Abstract
Males of advanced age represent a rapidly growing population at risk for prostate cancer. In the contemporary setting of earlier detection, a majority of prostate carcinomas are still clinically localized and often treated using radiation therapy. Our recent studies have shown that premature cellular senescence, rather than apoptosis, accounts for most of the clonogenic death induced by clinically-relevant doses of irradiation in prostate cancer cells. We show here that this treatment-induced senescence was associated with a significantly increased release of exosome-like microvesicles. In premature senescence, this novel secretory phenotype was dependent on the activation of p53. In addition, the release of exosome-like microvesicles also increased during proliferative senescence in normal human diploid fibroblasts. These data support the hypothesis that senescence, initiated either by telomere attrition (e.g., aging) or DNA damage (e.g., radiotherapy), may induce a p53-dependent increase in the biogenesis of exosome-like vesicles. Ultrastructural analysis and RNAi-mediated knockdown of Tsg101 provided significant evidence that the additional exosomes released by prematurely senescent prostate cancer cells were principally derived from multivesicular endosomes (MVEs). Moreover, these exosomes were enriched in B7-H3 protein, a recently identified diagnostic marker for prostate cancer, and an abundance of what has recently been termed “exosomal shuttle RNA”. Our findings are consistent with the proposal that exosomes can transfer cargos, with both immunoregulatory potential and genetic information, between cells through a novel mechanism that may be recruited to increase exosome release during accelerated and replicative cellular senescence.
Keywords: Irradiation, Tsg101, B7-H3, p53, exosome shuttle RNA, premature senescence, proliferative senescence
Introduction
Advanced age is the dominant risk factor for developing carcinoma of the prostate. The incidence of prostate cancer increases dramatically in men beyond 50 years of age and reaches a frequency of one-in-seven between ages 60 and 79 years (1). Once a prostatic intraepithelial neoplasia (PIN) has been initiated, a host of age-related alterations have the potential to influence the evolution and growth of malignant lesions; including chronic oxidative stress and inflammation, genomic instability, a loss of immune surveillance and telomere attrition. Telomere attrition limits the reproductive life span of normal human cells by inducing a permanent cell cycle arrest that is termed replicative senescence (2). Although replicative senescence is generally considered a natural tumor suppressor mechanism, owing to its antiproliferative effects, there is now evidence that senescent prostate fibroblasts populating aged tissue may secrete soluble growth factors capable of altering the host microenvironment (3). Understanding the relationships between biological ageing, this “senescent secretory phenotype” and age-related diseases such as prostate cancer represents an emerging area of active research.
Although no longer dividing, senescent fibroblasts remain metabolically active and secrete high levels of epithelial growth factors and matrix metalloproteinases (4–8). Thus, an accumulation of senescent fibroblasts can alter the surrounding microenvironment; the so-called “reactive stroma” associated with some carcinomas, and promote the growth and tumor progression of initiated human prostate epithelial cells (9). The factors secreted by senescent cells vary depending on cell type and the vast majority of studies to date have analyzed this senescent secretory phenotype using normal fibroblasts that have reached replicative exhaustion as a cell model (4–9). These studies have convincingly demonstrated that the p53, p21WAF1/Cip1 (p21), p16INK4a (p16) and pRb tumor suppressors are important senescence regulators (10). The functional collaboration between the p16/pRb and p53/p21 pathways responsible for initiating a permanent growth arrest have been described in detail (11, 12), yet we still understand little about the age-related mechanisms involved in initiating or maintaining the senescent secretory phenotype.
External beam radiation has long been one of the major curative treatment options for patients with clinically localized prostate cancer. We have recently presented evidence that the in vitro effectiveness of this treatment modality may be principally attributed to its ability to induce a p53-dependent terminal growth arrest, termed accelerated or “premature senescence,” rather than a direct cell kill (13). There is no direct evidence that the clinical use of irradiation induces premature senescence in prostate tumor cells in vivo. However, there is evidence that chemotherapeutics and irradiation selectively induce premature senescence within the malignant foci of breast cancer specimens (14).
Much recent attention has been focused on the task of identifying soluble factors secreted by senescent fibroblasts and tumor cells and characterizing their paracrine activities (3–8, 15). It is important to note, however, that in addition to soluble paracrine factors, many tumor cells also release microvesicles that have been termed “exosomes” (16). Exosomes are small, cup-shaped, vesicles of ~100nm diameter that are initially formed within the endosomal compartment and are secreted when a multivesicular endosome (MVE) fuses with the plasma membrane (17, 18). Interestingly, the abundance of exosomes released generally correlates positively with advanced stages of cancer progression (16). Moreover, there is recent evidence that the irradiation-induced activation of p53 has the potential to increase exosome release from nonquiescent human lung cancer cells (19).
In the present study, we sought to determine whether a relationship exists between the irradiation-induced activation of p53, premature cellular senescence and alterations in exosome biogenesis in human prostate cancer cells. We compared presenescent (i.e., mock-irradiated) and senescent (irradiated) prostate cancer cells for alterations in the senescence-associated secretion of exosome-like particles. This analysis identified a novel and previously unrecognized connection between the induction of p53-dependent senescence and the release of endosome-derived exosomes in human prostate cancer cells. Moreover, our analysis of replicative senescence in normal human fibroblasts provided preliminary evidence of a more general functional relationship that may be intrinsic to this mode of clonogenic death. Importantly, results of our biochemical analyses also support the concept that senescence-associated exosomes can transfer cargos, with both immunoregulatory potential and genetic information, between cells through a novel mechanism that may be recruited to increase exosome release during cellular senescence.
Materials and Methods
Cell culture
LNCaP, 22Rv1 and DU145 cells were acquired from the American Type Culture Collection (Manassas, VA USA; ATCC CRL-1740, CRL-2505 and HTB-81, respectively). LNCaP and 22Rv1 cultures were maintained in RPMI 1640 media containing 2mM L-glutamine, 10 mM HEPES, 1mM sodium pyruvate, 4.5 g/L glucose and 1.5 g/L sodium bicarbonate, supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 mg/mL streptomycin. The effects of p53 inactivation on irradiation-induced cellular senescence and exosome released were examined by infecting LNCaP and 22Rv1 cells with a retroviral vector encoding dominant negative (DN) p53 as described (13).
DU145 cells were cultured in minimum essential medium (MEM) with 2 mM L-glutamine and Earle’s balanced salt solution (BSS) adjusted to contain 1.5 g/L sodium bicarbonate, 0.1 mM nonessential amino acids, 0.1 mM sodium pyruvate, supplemented with 10% FBS and 100 U/ml penicillin and 100 mg/ml streptomycin. Normal human dermal fibroblasts (NHDF) were obtained from Dr. Warren Knudson (Department of Anatomy and Cell Biology, East Carolina University, Greenville, NC) and were grown as an adherent culture with MEM containing 2 mM L-glutamine and Earle’s BSS adjusted to contain 1.5 g/L sodium bicarbonate, 0.1 mM nonessential amino acids, 0.1 mM sodium pyruvate, supplemented with 10% FBS and 100 U/ml penicillin and 100 mg/ml streptomycin.
