma sCD163 and CSF neopterin levels were significantly correlated (Spearman rho = 0.37, P = 0.03) (Fig. 2c).
Fig. 2. Elevated neopterin in the CSF correlates with neurocognitive impairment in HIV-infected patients with long-term viral suppression.
HIV-infected patients were classified as impaired or normal for GDS (global deficit score) and executive function cognitive tests. Neopterin was elevated in the CSF of HIV-infected patients with GDS impairment (a, P = 0.03) and executive impairment (b, P = 0.037). (c) Neopterin in the CSF correlates with sCD163 in the plasma of HIV-infected patients with long-term viral suppression. A Spearman correlation was used to determine that neopterin in the CSF (nmol/l) correlates with sCD163 in the plasma (ng/ml) (P = 0.03, rho = .37).
Discussion
Here we have demonstrated that neuropsychological impairment in virologically suppressed HIV-infected individuals was associated with a sustained elevation of the monocyte/macrophage activation marker sCD163 as compared to NP-nml patients, in whom sCD163 levels declined. We report elevated levels of sCD163 in patients with impaired GDS and MND demonstrating persistent monocyte/macrophage activation in HIV-related neuropsychological impairment despite virologic suppression.
Prior studies have shown that sCD14 [13–16] and MCP-1 [17–19] were associated with HIV and/or HAD, but these markers are less reliable as correlates of HAND in patients with durable virologic suppression on ART [15,29,30]. Unlike sCD163, sCD14, and MCP-1 in our study did not correlate with HIV-associated neuropsychological impairment. Elevated intracellular HIV-DNA in circulating monocytes functions as a marker of HAND in ART patients with suppressed plasma HIV RNA [31]. Additionally, HIV DNA in monocytes increased in patients with persistent HAND [32,33]. We find similar observations in our studies with regard to sCD163. Together these data are consistent with the hypothesized role of persisting monocyte/macrophage infection and activation in HIV-related neurocognitive impairment despite virologic suppression [23,34]. Moreover, these results underscore the utility of sCD163 as a plasma marker of neuropsychological impairment in HIV and focus attention on the role of monocyte/macrophage outside the CNS in CNS diseases.
A recent study (CHARTER) [23] used the Frascati criteria [27] to diagnostically classify 1555 HIV-infected individuals at six university clinics across the United States. Most of these (71%) were taking combination antiretroviral therapies and had experienced substantial immune reconstitution. In the total cohort, 52% were neuropsychologically impaired and the most frequently affected domains were learning, executive functioning, recall, and working memory. Rates of specific HAND diagnoses were as follows: ANI, 32.7%; MND, 11.7%; HAD, 2.4%. Extrapolating these figures to the larger population of HIV-infected individuals, the estimated prevalence of HAND is equal to or greater than that for multiple sclerosis in the United States. Consistent with other studies [35,36] and compared to HAND estimates from before the introduction of highly effective combination antiretroviral therapies, these figures demonstrate that the overall prevalence of HAND has not declined. Although the burden of the most severe form, HAD, has dropped markedly, milder degrees of impairment continue to be as prevalent as they were in previous eras. This has raised questions about the causes of persisting impairment.
Even milder HIV-associated neurocognitive disorders such as MND can have considerable impact on daily functioning. The burden of this functional decline is amplified by the extended survival that many of these individuals can expect on effective antiretroviral therapy. Deficits in everyday, ‘real world’ functioning affect only a subset of individuals and are generally only evident in more complex tasks such as financial management, meal preparation, and medication adherence. HIV-associated neurocognitive impairment has been established as a robust risk factor for unemployment and decreased job performance [24], nonadherence to antiretroviral medications [37], and impairment in certain driving abilities [38].
Higher plasma sCD163 levels were specifically associated with impairments in the neuropsychological domains of executive functions and learning, two of the most common and consistent areas of cognitive impairment in the ART era [23]. Global impairment is higher among asymptomatic patients in the ART versus pre-ART era, and this increase in global impairment is primarily driven by an escalation of deficits in episodic memory and executive functions [23].
We did not find significant levels of sCD163 in the CSF of HIV-infected patients with durable virologic suppression compared with seronegative individuals and sCD163 in CSF did not correlate with neurological impairment. Whether sCD163 is shed in the CSF as efficiently as plasma is not known. CNS macrophages including perivascular macrophages that are in the perivascular space, which is in contact with CSF, and some parenchymal microglia have CD163 on their surface [39,40]. The majority of monocytes express CD163 and activated CD14+CD16+ monocyte/macrophage are implicated as a significant source of sCD163 in plasma [9,39,41]. Our data and others on CD163 levels on CD14+CD16+ monocytes [39,40], as well as data demonstrating an association of HIV-DNA in circulating monocytes with HAND [31–33,42], suggest that the pathological events that led to nervous system injury may originate outside of the nervous system.
