Abstract
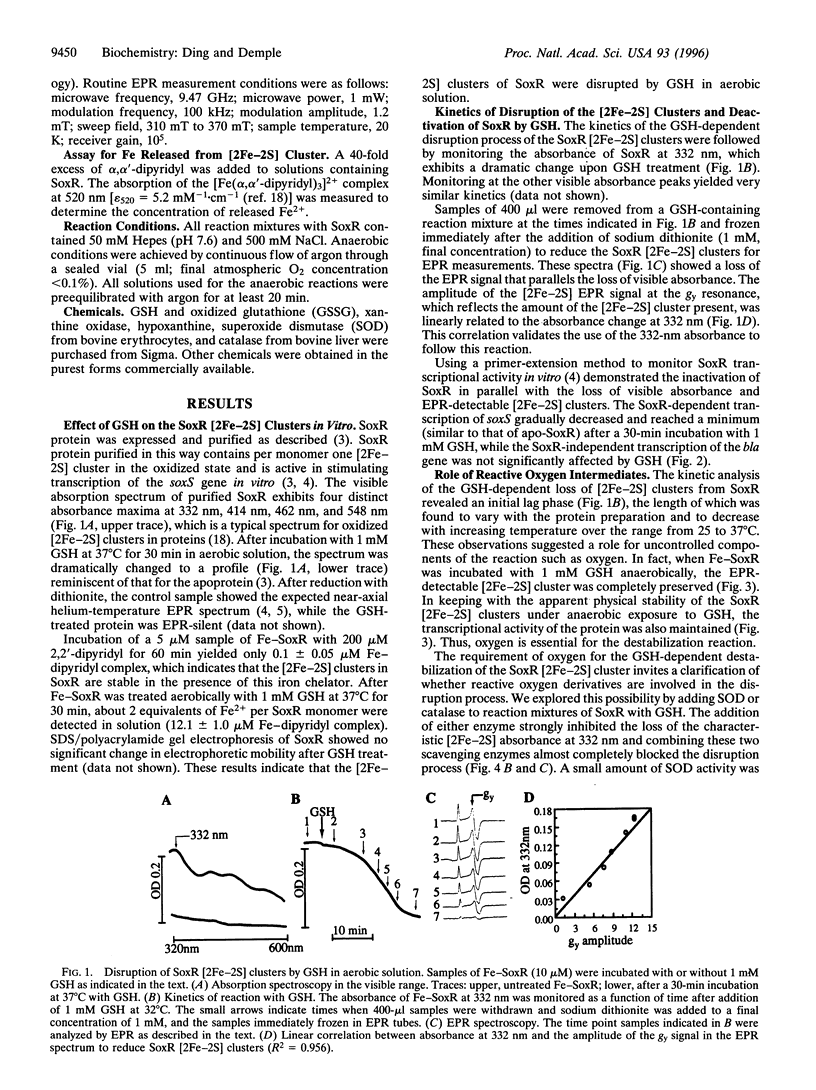
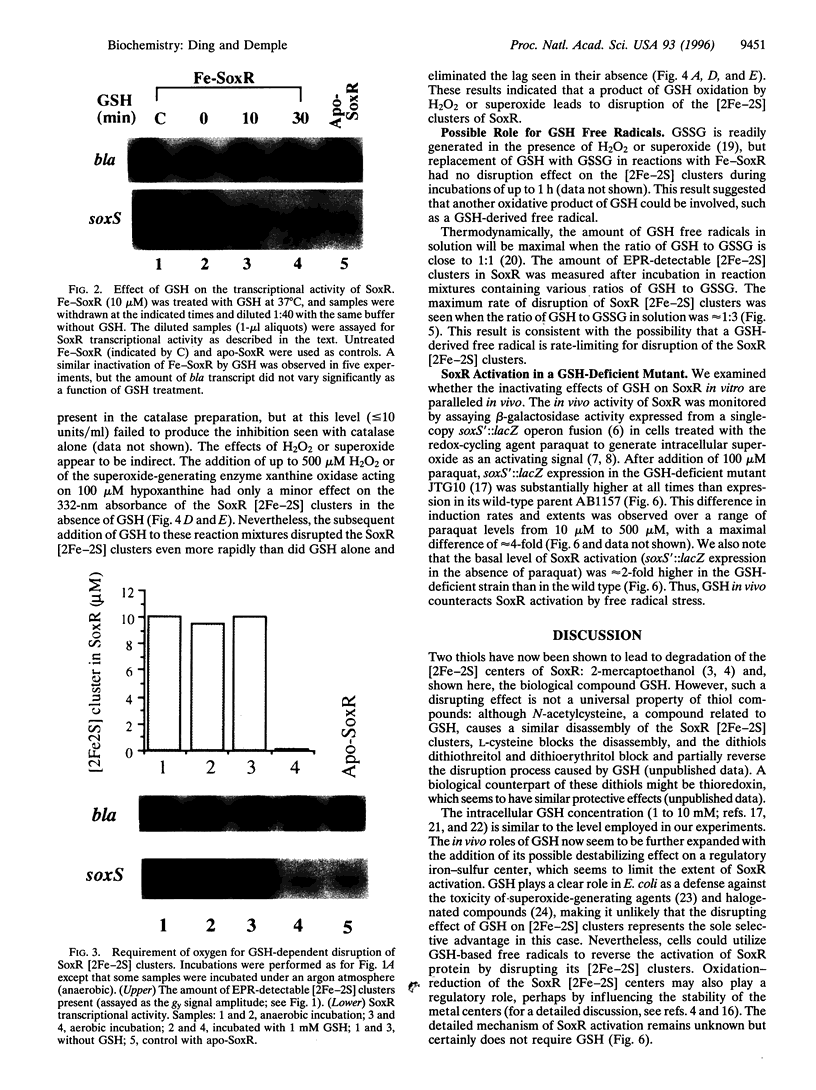
SoxR is a transcription factor that governs a global defense against the oxidative stress caused by nitric oxide or excess superoxide in Escherichia coli. SoxR is a homodimer containing a pair of [2Fe-2S] clusters essential for its transcriptional activity, and changes in the stability of these metal centers could contribute to the activation or inactivation of SoxR in vivo. Herein we show that reduced glutathione (GSH) in aerobic solution disrupts the SoxR [2Fe-2S] clusters, releasing Fe from the protein and eliminating SoxR transcriptional activity. This disassembly process evidently involves oxygen-derived free radicals. The loss of [2Fe-2S] clusters does not occur in anaerobic solution and is blocked in aerobic solution by the addition of superoxide dismutase and catalase. Although H2O2 or xanthine oxidase and hypoxanthine (to generate superoxide) were insufficient on their own to cause [2Fe-2S] cluster loss, they did accelerate the rate of disassembly after GSH addition. Oxidized GSH alone was ineffective in disrupting the clusters, but the rate of [2Fe-2S] cluster disassembly was maximal when reduced and oxidized GSH were present at a ratio of approximately 1:3, which suggests the critical involvement of a GSH-based free radical in the disassembly process. Such a reaction might occur in vivo: we found that the induction by paraquat of SoxR-dependent soxS transcription was much higher in a GSH-deficient E. coli strain than in its GSH-containing parent. The results imply that GSH may play a significant role during the deactivation process of SoxR in vivo. Ironically, superoxide production seems both to activate SoxR and, in the GSH-dependent disassembly process, to switch off this transcription factor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amábile-Cuevas C. F., Demple B. Molecular characterization of the soxRS genes of Escherichia coli: two genes control a superoxide stress regulon. Nucleic Acids Res. 1991 Aug 25;19(16):4479–4484. doi: 10.1093/nar/19.16.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari A. Z., Bradner J. E., O'Halloran T. V. DNA-bend modulation in a repressor-to-activator switching mechanism. Nature. 1995 Mar 23;374(6520):371–375. doi: 10.1038/374370a0. [DOI] [PubMed] [Google Scholar]