Senescence induction
Accelerated senescence: Subconfluent cultures of human prostate cancer cells (LNCaP, 22Rv1, and DU145) were routinely seeded in triplicate and allowed to proliferate to ~70% confluence prior to mock-irradiation (0 Gy) or exposure to an irradiation dose of 4 Gy, using a Gammacell 40 (Atomic Energy of Canada Limited) Cs137 source. After treatment, the cells were left to recover 4 days in complete medium. At this time post-irradiation, a majority of LNCaP and 22Rv1, but not DU145, cells test positive for various phenotypic markers of cellular senescence (13) and could be used for comparisons of exosome release from presenescent (mock-irradiated) versus prematurely senescent (irradiated) cells. Replicative senescence: To establish cells at replicative senescence, NHDF cells were cultured until cell doubling time exceeded 12 days (>10 cell doublings). At this time, the cells were considered to have reached replicative exhaustion. Induction of senescence was routinely verified by measuring the increased senescence-associated β-galactosidase (SA β-Gal) activity, as described previously (13).
Exosome purification and analysis
Exosomes were isolated from medium conditioned by LNCaP, 22Rv1 and DU145 cells using the differential centrifugation protocol of Thery et al. (20), with the following modifications. Subconfluent cultures were washed twice in PBS and incubated 48 h in serum-free medium. In our studies of irradiation-induced senescence, this period of conditioning was initiated 4 days after a single exposure to either 0 or 4 Gy. This conditioned medium was decanted, centrifuged at 500g for 10 min to sediment cells and at 14,000g for 15 min to eliminate cell debris. Exosomes were sedimented by ultracentrifugation at 100,000g for 70 min and the resultant pellets were washed once in PBS. The washed pellets were examined by electron microscopy (see below) and examined for their protein or lipid content using SDS-PAGE and Daiichi silver staining, immunoblotting or stained using Vybrant DiI (Molecular Probes, Portland, OR), a fluorescent carbocyanine lipid analogue that uniformly stains biological membranes (21). For lipid staining, equal aliquots of the final exosome suspensions were incubated 20 min at 37°C in the presence of 5 µM DiI and excess dye was removed by washing in PBS. The fluorescent intensity (Ex530, Em590) of these DiI-stained membrane fractions were measured using a Synergy HT microplate reader (Bio-Tek, Winooski, VT) and used for comparisons of vesicle secretion. All values were corrected for differences in the total number of viable cells left on the plate after the 48 h conditioning period, measured using the Trypan Blue-exclusion assay.
Electron microscopy
Exosome preparations from LNCaP and 22Rv1 cells were mixed with equal quantities of freshly prepared 4% paraformaldehyde and 5 µl of the sample was loaded onto carbon-coated formvar grids. After incubation in a moist atmosphere for 20 min, the samples were washed three times in PBS and then fixed for 5 min in 1% glutaraldehyde. After three washes, the exosome samples were stained for 10 min with saturated aqueous uranyl. Exosomes were examined with a JEOL 1200EX electron microscope at 60 kV.
Western blot analysis
Immunoblots were performed as described previously (13). Antibodies purchased from R&D Systems (Minneapolis, MN) were raised against human B7-H3. Anti-Tsg101 antibody (C-2) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-β-actin antibody (Ab-1) was purchased from Calbiochem (La Jolla, CA). Where specified, the relative intensity of immunospecific bands were analyzed with a Molecular Dynamics densitometer and ImageQuant software (GE Healthcare, Piscataway, NJ).
RNA interference
22Rv1 cells were transfected with oligomer-Lipofectamine 2000 complexes using 100 nM human Tsg101 SMARTpool siRNAs (NM_006292) or a non-targeting siRNA control, according to the manufacturer’s protocol (Dharmacon Inc., Lafayette, CO). Briefly, three days after cells had either been mock-irradiated or exposed to a 4 Gy dose the cultures were washed with PBS and incubated 24 h in serum-free medium. 22Rv1 cells were then incubated 24 h in the presence of 100 nM siRNA-Lipofectamine complexes before the medium was replaced with serum- and siRNA-free medium. After 72 h, the conditioned medium was removed for exosome purification and cell lysates were prepared using the corresponding adherent cells.
Total RNA extraction and analysis
Exosome fractions were resuspended in RNase-free water, briefly sonicated, and total nucleic acid was extracted using TRIzol reagent, according to the manufacturer’s protocol (Invitrogen Corp.). RNA precipitates were resuspended in RNase-free PBS and divided into three equal aliquots. Control samples were incubated 10 min at 37 °C, while the remaining two were incubated, under identical conditions, in the presence of either 0.4 µg/µl RNase or 0.083 U/µl DNase. These samples were separated on 2% agarose gels and stained with ethidium bromide.
Analysis of clonogenic survival and cell fate
Assays of clonogenic survival after a single exposure to 2 Gy (SF2), apoptosis (i.e., TdT-mediated dUTP nick end labeling, flow cytometric analysis of DNA content, and acridine orange/ethidium bromide staining), and cellular senescence (SA β-Gal and carboxyfluorescein diacetate succinimidyl ester (CFDA) flow cytometry) were performed as previously described (13).
Data analysis
Values shown are representative of triplicate determinations in two or more experiments, unless otherwise specified, and treatment effects were evaluated using a two-sided Student's t test. Errors are standard errors of the mean (s.e.) of averaged results and values of P < 0.05 were taken as a significant difference between means.
Results and Discussion
The three primary responses mediated by p53 in response to irradiation-induced DNA damage are cell cycle arrest, apoptosis or cellular senescence. Collectively, data from our previous study strongly supported the idea that, within four days, a single exposure to radiation could lead to the emergence of a prematurely senescent subpopulation of prostate cancer cells (13). Other studies suggest that senescent cells such as these have the potential to influence a tumor microenvironment through secretion of soluble pro- and anti-tumorigenic factors (3, 6, 8, 12, 15). However, the assortment of factors secreted appears to be cell-type and tissue-specific (12, 15). Senescent prostate fibroblasts have recently been shown to secrete soluble growth factors (3). Proteomic profiling the soluble factors secreted by a variety of senescent cell-types is currently a subject of intense investigation. However, we have found that the particulate material secreted by senescent prostate cancer cells may be equally informative.
