Significance
Mechanical forces, which guide cellular functions, can be sensed and translated into biochemical information at focal adhesions, where cells physically connect to extracellular matrix (ECM) through transmembrane receptor integrins. Our results have identified that different ECM proteins, type 1 collagen (Col I) and fibronectin (FN), can either transmit or shield from mechanical forces when regulating crucial intracellular signaling, focal adhesion kinase (FAK), with their different accessibility to the corresponding integrin receptors. Whereas the integrin α2 binding site in Col I is constitutively accessible, mechanical tension is required to expose the integrin α5 binding motif in FN. This finding should advance our understanding on how cells perceive extracellular mechanical cues through natural surface materials.
Keywords: FRET biosensor, intracellular tension, substrate rigidity
Abstract
Matrix mechanics controls cell fate by modulating the bonds between integrins and extracellular matrix (ECM) proteins. However, it remains unclear how fibronectin (FN), type 1 collagen, and their receptor integrin subtypes distinctly control force transmission to regulate focal adhesion kinase (FAK) activity, a crucial molecular signal governing cell adhesion/migration. Here we showed, using a genetically encoded FAK biosensor based on fluorescence resonance energy transfer, that FN-mediated FAK activation is dependent on the mechanical tension, which may expose its otherwise hidden FN synergy site to integrin α5. In sharp contrast, the ligation between the constitutively exposed binding motif of type 1 collagen and its receptor integrin α2 was surprisingly tension-independent to induce sufficient FAK activation. Although integrin α subunit determines mechanosensitivity, the ligation between α subunit and the ECM proteins converges at the integrin β1 activation to induce FAK activation. We further discovered that the interaction of the N-terminal protein 4.1/ezrin/redixin/moesin basic patch with phosphatidylinositol 4,5-biphosphate is crucial during cell adhesion to maintain the FAK activation from the inhibitory effect of nearby protein 4.1/ezrin/redixin/moesin acidic sites. Therefore, different ECM proteins either can transmit or can shield from mechanical forces to regulate cellular functions, with the accessibility of ECM binding motifs by their specific integrin α subunits determining the biophysical mechanisms of FAK activation during mechanotransduction.
Cells can sense and respond to the mechanical microenvironment by converting forces into biochemical signals inside the cells; that is, mechanotransduction (1). Focal adhesions (FAs) are the major sites of interaction between a cell and its extracellular matrix (ECM) microenvironment, and thus, outside mechanical signals can be sensed at FAs through transmembrane receptor integrins. In particular, it has been shown that matrix elasticity can control the cell fate (2) by modulating the interactions between ECM proteins and their receptor integrins (3, 4). The modifications in ECM–integrin bonds can be further translated into biochemical signals through FA proteins (5). For example, mechanical stretching of Crk-associated substrate (p130CAS) promotes its phosphorylation by Src family kinases (6). Unfolding purified talin rod domain by mechanical stretching allows the recruitment of vinculin, potentially reinforcing the connection between integrin and actin (7). Myosin II–dependent endogenous tension has also been shown to directly unfold vinculin in living cells (8). However, it remains unclear how focal adhesion kinase (FAK), a major downstream molecule of integrin signaling, is regulated by mechanical signals.
Various ECM proteins including fibronectin (FN) and type 1 collagen (Col I) are specifically recognized by integrin subtypes with different combinations of α and β subunits, allowing diverse cellular responses to the same mechanical stimulations (9). For example, M2 melanoma cells, lacking an actin cross-linker filamin A, spread on the rigid surface coated with FN, but not Col I (10). Shear stress stimulates the activation of integrin α5β1 and αvβ3 in endothelial cells seeded on FN, which then activates PKCα and inhibits integrin α2β1. However, on Col I, shear stress can activate integrin α2β1 and PKA to suppress integrin α5β1 and αvβ3 (11). A recent study also showed that normal and nonmetastatic tumor cells respond to substrate stiffness on FN, but not Col I (12). However, the fundamental mechanisms of how these engagements of different ECM and integrin subtypes regulate mechanotransduction remain elusive. In this study, we present, using a fluorescence resonance energy transfer (FRET)-based biosensor, the biophysical and molecular mechanisms of FAK mechanoactivation via different ECMs and their corresponding integrin subtypes.