Our data are consistent with persistent monocyte/macrophage activation in HAND. The cause of persistent activation is not known, but likely results in part from bacterial translocation, coinfection (although our patients were CMV negative), and other factors. Because we did not find that sCD14 was a marker of neuropsychological impairment, future studies on monocyte/macrophage mechanisms of HIV-associated neuropsychological impairment may point to differential activation of monocyte/macrophages.
That plasma sCD163 correlates with neurological impairment is opportune, as plasma is easier to obtain than CSF. Additionally, plasma sCD163 measurements may guide Neuropsychologists in determining if a comprehensive neurocognitive test battery is needed. Elevated plasma sCD163 is found in other diseases with neurologic symptoms, but it is associated but not correlative with disease. This is likely because diseases like multiple sclerosis that have elevated sCD163 are not driven only by macrophages [12]. sCD163 does correlate in Gaucher disease, hemophagocytic syndrome and macrophage activation syndrome [12], all of which are primarily macrophage driven. These data underscore the potential importance of chronic innate immune activation in general, and monocyte/macrophage in particular with regard to CNS disease during HIV.
Our previous study of 30 chronic and 14 early HIV-infected patients found an association of sCD163 with CD14+CD16+ monocytes and HIV activity [9]. We also found a correlation of sCD163 with noncalcified plaques in 102 HIV-positive men [10] and with arterial inflammation in 27 HIV-infected patients with cardiac disease [11]. Additionally, sCD163 was significantly increased in 65 obese patients compared with 30 normal-weight controls [43]. Recently, there have been reports of correlations between patients who are obese or have cardiovascular disease with dementia. It is likely all of these contribute to HIV-associated neuropsychological impairment, wherein macrophages are central players.
Further studies on HIV-associated neuropsychological impairment should focus on CD14+CD16+ monocytes in blood that may drive HAND neuropathogenesis. In addition, research focused on chronic CNS inflammation and neuroimaging studies with plasma sCD163 may be informative. Overall, these data underscore the important role of immune activation in neurological impairment of HIV-infected patients. We suggest that sCD163, a marker of macrophage activation, is a potential biomarker in the plasma for neurocognitive impairment in HIV-infected individuals.
Acknowledgements
This work was supported by R01-NS082116 (T.H.B.), R01-NS37654 (K.C.W.), R01-NS40237 (K.C.W.), R01-MH58076 (R.J.E., S.L.), P30-MH62512 (R.J.E., S.L.), R21-MH85610 (R.J.E., S.L.), R01-MH073419 (S.P.W.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
The author’s contributions to this manuscript are as following: concept and design (T.H.B., R.J.E., K.C.W.) acquisition of data (T.H.B., A.W.), analysis and interpretation of data (T.H.B., S.W., S.L., R.J.E. and K.C.W.), drafting of manuscript (T.H.B. and K.C.W.), critical revision of the manuscript for important intellectual content (A.W., S.W., S.L. and R.J.E.), statistical analysis (T.H.B., S.W. and R.J.E.) and obtaining funding (S.W., S.L., R.J.E. and K.C.W.).
Conflicts of interest
There are no conflicts of interest.