Evidence of an endosomal origin for irradiation-induced exosome release
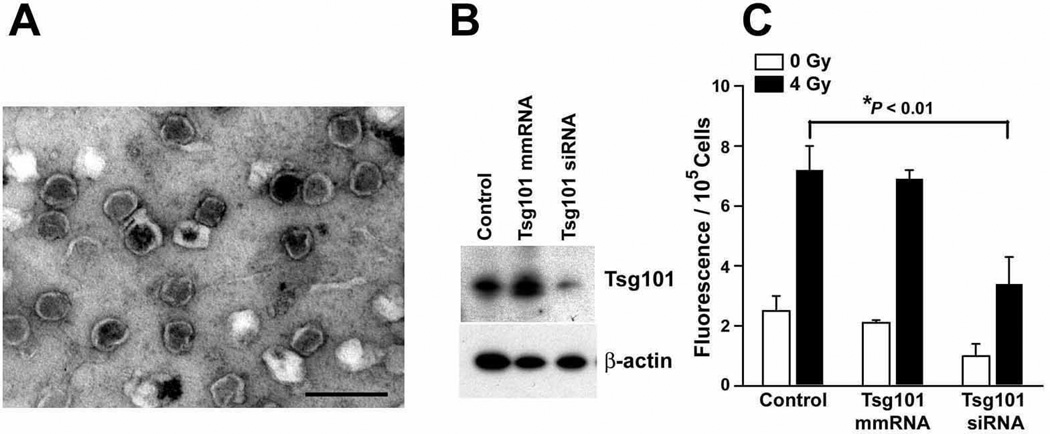
To establish a subpopulation of prematurely senescent cells, LNCaP and 22Rv1 cells were irradiated and allowed to accumulate over a period of four days before the medium was replenished with fresh serum-free medium and conditioned two days, as previously described (13). Microvesicles secreted by these presenescent cells were isolated from the conditioned medium and the final fractions were examined by electron microscopy or stained using the lipid probe Vybrant DiI (21). Electron micrographs revealed that 22Rv1 cells released cup-shaped, rounded, vesicles with a size of approximately 80 – 150 nm (Fig. 1a), similar to exosomes released by LNCaP prostate cancer cells (data not shown and Ref. 18) and other cell types (18–20). Of note was also the absence of any visual evidence of contamination by membrane fragments in our preparations of exosome-like particles. Additional evidence that microvesicles released by 22Rv1 cells were of endosomal origin is presented below (Fig. 1b).
Figure 1.
Ultrastructural and functional analysis of exosomes released from 22Rv1 prostate cancer cells. (a) Electron micrograph of 22Rv1 exosome-like particles. The image shows small cup-shaped vesicles of approximately 60–150 nm in diameter, which is representative of the exosome-like vesicles released by both 22Rv1 and LNCaP cells. Exosome fractions were isolated from serum-free medium conditioned by 22Rv1 cells for 48 h, beginning 4 days after irradiation (4 Gy). Scale bar equals 200 nm. (b) Western blot analysis of Tsg101 downregulation in irradiated 22Rv1 cells. A mock transfection (control) or transfection with a Tsg101 mismatch (mm) RNA or Tsg101 siRNA (both 100 nM) on day 3 after irradiation (4 Gy) was followed by harvesting of cell lysates on day 6. (c) Fluorescence assay of particulate membranes collected from media conditioned on days 5–6 post-irradiation. 22Rv1 cells were exposed to 0 Gy or 4 Gy, incubated 4 days and transfected with Tsg101 mmRNA, siRNA or untreated. After an additional 2 days, particulate fractions were isolated and stained. All values were then corrected for differences in viable cells left on the plate after 0 Gy or 4 Gy. Data are the means (± s.e.) derived from two independent experiments done in triplicate. *P < 0.01, Student’s t-test.
Vybrant DiI was used as a sensitive fluorescent probe for measuring changes in the bulk level of microvesicles released by cells. The fluorescent intensity of these DiI-stained fractions were corrected for differences in the total number of viable cells left on each plate after the 48 h conditioning period. The results of this assay provided evidence that the irradiation-induced senescence of 22Rv1 cells (13) was associated with an approximate three-fold increase in the bulk level of microvesicles released by their unirradiated controls (Fig. 1c). These data indicate that irradiation therapy has the potential to induce a senescent phenotype associated with increased production and release of exosome-like particles into the microenvironment of neighboring cells that could potentially influence tumor progression.
We next used RNA interference to determine the cellular origin of the studied exosome-like particles. Two modes of exosome biogenesis have been described, immediate or delayed (21). The immediate mode of exosome biogenesis generates secreted vesicles that bud outward (i.e., away from the cytoplasm) from certain domains of the plasma membrane. This mode of exosome formation has been reported to be independent of class E vacuolar protein sorting (VPS) function and MVEs (21). In contrast, the delayed mode of exosome biogenesis begins with outward vesicle budding at the limiting membrane of endosomes, generating MVEs. We targeted Tsg101 because this ubiquitin-binding protein is required for MVE vesicle formation (26) and a Tsg101 siRNA sequence had recently been shown to be effective in selectively decreasing the level of Tsg101 protein in PC3 prostate cancer cells (27). Four days after an exposure to mock, or 4 Gy irradiation, 22Rv1 cells were transfected with 100 nM Tsg101 siRNA and then incubated an additional three days in antibiotic- and serum-free medium. A three day exposure to Tsg101 siRNA resulted in an approximately 70% downregulation at the protein level (ImageQuant™ TL, Fig. 1b) and ~55% reduction in basal exosome release in subconfluent cultures of proliferating 22Rv1 cells (i.e., unirradiated controls, Fig. 1c). Neither the mismatch (mm) RNA nor untreated control samples showed a significant change in Tsg101 protein levels or exosome release. Identical treatments with Tsg101 siRNA resulted in a 65% reduction in the extent to which irradiation-induced senescence was associated with an upregulation of exosome release, when compared to the mmRNA and untreated controls (Fig. 1c, filled bars). Thus, electron microscopy and specific siRNA knockdown of Tsg101 provided significant evidence in favor of an endosomal/MVE origin for the vesicles under study.
p53-dependent and senescence-associated exosome release
A novel role for p53 in the control of intracellular protein trafficking has been proposed (8) and, more recently, it was demonstrated that irradiation-induced activation of p53 can lead to significantly increased levels of exosome biogenesis and secretion by cultured human lung cancer cells (24). Moreover, there is evidence that the intrinsic MVE-exosomal transport pathway may be upregulated in both ovarian cancer cells and erythroleukemic cells treated with the anticancer drug doxorubicin, which also activates a p53-dependent DNA damage response pathway (25, 26). Because prostate cancer cells also secrete exosome-like vesicles (18), we examined human prostate cancer cells for a potential functional relationship between irradiation-induced p53 activation, cellular senescence and exosome secretion.