Results and Discussion
FAK Activation Depends on Mechanical Tension with Fibronectin, but Not Collagen.
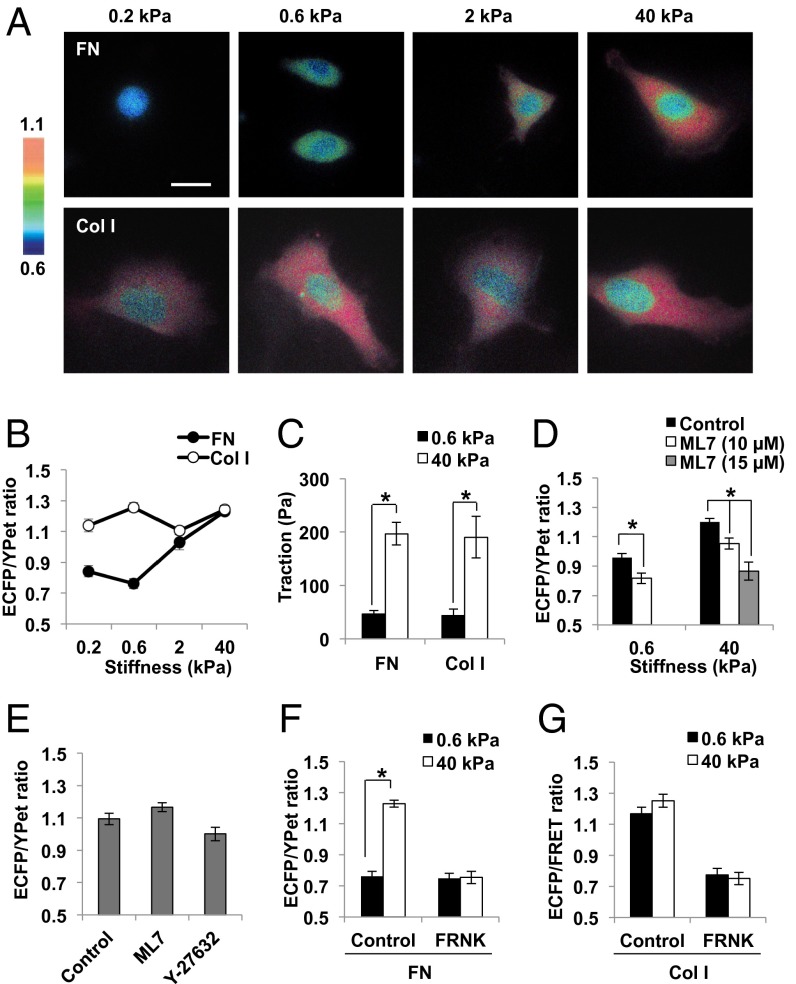
To investigate how different ECM molecules and integrin subtypes contribute to the mechanical tension-induced FAK activation, polyacrylamide gels of different stiffness were prepared (13) and coated with FN or Col I. We then applied a sensitive FAK FRET biosensor to visualize the FAK activity in live cells by detecting the autophosphorylation of Tyr397 and its subsequent binding to the Src SH2 domain with FRET change between enhanced CFP (ECFP) and a YFP variant (YPet) (SI Appendix, Fig. S1A) (14). In fact, this FAK biosensor was phosphorylated specifically by activated FAK on cell adhesion (SI Appendix, Fig. S1B). Extensive characterization of its sensitivity, selectivity, and reliability can be found in our previous publication (14). When human fibrosarcoma (HT1080) cells expressing the FAK biosensor were adhered on the FN-coated polyacrylamide (PA) gels through integrin α5β1 (SI Appendix, Fig. S2), the ECFP/YPet emission ratio of the FAK biosensor, representing the FAK activity, increased proportional to the substrate rigidity (0.2–40 kPa) (Fig. 1 A and B). This is consistent with a previous observation that FAK phosphorylation at Tyr397 in HT1080 cells increased on the rigid surface, whereas tethering-independent Tyr861 phosphorylation representing the FAK expression level remained constant on gels with different stiffness (15).
Fig. 1.