References
- 1.d’Ettorre G, Paiardini M, Zaffiri L, Andreotti M, Ceccarelli G, Rizza C, et al. HIV persistence in the gut mucosa of HIV-infected subjects undergoing antiretroviral therapy correlates with immune activation and increased levels of LPS. Curr HIV Res. 2011;9:148–153. doi: 10.2174/157016211795945296. [DOI] [PubMed] [Google Scholar]
- 2.Sachdeva M, Fischl MA, Pahwa R, Sachdeva N, Pahwa S. Immune exhaustion occurs concomitantly with immune activation and decrease in regulatory T cells in viremic chronically HIV-1-infected patients. J Acquir Immune Defic Syndr. 2010;54:447–454. doi: 10.1097/QAI.0b013e3181e0c7d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 4.Veazey RS, Lackner AA. Getting to the guts of HIV pathogenesis. J Exp Med. 2004;200:697–700. doi: 10.1084/jem.20041464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ancuta P, Kamat A, Kunstman KJ, Kim EY, Autissier P, Wurcel A, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One. 2008;3:e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa A, Liu H, Ling B, Borda JT, Alvarez X, Sugimoto C, et al. The level of monocyte turnover predicts disease progression in the macaque model of AIDS. Blood. 2009;114:2917–2925. doi: 10.1182/blood-2009-02-204263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burdo TH, Soulas C, Orzechowski K, Button J, Krishnan A, Sugimoto C, et al. Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog. 2010;6:e1000842. doi: 10.1371/journal.ppat.1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burdo TH, Lentz MR, Autissier P, Krishnan A, Halpern E, Letendre S, et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after antiretroviral therapy. J Infect Dis. 2011;204:154–163. doi: 10.1093/infdis/jir214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204:1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, et al. Arterial inflammation in patients with HIV. JAMA. 2012;308:379–386. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moller HJ. Soluble CD163. Scand J Clin Lab Invest. 2011;72:1–13. doi: 10.3109/00365513.2011.626868. [DOI] [PubMed] [Google Scholar]
- 13.Nockher WA, Bergmann L, Scherberich JE. Increased soluble CD14 serum levels and altered CD14 expression of peripheral blood monocytes in HIV-infected patients. Clin Exp Immunol. 1994;98:369–374. doi: 10.1111/j.1365-2249.1994.tb05499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lien E, Aukrust P, Sundan A, Muller F, Froland SS, Espevik T. Elevated levels of serum-soluble CD14 in human immunodeficiency virus type 1 (HIV-1) infection: correlation to disease progression and clinical events. Blood. 1998;92:2084–2092. [PubMed] [Google Scholar]
- 15.Ryan LA, Zheng J, Brester M, Bohac D, Hahn F, Anderson J, et al. Plasma levels of soluble CD14 and tumor necrosis factor-alpha type II receptor correlate with cognitive dysfunction during human immunodeficiency virus type 1 infection. J Infect Dis. 2001;184:699–706. doi: 10.1086/323036. [DOI] [PubMed] [Google Scholar]
- 16.Lyons JL, Uno H, Ancuta P, Kamat A, Moore DJ, Singer EJ, et al. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. J Acquir Immune Defic Syndr. 2011;57:371–379. doi: 10.1097/QAI.0b013e3182237e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cinque P, Vago L, Mengozzi M, Torri V, Ceresa D, Vicenzi E, et al. Elevated cerebrospinal fluid levels of monocyte chemo-tactic protein-1 correlate with HIV-1 encephalitis and local viral replication. AIDS. 1998;12:1327–1332. doi: 10.1097/00002030-199811000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Monteiro de Almeida S, Letendre S, Zimmerman J, Lazzaretto D, McCutchan A, Ellis R. Dynamics of monocyte chemoattractant protein type one (MCP-1) and HIV viral load in human cerebrospinal fluid and plasma. J Neuroimmunol. 2005;169:144–152. doi: 10.1016/j.jneuroim.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Sevigny JJ, Albert SM, McDermott MP, McArthur JC, Sacktor N, Conant K, et al. Evaluation of HIV RNA and markers of immune activation as predictors of HIV-associated dementia. Neurology. 2004;63:2084–2090. doi: 10.1212/01.wnl.0000145763.68284.15. [DOI] [PubMed] [Google Scholar]
- 20.Dunbar N, Pemberton L, Perdices M, Brew BJ. Clinical markers of the presence of dementia and neuropsychological impairment in HIV infection. J Neuro AIDS. 1996;1:31–48. doi: 10.1300/j128v01n04_04. [DOI] [PubMed] [Google Scholar]
- 21.Hagberg L, Cinque P, Gisslen M, Brew BJ, Spudich S, Bestetti A, et al. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther. 2010;7:15. doi: 10.1186/1742-6405-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gisslen M, Chiodi F, Fuchs D, Norkrans G, Svennerholm B, Wachter H, Hagberg L. Markers of immune stimulation in the cerebrospinal fluid during HIV infection: a longitudinal study. Scand J Infect Dis. 1994;26:523–533. doi: 10.3109/00365549409011810. [DOI] [PubMed] [Google Scholar]