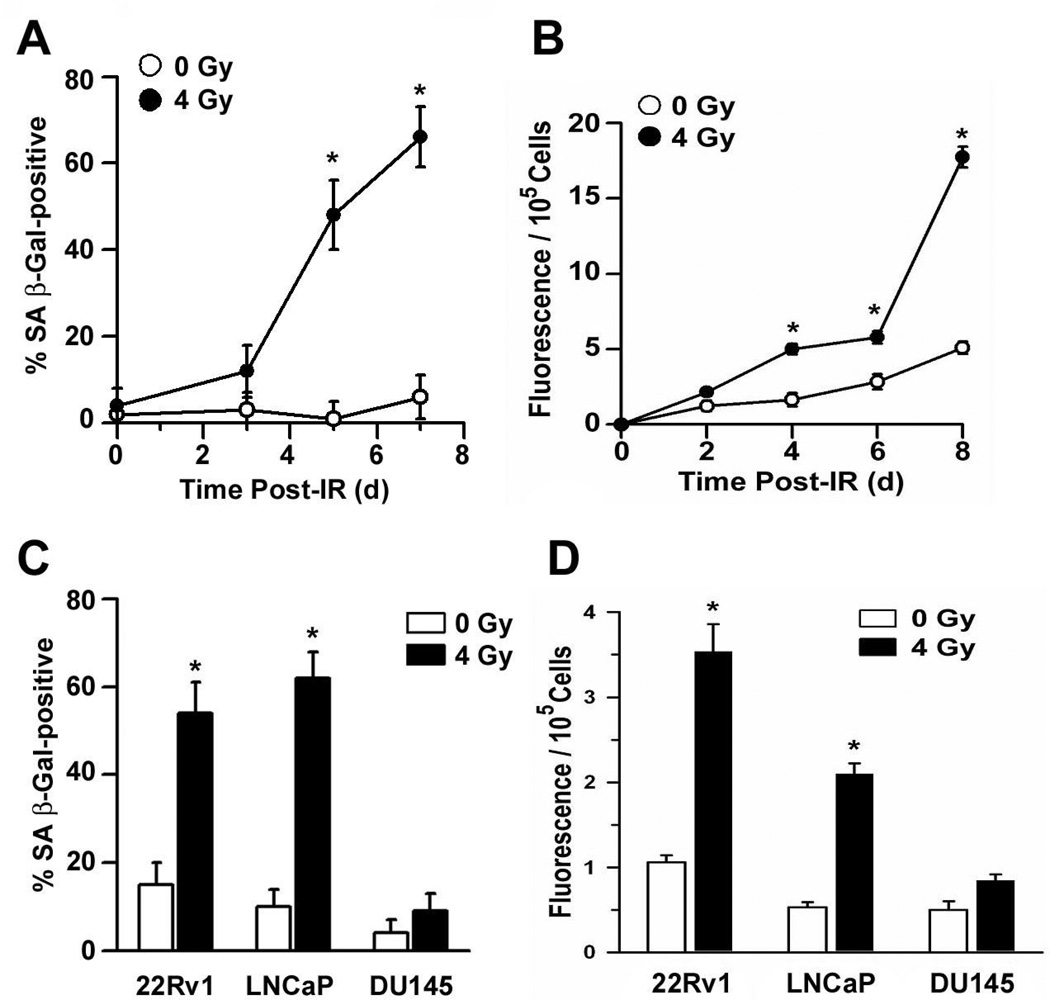
First, we analyzed conditioned medium from cultures of mock-treated (0 Gy) or irradiated (4 Gy) 22Rv1 cells for the presence of membrane vesicles at various times after treatment. Using SA β-Gal as an indicator of cellular senescence, the percentage of prematurely senescent 22Rv1 cells did not significantly increase within the three days after irradiation but had climbed to approximately 50% within five days post-irradiation (Fig. 2a). Concurrent with this sharp increase in the relative number of prematurely senescent cells was an increase in the release of exosome-like vesicles from irradiated 22Rv1 cells (Fig. 2b). Beginning on the day specified, cultures were washed with PBS and incubated an additional two days in fresh serum-free medium to eliminate the possibility of any contamination by FBS-derived exosomes. The bulk level of particulate membrane accumulated between 2 and 4 days after irradiation did not differ between mock controls and irradiated cultures. However, when the onset of conditioning was not initiated until the onset of premature cellular senescence (four days post-irradiation; 13) there was a significantly greater amount of membrane vesicles accumulated in the medium conditioned by irradiated versus unirradiated 22Rv1 cells (Fig. 2b). This discrepancy remained evident, and grew, during the subsequent four days post-irradiation (Fig. 2b).
Figure 2.
Senescence-associated release of exosomes from human prostate cancer cell lines. (a) Subconfluent cultures of 22Rv1 cells were exposed to either 0 Gy (mock control) or 4 Gy and, on the day specified, triplicate determinations of the percent SA β-galactosidase (SA β-Gal)-positive cells were taken from a total of 300 cells in three independent experiments. *P < 0.01, Student’s t-test. (b) 22Rv1 cells were exposed to either 0 Gy (mock control) or 4 Gy and serum-free medium was conditioned 48 h during the interval between 2–4, 4–6, 6–8 or 8–10 days after treatment. Exosome fractions were isolated from the conditioned medium and stained using Vybrant DiI. After normalizing for differences in cell survival, fluorescent intensity was plotted as a function of the time medium conditioning was initiated. Data represent the means (± s.e.) of three independent experiments done in triplicate. *P < 0.01, Student’s t-test. (c) LNCaP, 22Rv1 or DU145 cells were exposed to either 0 Gy (mock control) or 4 Gy and on day 5 after irradiation the percentage of SA β-Gal-positive cells were determined, as in (a). *P < 0.01, Student’s t-test. (d) LNCaP, 22Rv1 or DU145 cells were exposed to either 0 Gy (mock control) or 4 Gy and serum-free medium was conditioned from 4–6 days after treatment. Exosome fractions were isolated from the conditioned medium and stained using Vybrant DiI. After normalizing for differences in cell survival, fluorescent intensity was plotted as a function of treatment condition and cell line tested. Data represent the means (± s.e.) of triplicate determinations in two experiments. *P < 0.01, Student’s t-test.