FAK activation is dependent on substrate rigidity coupled with FN, but not Col I. (A and B) The representative images (A) and the average ECFP/YPet ratio values (B) of FAK biosensor in HT1080 cells cultured on the FN- or Col I-coated PA gels with different stiffness, as indicated (n = 18–31). The color bar on the left in A shows the ECFP/YPet ratio values, with cold and hot colors representing low and high ratio values, as indicated. FN and Col I group are displayed by black and white circles, respectively, in B. (C) The traction in HT1080 cells on the FN- or Col I-coated PA gels of 0.6 kPa (black) and 40 kPa (white) (n = 3). (D) The average ECFP/YPet ratios of FAK biosensor in HT1080 cells after 2 h of adhesion on the FN-coated PA gels of 0.6 kPa and 40 kPa, with (white, 10 µM; gray, 15 µM) or without (black) the pretreatment of ML7 (n = 24–34). (E) The average ECFP/YPet ratio values of FAK biosensor in HT1080 cells adhered on the Col I-coated 0.6 kPa gel for 2 h with or without 10 µM of ML7 or Y-27632 (n = 30–33). (F and G) The average ECFP/YPet ratio values of FAK biosensor in HT1080 cells with or without FAK mutant FRNK, on PA gels of 0.6 kPa (black) and 40 kPa (white) coupled with (F) FN (n = 18–21) or (G) Col I (n = 11–21). Graphs show mean ± SEM. *Significant difference (P < 0.05). (Scale bar, 10 µm.)
Cells can develop stronger intracellular tension on stiffer substrates, and accordingly exert higher traction force on their environment. Thus, we measured the root-mean-square traction on gels of varying stiffness (16) to assess the intracellular tension and examine its role in governing FAK activation. The results showed that the traction in HT1080 cells on the rigid 40-kPa gel (200 Pa) is significantly higher than on the soft 0.6-kPa gel (40 Pa) (Fig. 1C), confirming that cells in the rigid environment can develop higher intracellular tension to exert stronger traction force. We further investigated the effect of intracellular tension on FAK activity. The actin polymerization inhibitor, cytochalasin D, or an inhibitor for myosin light chain kinase, ML7, was applied to suppress the intracellular tension. The FAK activation during cell adhesion on FN-coated gels was inhibited by preincubation with ML7 (Fig. 1D). The direct addition of either cytochalasin D or ML7 also caused the decrease of the ECFP/YPet ratios of FAK biosensor and the FA disassembly, as visualized by mCherry-tagged paxillin (SI Appendix, Fig. S3; Movies S1 and S2). This manipulation of actomyosin-derived tension was further shown to cause the reduction of traction force faster than the decrease of FAK activity (SI Appendix, Fig. S4), suggesting that the loss of actomyosin-based tension led to a decreased FAK activity. A similar starting point followed by an overall time delay of several minutes between the decreases in tension and FAK activity suggests the existence of possibly two FAK populations: one physically associated with FAs, and hence the pretensioned actin cytoskeleton, whose signaling/enzymatic activity can be almost immediately affected by tension (representing a similar starting point with a time delay <<1 s) (17, 18), and the other physically separated from the cytoskeleton, whose activity relies on the recruitment into the FAs and hence changes slower than tension.
We further visualized the FAK activity at focal adhesions by targeting the FAK biosensor to focal adhesion sites, with focal adhesion targeting (FAT) domain in its C terminus (SI Appendix, Fig. S5A). In response to ML7, this FAT-FAK biosensor also showed a decrease in the ECFP/YPet ratio as well as FA disassembly (SI Appendix, Fig. S5B), confirming the tension-dependent regulation of FAK activity. Although the FAT-FAK biosensor can report the local FAK activity at focal adhesions, the main experimental conditions in the current study (e.g., on the gels) render very weak or no FA structures in HT1080 cells (SI Appendix, Fig. S6). Because it would be technically difficult to visualize and precisely compare the FAK activity at focal adhesions under these cell conditions, we used the cytosolic FAK biosensor, which in fact provides reliable and specific signals, as shown in our previous publication (14).