- Ansari A. Z., Chael M. L., O'Halloran T. V. Allosteric underwinding of DNA is a critical step in positive control of transcription by Hg-MerR. Nature. 1992 Jan 2;355(6355):87–89. doi: 10.1038/355087a0. [DOI] [PubMed] [Google Scholar]
- Beinert H., Kiley P. Redox control of gene expression involving iron-sulfur proteins. Change of oxidation-state or assembly/disassembly of Fe-S clusters? FEBS Lett. 1996 Mar 11;382(1-2):218–221. doi: 10.1016/0014-5793(96)00140-8. [DOI] [PubMed] [Google Scholar]
- Buettner G. R. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys. 1993 Feb 1;300(2):535–543. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- Chesney J. A., Eaton J. W., Mahoney J. R., Jr Bacterial glutathione: a sacrificial defense against chlorine compounds. J Bacteriol. 1996 Apr;178(7):2131–2135. doi: 10.1128/jb.178.7.2131-2135.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. Enzymatic/nonenzymatic sources of oxyradicals and regulation of antioxidant defenses. Ann N Y Acad Sci. 1994 Nov 17;738:8–14. doi: 10.1111/j.1749-6632.1994.tb21784.x. [DOI] [PubMed] [Google Scholar]
- Fawcett W. P., Wolf R. E., Jr Genetic definition of the Escherichia coli zwf "soxbox," the DNA binding site for SoxS-mediated induction of glucose 6-phosphate dehydrogenase in response to superoxide. J Bacteriol. 1995 Apr;177(7):1742–1750. doi: 10.1128/jb.177.7.1742-1750.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W., Jack R. F., Morgan T. V., Dean D. R., Johnson M. K. nifU gene product from Azotobacter vinelandii is a homodimer that contains two identical [2Fe-2S] clusters. Biochemistry. 1994 Nov 15;33(45):13455–13463. doi: 10.1021/bi00249a034. [DOI] [PubMed] [Google Scholar]
- Gardner P. R., Fridovich I. Effect of glutathione on aconitase in Escherichia coli. Arch Biochem Biophys. 1993 Feb 15;301(1):98–102. doi: 10.1006/abbi.1993.1120. [DOI] [PubMed] [Google Scholar]
- Greenberg J. T., Demple B. Glutathione in Escherichia coli is dispensable for resistance to H2O2 and gamma radiation. J Bacteriol. 1986 Nov;168(2):1026–1029. doi: 10.1128/jb.168.2.1026-1029.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo E., Bollinger J. M., Jr, Bradley T. M., Walsh C. T., Demple B. Binuclear [2Fe-2S] clusters in the Escherichia coli SoxR protein and role of the metal centers in transcription. J Biol Chem. 1995 Sep 8;270(36):20908–20914. doi: 10.1074/jbc.270.36.20908. [DOI] [PubMed] [Google Scholar]
- Hidalgo E., Demple B. Activation of SoxR-dependent transcription in vitro by noncatalytic or NifS-mediated assembly of [2Fe-2S] clusters into apo-SoxR. J Biol Chem. 1996 Mar 29;271(13):7269–7272. doi: 10.1074/jbc.271.13.7269. [DOI] [PubMed] [Google Scholar]