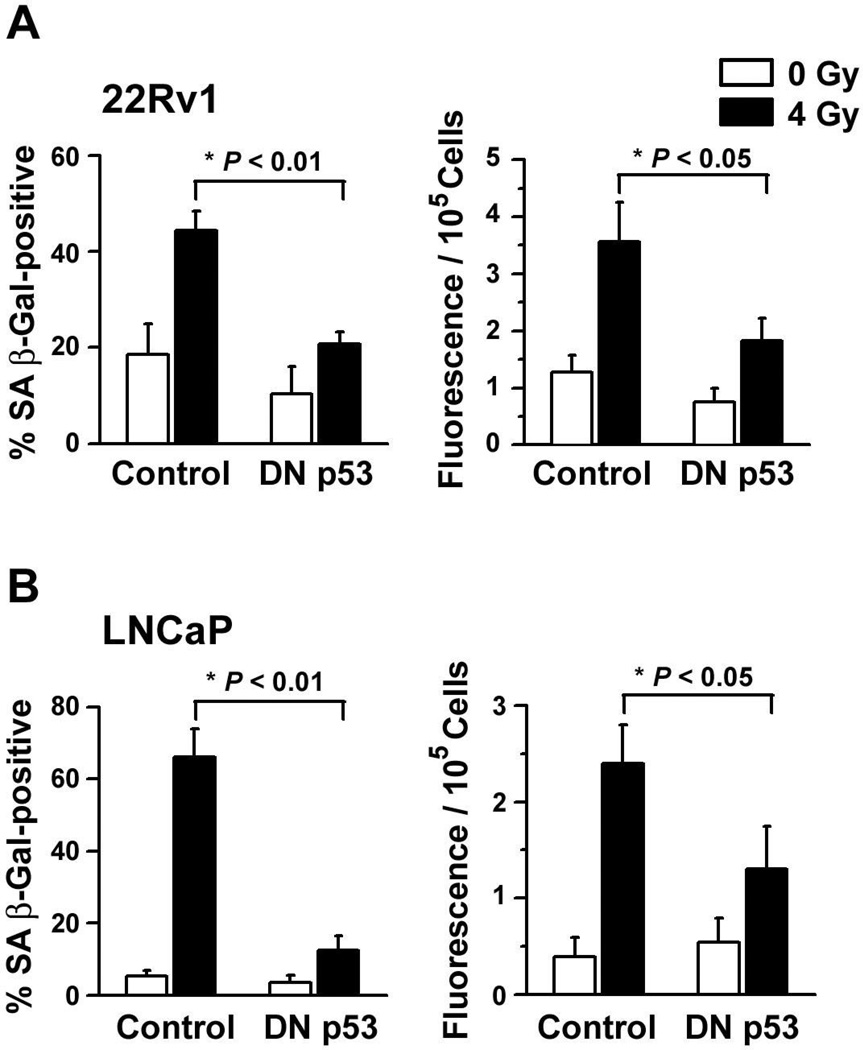
Second, to determine whether this secretory pattern was restricted to senescent populations of prostate cancer cells, we compared the bulk levels of particulate membranes accumulated between 4 – 6 days after cultured prostate cancer cells were exposed to either 0 Gy or 4 Gy. A significant percentage of LNCaP and 22Rv1 cells become senescent within four days after irradiation (13) and there was a significant increase in the release of membrane vesicles associated with the irradiation-induced initiation of this cellular senescence (Fig. 2c and d). In contrast, DU145 cells neither display several of the phenotypic characteristics of a senescent cell nor increase in vesicle release following a single exposure to IR (Fig. 2c and d and Ref. 13). Further evidence of a link between irradiation-induced p53 activation, premature senescence and increased exosome release is presented in Figure 3. Compared to their parental counterparts, fewer LNCaP and 22Rv1 cells transfected with a dominant negative p53 mutant (DN p53) stained positive for SA β-Gal four days after irradiation and the observed differences in the release of exosome-like vesicles were diminished; each of these differences were statistically significant (Fig. 3). These data are consistent with the hypothesis that an increased biogenesis and/or release of exosome-like vesicles may comprise an important and previously unrecognized feature of premature cellular senescence.
Figure 3.
p53-dependent release of exosomes from prematurely senescent human prostate cancer cell lines. (a) Parental 22Rv1 cells and derivatives expressing a dominant-negative p53 mutant (DN p53) were exposed to either 0 Gy or 4 Gy and on day 4 after irradiation (Left panel) the percentage of SA β-Gal-positive cells were determined as in Figure 2a or (Right panel) or cells were used to condition serum-free medium for 48 h. Exosome fractions were isolated from the conditioned medium and stained using Vybrant DiI. Fluorescence intensity was normalizing for differences in cell survival and data represent the means (± s.e.) of two independent experiments done in triplicate. (b) Results of experiments conducted using LNCaP cells under identical conditions to those described in (a), above.
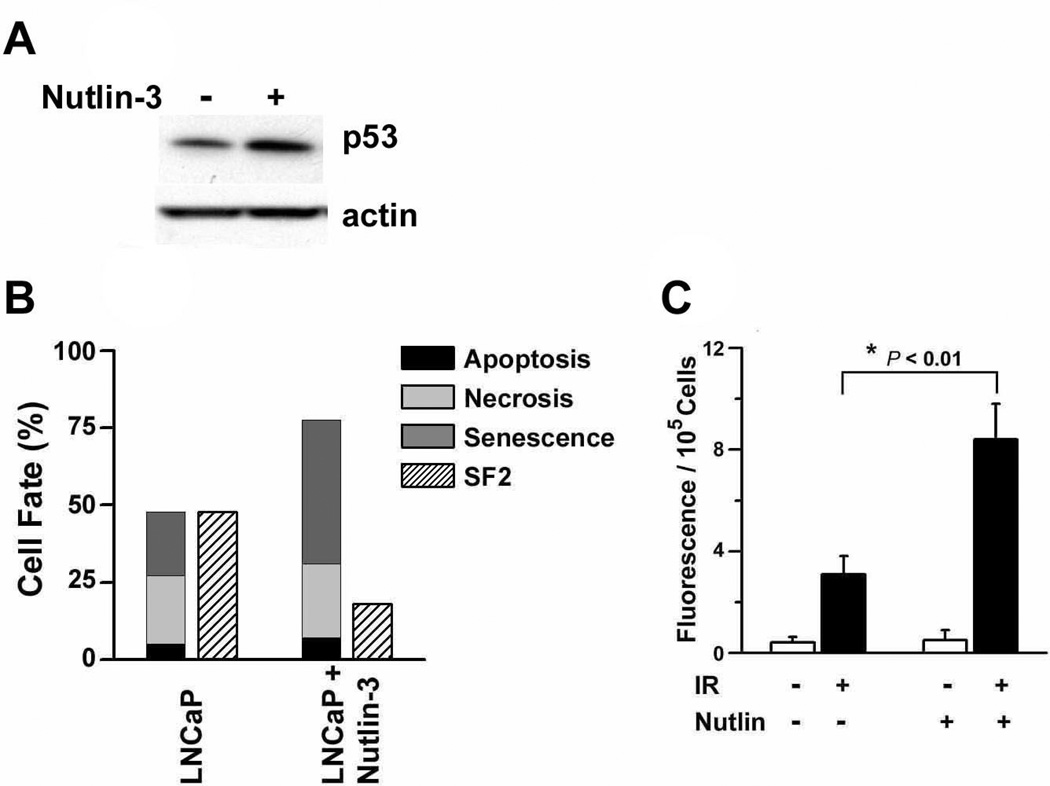
Third, we tested the hypothesis that an increase in p53 activity would both enhance irradiation-induced cellular senescence and exosome secretion. To do this we used nutlin-3, a small molecule antagonist of MDM2 that disrupts a negative feedback loop involving p53 ubiquitination (27). Nutlin-3 effectively primes the p53 pathway to respond to irradiation-induced DNA damage by increasing basal levels of p53 protein (Fig. 4a). Data shown in Figure 4b summarize our recent findings (13) that MDM2 inhibition significantly decreases the clonogenic survival of LNCaP cells following a single exposure to the clinically-relevant dose of 2 Gy (surviving fraction after 2 Gy = SF2; Fig. 4b). Moreover, this radiosensitizing effect of nutlin-3 has previously been shown to be blocked by the expression of a DN p53 mutant (13) and could be entirely attributed to an increased induction of p53-dependent cellular senescence, rather than an increased percentage of apoptotic and necrotic cells (Fig. 4b). Finally, the introduction of nutlin-3 30 min prior to irradiation (4 Gy) and throughout the following four days resulted in more than a two-fold increase in the release of exosome-like vesicles (Fig. 4c). This is the first investigation of the concept that p53 activity, premature senescence and exosome biogenesis may be functionally coupled by intracellular signals and events that remain uncertain and represent important gaps in our current understanding of the secretory pattern of senescent cells.
Figure 4.