We next investigated the effect of another major ECM molecule, Col I, on how FAK activity is modulated by substrate rigidity coated with Col I. Surprisingly, FAK activity in HT1080 cells on Col I was decoupled from surface stiffness (Fig. 1 A and B). The phosphorylation level of endogenous FAK Y397 was also similar on soft and hard gels coated with Col I (SI Appendix, Fig. S7A), supporting the observation of FRET signals. In fact, a high FAK activity was observed on soft 0.6-kPa gel, whereas the traction force on this soft gel was significantly lower than that on the stiffer 40-kPa gel (Fig. 1C). Consistently, this FAK activation on cell adhesion on the Col I-coated soft gel was not inhibited by the preincubation of ML7 or a Rho kinase inhibitor Y-27632 (Fig. 1E). Therefore, in stark contrast to the FN-coated surface, Col I-induced FAK activation in HT1080 cells was independent of mechanical tension. We further used the Lyn-FAK biosensor (14), which is capable of detecting the specific FAK activity at the plasma membrane raft-domains and focal adhesion sites (SI Appendix, Fig. S7B). Consistently, this membrane-tethered biosensor reported that FAK activity is dependent on substrate rigidity on FN, but not on Col I (SI Appendix, Fig. S7C). Similar results were also observed in other types of cells [SI Appendix, Fig. S8 A and B; human breast adenocarcinoma cells (MDA-MB-231); S8 D and E, human glioblastoma (U87MG) cells]. Both FN- and Col I-induced FAK activation was abolished by a FAK negative mutant, FRNK (Fig. 1 F and G), which contains the FAT domain for the occupation of FA sites to block the recruitment of endogenous FAK at FAs (19), suggesting that the proper localization of FAK at FA is crucial for its activation. It has been previously shown that the matrix compliance can guide the differentiation of stem cells cultured on Col I-coated polyacrylamide gels (2–4). It is possible that FAK activation may represent an early event triggered by Col I–integrin interactions, which may then require mechanical cues for the completion of signaling transduction governing the stem cell differentiation.
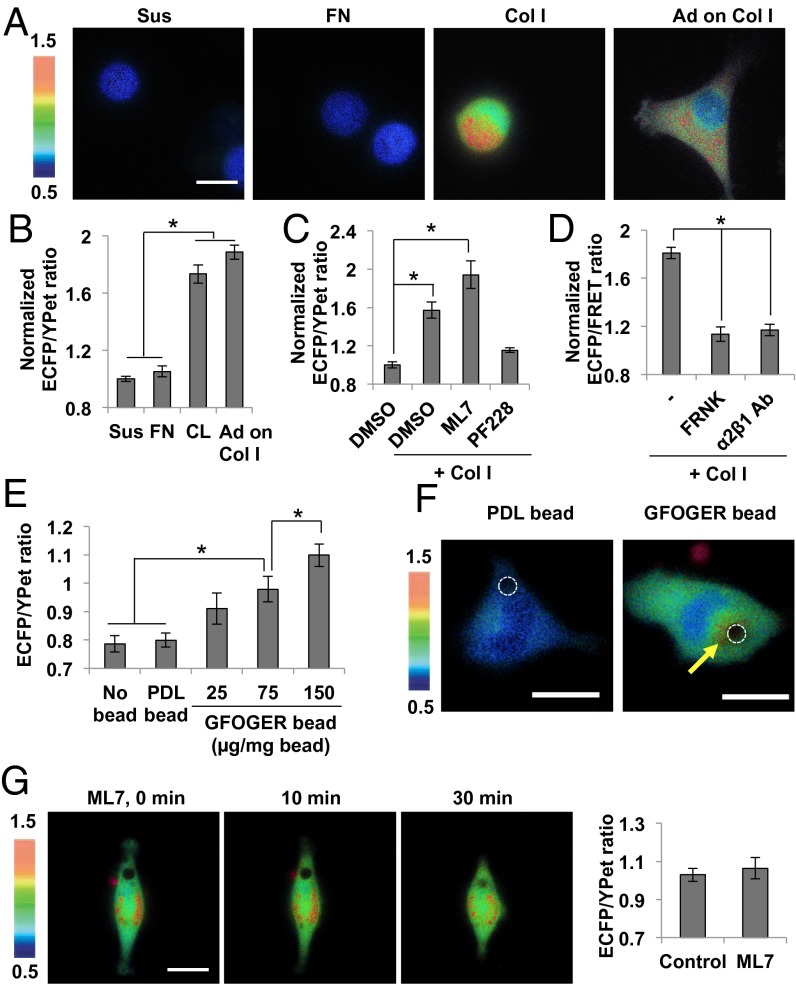
We further examined whether Col I can induce the FAK activation in the suspended cells, where no significant intracellular tension can be developed (20). When soluble Col I was directly added to HT1080 cells in suspension, a strong FRET response was observed (Fig. 2 A and B). The Col I-induced FAK activation in suspension was also observed in other cell types (SI Appendix, Fig. S8C; MDA-MB-231 cells; S8F, U87-MG cells). This increased ECFP/YPet ratio of FAK biosensor by Col I in suspension was specifically caused by FAK activation but was independent of intracellular tension, as a FAK inhibitor PF228 completely blocked this ratio change, whereas ML7 had no effect on it (Fig. 2C). In contrast to Col I, no FAK activation in the suspended HT1080 cells was observed when soluble FN was applied (Fig. 2 A and B), consistent with the notion that the FN-induced FAK activation is dependent on the intracellular tension. The Col I-induced FAK activation in suspension was also blocked by inhibitory antibody against integrin α2β1 or by expressing FRNK (Fig. 2D), confirming the primary roles of the specific interaction between Col I and its receptor integrin α2β1 and the correct localization of FAK via the FAT domain in the Col I-induced FAK activation in suspension.