- Hidalgo E., Demple B. An iron-sulfur center essential for transcriptional activation by the redox-sensing SoxR protein. EMBO J. 1994 Jan 1;13(1):138–146. doi: 10.1002/j.1460-2075.1994.tb06243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang C., Sinskey A. J., Lodish H. F. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992 Sep 11;257(5076):1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- Li Z., Demple B. Sequence specificity for DNA binding by Escherichia coli SoxS and Rob proteins. Mol Microbiol. 1996 Jun;20(5):937–945. doi: 10.1111/j.1365-2958.1996.tb02535.x. [DOI] [PubMed] [Google Scholar]
- Li Z., Demple B. SoxS, an activator of superoxide stress genes in Escherichia coli. Purification and interaction with DNA. J Biol Chem. 1994 Jul 15;269(28):18371–18377. [PubMed] [Google Scholar]
- Meister A. Glutathione-ascorbic acid antioxidant system in animals. J Biol Chem. 1994 Apr 1;269(13):9397–9400. [PubMed] [Google Scholar]
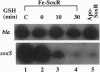
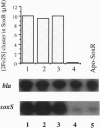
- Nunoshiba T., Hidalgo E., Amábile Cuevas C. F., Demple B. Two-stage control of an oxidative stress regulon: the Escherichia coli SoxR protein triggers redox-inducible expression of the soxS regulatory gene. J Bacteriol. 1992 Oct;174(19):6054–6060. doi: 10.1128/jb.174.19.6054-6060.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunoshiba T., Hidalgo E., Li Z., Demple B. Negative autoregulation by the Escherichia coli SoxS protein: a dampening mechanism for the soxRS redox stress response. J Bacteriol. 1993 Nov;175(22):7492–7494. doi: 10.1128/jb.175.22.7492-7494.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C., Metodiewa D. Reaction of superoxide with glutathione and other thiols. Methods Enzymol. 1995;251:81–86. doi: 10.1016/0076-6879(95)51112-1. [DOI] [PubMed] [Google Scholar]
- Wu J., Dunham W. R., Weiss B. Overproduction and physical characterization of SoxR, a [2Fe-2S] protein that governs an oxidative response regulon in Escherichia coli. J Biol Chem. 1995 Apr 28;270(17):10323–10327. doi: 10.1074/jbc.270.17.10323. [DOI] [PubMed] [Google Scholar]
- Wu J., Weiss B. Two-stage induction of the soxRS (superoxide response) regulon of Escherichia coli. J Bacteriol. 1992 Jun;174(12):3915–3920. doi: 10.1128/jb.174.12.3915-3920.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]