Nutlin-3-induced radiosensitization, premature senescence and release of exosome-like particles. (a) Western blot of p53 in whole cell lysates prepared from subconfluent cultures of LNCaP cells 48 h after the addition of 2 µM nutlin-3. The same lysates were also probed using an anti-β-actin antibody as a loading control. (b) An overview of cell fate determinants (apoptosis, necrosis, senescence) in irradiated LNCaP cells at 48 h post-irradiation (2 Gy). Data shown summarize the results of a previous study (13) are the mean percentages of cells scored positive for apoptosis (i.e., using a combination of TdT-mediated dUTP nick end labeling, sub-G1 DNA content, and acridine orange/ethidium bromide (EtBr) staining), necrosis (i.e., intact cells that failed to exclude EtBr and displayed EtBr-positive nuclei) or senescence (i.e., SA β-Gal and CFDA flow cytometry) of triplicate determinations in two experiments. The fractions of cells surviving an exposure to 2 Gy (SF2) were determined using clonogenic assays. Methods used to perform each of these assays have been described (13). (c) LNCaP cells were incubated in the presence or absence of nutlin-3 (2 µM) for 30 min and exposed to either 0 Gy or 4 Gy. After an additional 4 days, particulate fractions were isolated and stained using Vybrant DiI. Data are the means (± s.e.) of two independent experiments done in triplicate. *P < 0.01, Student’s t-test.
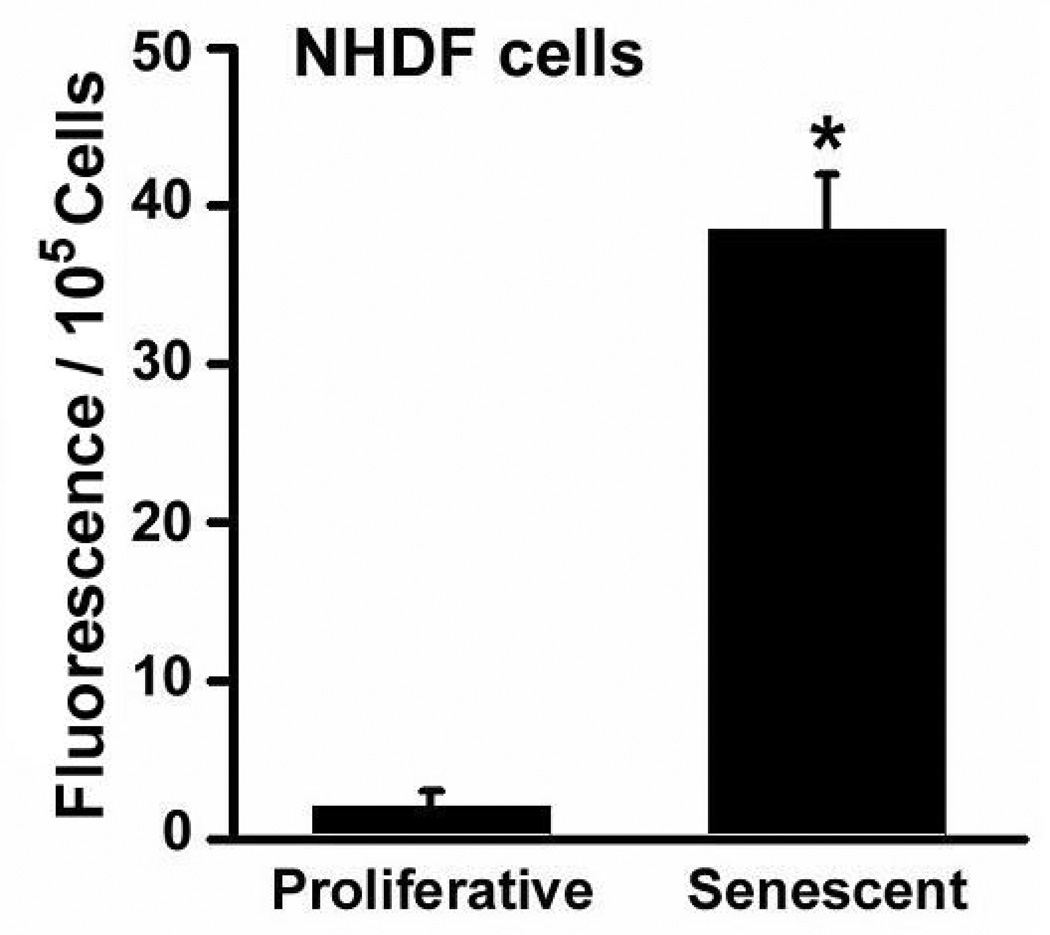
To determine whether this secretory pattern was restricted to prematurely senescent populations of prostate cancer cells, we examined cultures of normal human dermal fibroblasts (NHDF). NHDF cells have a finite lifespan in vitro and generally remain proliferative for less than twelve population doublings. When the bulk levels of particulate membranes released by proliferating and nonproliferating (i.e., senescent) cultures were compared, we observed a more than 15-fold increase in the release of exosome-like particles from NHDF cells that had naturally reached a state of replicative senescence (Fig. 5). These data provide strong support for the hypothesis that senescence, initiated either by telomere attrition (e.g., aging) or DNA damage (e.g., radiotherapy), may induce a p53-dependent increase in the biogenesis of exosome-like vesicles. This previously unrecognized feature of cellular senescence may hold important clues about the basic biology of both aging and the premature senescence of cancer cells.
Figure 5.
Proliferating (low passage) and nonproliferative (passage > 10) cultures of NHDF cells were incubated 48 h in the recommended medium, containing 5% exosome-depleted FBS (i.e., post-100,000×g supernatant). Exosome fractions were isolated from the conditioned medium and stained using Vybrant DiI. Fluoresence intensity is a function of lipid content, after normalizing for differences in cell survival. Data represent the means (± s.e.) of triplicate determinations. *P < 0.01, Student’s t-test.