Fig. 2.
Soluble collagen can induce FAK activation via integrin α2β1 in low-tensional states. (A and B) The representative images (A) and the average ECFP/YPet ratio values (B) of FAK biosensor in HT1080 cells suspended for 1 h and subsequently treated with or without 40 µg/mL of Col I or FN for another 1 h (n = 45–50). The “Ad on Col I” group represents the FAK activity in the cell adhered on the Col I-coated glass surface. The ECFP/YPet ratio of each group was normalized by the value of suspension group without any treatment. (C) The average ECFP/YPet ratios of FAK biosensor in the suspended HT1080 cells treated with 1% DMSO as a control, or with 40 µg/mL Col I together with 1% DMSO, 10 µM ML7, or 1 µM PF228 (n = 14–19). The ECFP/YPet ratio of each group was normalized by the value of the suspension DMSO group. (D) The average ECFP/YPet ratios of FAK biosensor by the incubation of 40 µg/mL of Col I in the suspended HT1080 cells expressing FRNK or treated by 10 µg/mL inhibitory antibody of integrin α2β1 (n = 20–37). The ECFP/YPet ratio of each group was normalized by the value of the control suspension group. (E) The average ECFP/YPet ratio values of FAK biosensor near the attachment area of beads coated with 75 µg/mg bead of PDL or 25, 75, or 150 µg/mg bead of GFOGER peptide, in cells adhered on PDL-coated surface (n = 12–30). (F) The representative ECFP/YPet ratio images of FAK biosensor in cells with beads coated with PDL or GFOGER peptide (75 µg/mg bead). (G) The representative images and the average ECFP/YPet ratio values of FAK biosensor near the attachment area of GFOGER bead before (Control) and after the treatment with 10 µM of ML7 (n = 14–26). Graphs show mean ± SEM. *Significant difference (P < 0.05). (Scale bar, 10 µm.)
Collagen can interact with integrin α2 via its GFOGER (Gly-Phe-hydroxyproline-Gly-Glu-Arg) motif; thus, we examined whether FAK can be sufficiently activated by GFOGER peptide, a triple-helical collagen mimetic peptide (21). Although the GFOGER peptide can indeed compete with Col I (SI Appendix, Fig. S9A), it could not cause sufficient FAK activation under different dosages (SI Appendix, Fig. S9 B and C). As the integrin recognition of Col I through the GFOGER motif is largely dependent on the Col I triple-helical structure (21), this peptide may have less efficiency than Col I in activating integrin α2β1. GFOGER peptides are also more separated in solution than Col I. Indeed, when we clustered GFOGER peptides by immobilizing them on the bead surface, the ECFP/YPet ratio of FAK biosensor in cells seeded on poly-d-lysine (PDL)-coated surface increased near the attachment area of GFOGER-coated beads in a concentration-dependent manner (Fig. 2 E and F). The same bead coated with PDL was not able to induce the FAK activation (Fig. 2 E and F), and ML7 did not inhibit the FAK activation by GFOGER beads (Fig. 2G), suggesting that this FAK activation is not caused by the mechanical cues but, rather, by the clustered GFOGER ligands. In fact, the more clustered GFOGER ligands (of higher concentration) induced, the stronger FAK activation (Fig. 2E), supporting this ligand-clustering effect on FAK activation. Although a higher FAK activation was observed near the GFOGER bead (Fig. 2F, Right), the ECFP/YPet ratio was globally elevated in the whole-cell areas, likely because of the diffusion effect of the cytosolic biosensors, as we previously observed (22).