- 23.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10:317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- 25.Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004;26:307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- 26.Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol. 2004;26:759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
- 27.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blackstone K, Moore DJ, Heaton RK, Franklin DR, Jr, Woods SP, Clifford DB, et al. Diagnosing symptomatic HIV-associated neurocognitive disorders: self-report versus performance-based assessment of everyday functioning. J Int Neuropsychol Soc. 2012;18:79–88. doi: 10.1017/S135561771100141X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McArthur JC, McDermott MP, McClernon D, St Hillaire C, Conant K, Marder K, et al. Attenuated central nervous system infection in advanced HIV/AIDS with combination antiretroviral therapy. Arch Neurol. 2004;61:1687–1696. doi: 10.1001/archneur.61.11.1687. [DOI] [PubMed] [Google Scholar]
- 30.Chang L, Ernst T, St Hillaire C, Conant K. Antiretroviral treatment alters relationship between MCP-1 and neurometabolites in HIV patients. Antivir Ther. 2004;9:431–440. doi: 10.1177/135965350400900302. [DOI] [PubMed] [Google Scholar]
- 31.Valcour VG, Shiramizu BT, Sithinamsuwan P, Nidhinandana S, Ratto-Kim S, Ananworanich J, et al. HIV DNA and cognition in a Thai longitudinal HAART initiation cohort: the SEARCH 001 Cohort Study. Neurology. 2009;72:992–998. doi: 10.1212/01.wnl.0000344404.12759.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiramizu B, Ananworanich J, Chalermchai T, Siangphoe U, Troelstrup D, Shikuma C, et al. Failure to clear intra-monocyte HIV infection linked to persistent neuropsychological testing impairment after first-line combined antiretroviral therapy. J Neurovirol. 2012;18:69–73. doi: 10.1007/s13365-011-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kusao I, Shiramizu B, Liang CY, Grove J, Agsalda M, Troelstrup D, et al. Cognitive performance related to HIV-1-infected monocytes. J Neuropsychiatry Clin Neurosci. 2012;24:71–80. doi: 10.1176/appi.neuropsych.11050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Letendre SL, Ellis RJ, Everall I, Ances B, Bharti A, McCutchan JA. Neurologic complications of HIV disease and their treatment. Top HIV Med. 2009;17:46–56. [PMC free article] [PubMed] [Google Scholar]
- 35.McArthur JC, Sacktor N, Selnes O. Human immunodeficiency virus-associated dementia. Semin Neurol. 1999;19:129–150. doi: 10.1055/s-2008-1040831. [DOI] [PubMed] [Google Scholar]
- 36.McArthur JC. HIV dementia: an evolving disease. J Neuroimmunol. 2004;157:3–10. doi: 10.1016/j.jneuroim.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 37.Hinkin CH, Castellon SA, Durvasula RS, Hardy DJ, Lam MN, Mason KI, et al. Medication adherence among HIV+ adults: effects of cognitive dysfunction and regimen complexity. Neurology. 2002;59:1944–1950. doi: 10.1212/01.wnl.0000038347.48137.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marcotte TD, Wolfson T, Rosenthal TJ, Heaton RK, Gonzalez R, Ellis RJ, Grant I. A multimodal assessment of driving performance in HIV infection. Neurology. 2004;63:1417–1422. doi: 10.1212/01.wnl.0000141920.33580.5d. [DOI] [PubMed] [Google Scholar]
- 39.Fischer-Smith T, Tedaldi EM, Rappaport J. CD163/CD16 coexpression by circulating monocytes/macrophages in HIV: potential biomarkers for HIV infection and AIDS progression. AIDS Res Hum Retroviruses. 2008;24:417–421. doi: 10.1089/aid.2007.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L’Heureux D, Regulier EG, et al. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001;7:528–541. doi: 10.1080/135502801753248114. [DOI] [PubMed] [Google Scholar]
- 41.Davis BH, Zarev PV. Human monocyte CD163 expression inversely correlates with soluble CD163 plasma levels. Cytometry B Clin Cytom. 2005;63:16–22. doi: 10.1002/cyto.b.20031. [DOI] [PubMed] [Google Scholar]
- 42.Shiramizu B, Williams AE, Shikuma C, Valcour V. Amount of HIV DNA in peripheral blood mononuclear cells is proportional to the severity of HIV-1-associated neurocognitive disorders. J Neuropsychiatry Clin Neurosci. 2009;21:68–74. doi: 10.1176/appi.neuropsych.21.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zanni MV, Burdo TH, Makimura H, Williams KC, Grinspoon SK. Relationship between monocyte/macrophage activation marker soluble CD163 and insulin resistance in obese and normal-weight subjects. Clin Endocrinol (Oxf) 2012;77:385–390. doi: 10.1111/j.1365-2265.2011.04284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]