Preliminary analysis of exosome cargo secreted by prematurely senescent prostate cancer cells
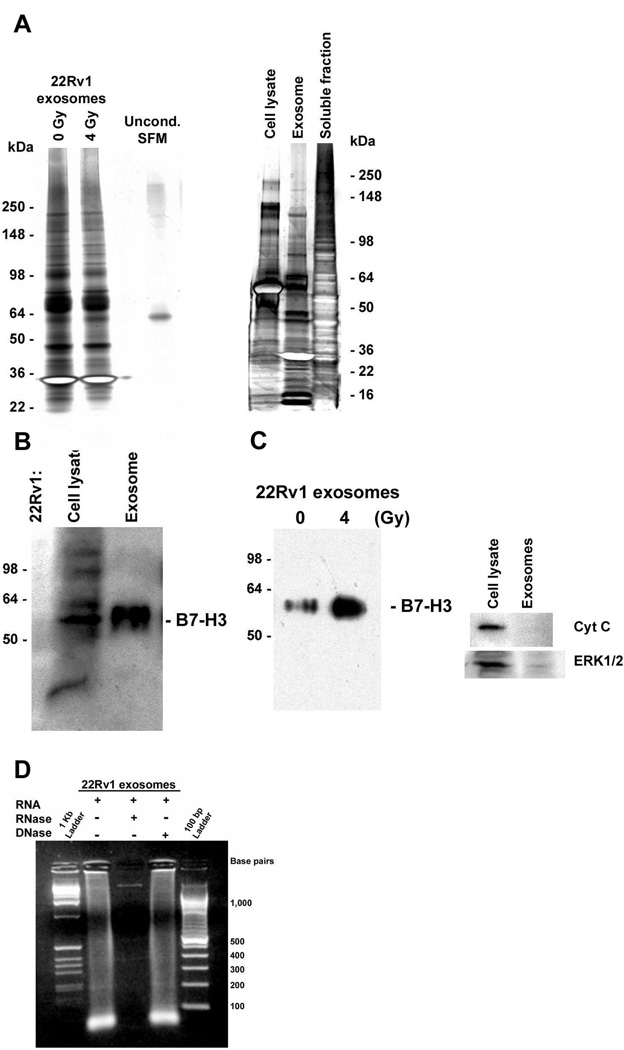
To determine whether irradiation-induced senescence was associated with qualitative, as well as quantitative, changes in MVE cargo sorting, we performed a SDS-PAGE analysis of the proteins associated with exosomal fractions collected from unirradiated and irradiated LNCaP and 22Rv1 cells. These assays showed that proteins detected in these gels were not derived from serum contamination of the exosome fractions (left panel, Fig. 6, lane 4) and confirmed that irradiation-induced senescence did not alter the overall profile of exosome proteins released (Fig. 6a). Moreover, comparisons of the proteins present in 22Rv1-derived whole cell lysates, exosome fractions, and soluble proteins indicated that exosomes were enriched in various subclasses of cellular proteins (right panel, Fig. 6; e.g., bands of ~ 13, 16, 30, 47, 58, 65 and 90 kDa). We have established that B7-H3 (CD276) represents one of the proteins enriched in the exosomes released by 22Rv1 cells (Fig. 6b). However, the presence of this protein has not been detected in exosomes released by LNCaP cells, possibly due to the relatively slow rate of exosome release from this cell line (Fig. 6a). In 22Rv1 cells, irradiation-induced senescence was associated with an increased release of B7-H3-positive exosomes that were free of contamination by cytosolic or mitochondrial proteins (e.g., ERK1/2 or cytochrome C; Fig. 6c); thus, providing independent confirmation of results obtained using DiI as a lipid probe to detect changes in exosome release. This is a novel and important finding, as B7-H3 is a member of the B7 family of proteins that are capable of modulating CD4 T-cell responses and antitumoral immunity (28–31). It was recently reported that increased B7-H3 expression provides an extremely reliable marker of prognosis for differentiating indolent from aggressive prostate cancers (32). The precise role of B7-H3 in prostate cancer and immunosurveillance has yet to be established. However, as a prognostic biomarker for aggressive cases of prostate cancer, B7-H3 has shown promise in immunohistochemical analyses of tissue sections (33). Because B7-H3 protein can be detected in the exosomes released by senescent 22Rv1 cells, it is possible that meaningful changes in the levels of circulating B7-H3-positive exosomes in the blood may provide a non-invasive way to survey the efficacy of radiotherapy for prostate cancer patients.
Figure 6.
Secretion patterns of human prostate cancer cells. (a) Silver staining analysis of the exosome fractions released, 4 – 6 days after exposure to either 0 Gy or 4 Gy, by 22Rv1 cells. Left panel: Electrophoresis was performed on 8 – 16% Tris-glycine gels; lane 4 is a negative control loaded with unconditioned serum-free medium (Uncond. SFM). Right panel: Comparison of the silver-stained protein profiles for 22Rv1 total cell lysates, exosome fractions, and exosome-free medium (soluble fraction) conditioned 4 – 6 days after irradiation (4 Gy). Electrophoresis performed on 4 – 20% gels. (b) Western blot analysis of B7-H3 protein in a whole cell lysate and exosome fraction isolated from medium conditioned 48 h by irradiated 22Rv1 cells. (c) Western blot analysis of B7-H3 protein in exosome fractions and an absence of contamination by either cytochrome C or ERK1/2. Exosomes were isolated 4 days after an exposure of 22Rv1 cells to either 0 Gy or 4 Gy; total cell lysates were prepared from remaining adherent cells. Final exosome pellets were resuspended in equal volumes of lysis buffer and 20% of the total volumes for each sample were loaded. The position of molecular weight markers (kDa) are indicated on the left. (d) Large amounts of small RNA (< 100 bp) were detected in 22Rv1 derived exosomes on a 2% agarose gel stained by EtBr; treatments with either RNase or DNase confirmed the presence of both DNA and RNA.
Finally, we have extended the recent discovery by Valadi et al. (34) that exosomes contain small RNA. In this important study the authors demonstrated that exosomes released from mouse and human mast cells contain an abundance of small mRNAs and microRNAs (34). In addition, it was established that RNA from mouse mast cells was transferable to recipient human mast cells and can be translated after entering the recipient cell. In our preliminary analysis, we have found that prematurely senescent (irradiation-induced) 22Rv1 cells also release exosomes containing substantial amounts of small RNAs (Fig. 6d; <100 bp). To confirm that the nucleotides detected by agarose gel electrophoresis were RNA, exosome extracts were treated with either RNase or DNase in solution. The results showed minimal degradation by DNase, while added RNase degraded exosome RNA and revealed the presence of a single DNA band of >1,000 bp (Fig. 6d). The studies described here present the challenges of addressing the basic questions of whether the RNA cargo contained in MVE-derived exosomes are generically capable of mediating cell-cell communication, as in the case of human mast cells (34), and to investigate the mechanisms of “exosomal shuttle RNA” (esRNA) uptake by neighboring cells.
Clinically-relevant doses of irradiation induce a premature state of cellular senescence that accounts for most of the clonogenic death observed in cultures of human prostate cancer cells retaining a functional p53 DNA damage-response pathway (13). We show here for the first time that this treatment-induced cell cycle arrest was associated with a senescence-associated increase in exosome release. In accelerated senescence, this novel secretory phenotype was dependent on the activation of p53. However, the release of exosome-like microvesicles also increased during proliferative senescence in normal human fibroblasts. This suggests that senescence, initiated either by telomere attrition (e.g., aging) or DNA damage (e.g., radiotherapy), may induce a p53-dependent increase in the biogenesis of exosome-like vesicles. Ultrastructural analysis and RNAi-mediated knockdown of Tsg101 provided significant evidence that the additional exosomes released by prematurely senescent prostate cancer cells were principally derived from MVEs. Moreover, these exosomes were enriched in B7-H3 protein, a recently identified diagnostic marker for prostate cancer, and an abundance of esRNA. Many details regarding the mechanisms that control exosome biogenesis and their potential role in intercellular communication remain obscure. Our findings are consistent with the proposal that exosomes can transfer cargos, with both immunoregulatory potential and genetic information, between cells through a novel mechanism that may be recruited to increase exosome release during treatment-induced and replicative cellular senescence.