Distinct Biophysical Mechanism of FAK Mechanoactivation Determined by Accessibility of ECM Binding Motifs to Integrin α Subunits.
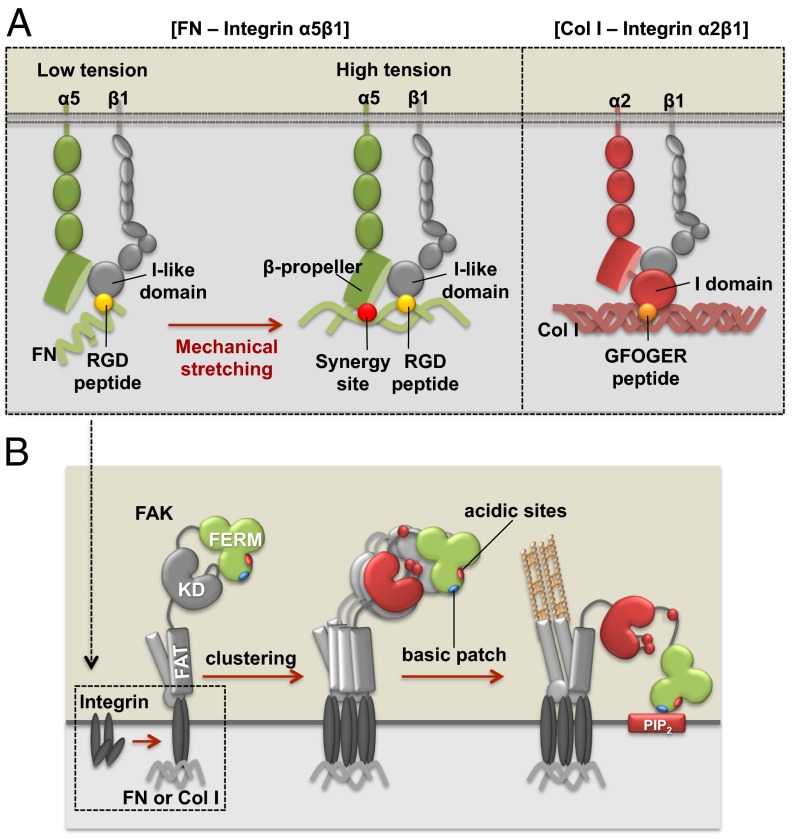
Integrin α2β1 directly binds to GFOGER peptide in Col I through the unique I-domain of the α2 subunit (23). This ligation can cause the conformational changes and activation of the β subunit to result in the full activation of integrin α2β1 (9), which then triggers the FAK activation. Our results suggest the GFOGER peptide in Col I is readily exposed to mediate this initial ligation, resulting in the tension-independent FAK activation. In contrast, integrin α5β1 can bind to FN either as relaxed or tensioned state (15). Although the relaxed integrin α5β1 binds to the RGD (Arg-Gly-Asp) motif in FN via a β1 subunit, in the tensioned state supported by actomyosin-derived contractility, the additional binding of the FN synergy site to α5 subunit can result in FAK activation (15, 24). Thus, the role of mechanical tension in FN-mediated FAK activation would be to stretch the FN molecule (25), exposing its otherwise hidden synergy site; in contrast, the tension-independent FAK activation by Col I is caused by the direct engagement of integrin α2 with the readily exposed GFOGER motif.