Acknowledgements
We thank Warren Knudson for the NHDF cells and members of the Anatomy and Cell Biology Workshop Series for their many helpful suggestions.
Supported in part by grants from the NIH (R01CA098195 to J.A.M.) and the Brody Brother’s Foundation Endowment (#997729 to D.M.T. and J.A.M.).
References
- 1.Cooperberg MR, Broering JM, Litwin MS, et al. CaPSURE Investigators. The contemporary management of prostate cancer in the United States: lessons from the cancer of the prostate strategic urologic research endeavor (CapSURE), a national disease registry. J Urology. 2004;171:1393–1401. doi: 10.1097/01.ju.0000107247.81471.06. [DOI] [PubMed] [Google Scholar]
- 2.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 3.Bavik C, Coleman H, Dean JP, Knudsen B, Plymate S, Nelson PS. The gene expression program of prostate fibroblast senescence modulates neoplastic epithelial cell proliferation through paracrine mechanisms. Cancer Res. 2006;66:794–802. doi: 10.1158/0008-5472.CAN-05-1716. [DOI] [PubMed] [Google Scholar]
- 4.Chang B-D, Watanabe K, Broude EV, et al. Effects of p21Waf1/Cip1/Sdi1 on cellular gene expression: implications for carcinogenesis, senescence, and age-related diseases. Proc Nat Acad Sci USA. 2000;97:4291–4296. doi: 10.1073/pnas.97.8.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Nat Acad Sci USA. 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coppe J-P, Kauser K, Campisi J, Beausejour CM. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J Biol Chem. 2006;281:568–574. doi: 10.1074/jbc.M603307200. [DOI] [PubMed] [Google Scholar]
- 7.Currid CA, O’Connor DP, Chang BD, et al. Proteomic analysis of factors released from p21-overexpressing tumour cells. Proteomics. 2006;6:3739–3753. doi: 10.1002/pmic.200500787. [DOI] [PubMed] [Google Scholar]
- 8.Khwaja FW, Svoboda P, Reed M, Pohl J, Pyrzynska B, Van Meir EG. Proteomic identification of the wt-p53-regulated tumor cell secretome. Oncogene. 2006;25:7650–7661. doi: 10.1038/sj.onc.1209969. [DOI] [PubMed] [Google Scholar]
- 9.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tisty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z, Trotman LC, Shaffer D, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roninson IB. Oncogenic functions of tumour suppressor p21(Waf1/Cip1/Sdi1): association with cell senescence and tumour-promoting activities of stromal fibroblasts. Cancer Lett. 2002;179:1–14. doi: 10.1016/s0304-3835(01)00847-3. [DOI] [PubMed] [Google Scholar]
- 12.Campisi J. Suppressing cancer: the importance of being senescent. Science. 2005;309:886–887. doi: 10.1126/science.1116801. [DOI] [PubMed] [Google Scholar]
- 13.Lehmann BD, McCubrey JA, Jefferson HS, Paine MS, Chappell WH, Terrian DM. A dominant role for p53-dependent cellular senescence in radiosensitization of human prostate cancer cells. Cell Cycle. 2007;6:595–605. doi: 10.4161/cc.6.5.3901. [DOI] [PubMed] [Google Scholar]
- 14.Roninson IB. Tumor cell senescence in cancer treatment. Cancer Res. 2003;63:2705–2715. [PubMed] [Google Scholar]
- 15.Shay JW, Roninson IB. Hallmarks of senescence in carcinogenesis and cancer therapy. Oncogene. 2004;23:2919–2933. doi: 10.1038/sj.onc.1207518. [DOI] [PubMed] [Google Scholar]
- 16.Taylor DD, Gercel-Taylor C. Tumour-derived exoxomes and their role in cancer-associated T-cell signaling defects. Br J Cancer. 2005;92:305–311. doi: 10.1038/sj.bjc.6602316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: from biogenesis and secretion to biological function. Immunol Lett. 2006;07:102–108. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Abusamra AJ, Zhong Z, Zheng X, et al. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol Dis. 2005;35:169–173. doi: 10.1016/j.bcmd.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Fevrier B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opinion Cell Biol. 2004;16:415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Thery C, Regnault A, Garin J, et al. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:500–510. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sengupta P, Holowka D, Baird B. Fluorescence resonance energy transfer between lipid probes detects nanoscopic heterogeneity in the plasma membrane of live cells. Biophys J. 2007;92:3564–3574. doi: 10.1529/biophysj.106.094730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Razi M, Futter CE. Distinct roles for Tsg101 and Hrs in multivesicular body formation and inward vesiculation. Mol Biol Cell. 2006;17:3469–3483. doi: 10.1091/mbc.E05-11-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu G, Gilchrist R, Borley N, et al. Reduction of TSG101 protein has a negative impact on tumor cell growth. Int J Cancer. 2004;109:541–547. doi: 10.1002/ijc.20014. [DOI] [PubMed] [Google Scholar]
- 24.Yu X, Harris SL, Levine AJ. The regulation of exoxome secretion: a novel function of the p53 protein. Cancer Res. 2006;66:4795–4801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- 25.Shedden K, Xie XT, Chandaroy P, Chang YT, Rosania GR. Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer Res. 2003;63:4331–4337. [PubMed] [Google Scholar]
- 26.Chen VY, Posada MM, Blazer LL, Zhao T, Rosania GR. The role of the VPS4A-exosome pathway in the intrinsic egress route of a DNA-binding anticancer drug. Pharm Res. 2006;23:1687–1695. doi: 10.1007/s11095-006-9043-0. [DOI] [PubMed] [Google Scholar]
- 27.Vassilev LT, Vu BT, Graves B, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 28.Booth AM, Fang Y, Fallon JK, Yang JM, Hildreth JE, Gould SJ. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol. 2006;172:923–935. doi: 10.1083/jcb.200508014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang LQ, Fraser CC, Kikly K, et al. B7-H3 promotes acute and chronic allograft rejection. Eur J Immunol. 2005;35:428–438. doi: 10.1002/eji.200425518. [DOI] [PubMed] [Google Scholar]
- 30.Suh WK, Gajjewska BU, Okada H. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nature Immunol. 2003;4:899–906. doi: 10.1038/ni967. [DOI] [PubMed] [Google Scholar]
- 31.Prasad DV, Nguyen T, Li Z, Yang Y, Duong J, Wang Y, Dong C. Murine B7-H3 is a negative regulator of T cells. J Immunol. 2004;173:2500–2506. doi: 10.4049/jimmunol.173.4.2500. [DOI] [PubMed] [Google Scholar]
- 32.Roth TJ, Sheinin Y, Lohse CM, et al. B7-H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res. 2007;67:7893–7900. doi: 10.1158/0008-5472.CAN-07-1068. [DOI] [PubMed] [Google Scholar]
- 33.Steinberger P, Majdic O, Derdak SV, et al. Molecular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-Like domains. J Immunol. 2004;172:2352–2359. doi: 10.4049/jimmunol.172.4.2352. [DOI] [PubMed] [Google Scholar]
- 34.Valad H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]