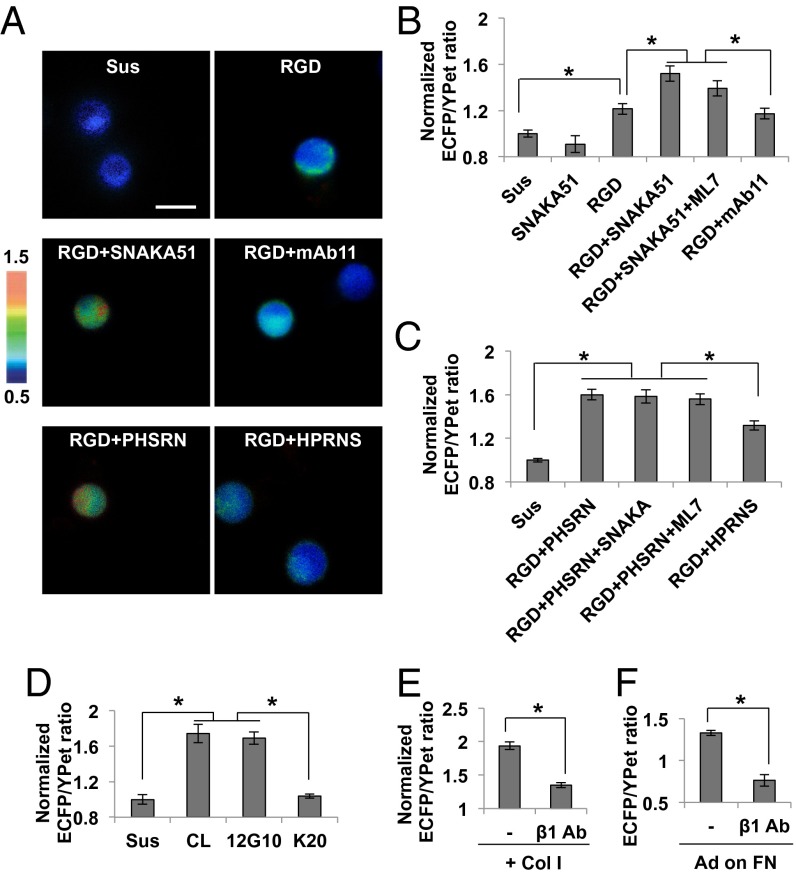
To examine whether FAK can be activated independent of tension by integrin α5β1, we applied the integrin β1-binding RGD peptide together with an integrin α5-activating antibody SNAKA51 (26) to the suspended cells. A strong FAK activation was observed with RGD and SNAKA51, but not its control nonfunctional antibody mAb11 (Fig. 3 A and B). The incubation with SNAKA51 alone did not cause any FAK activation, whereas RGD peptide, which binds to integrin subunit β1 at the interface between subunit α5 and β1 (15, 27), can only partially activate FAK (Fig. 3 A and B). FN, FNIII7-10 domain (seventh to tenth type 3 FN repeats), and RGD peptide at various concentrations could not cause a full FAK activation without an α5 activating antibody (SI Appendix, Fig. S10). These results suggest that FAK can be fully activated only when both α5 and β1 subunits are engaged. The incubation of ML7 did not inhibit the FAK activation induced by RGD and SNAKA51 (Fig. 3B), confirming the tension-independent FAK activation mechanism in this system.
Fig. 3.
Activation of FAK in suspended and adhesion cells. (A) The representative ECFP/YPet ratio images of FAK biosensor in the suspended HT1080 cells treated with 40 µg/mL of RGD only or together with SNAKA51 (α5 activating antibody, 10 µg/mL), mAb11 (control antibody, 10 µg/mL), PHSRN (synergy peptide, 240 µg/mL), or HPRNS (control peptide, 240 µg/mL). (B) The average ECFP/YPet ratio values of FAK biosensor in the suspended HT1080 cells with the incubation of SNAKA51 (10 µg/mL), RGD peptide (40 µg/mL), RGD and SNAKA51 with or without 10 µM ML7, or RGD and mAb11 (10 µg/mL) (n = 23–38). The ECFP/YPet ratio of each group was normalized by the value of the control suspension group. (C) The average ECFP/YPet ratio values of FAK biosensor in the suspended HT1080 cells with the incubation of RGD peptide (40 µg/mL) and PHSRN (240 µg/mL), without or together with SNAKA51 (10 µg/mL) or 10 µM ML7, or the incubation of RGD and HPRNS (240 µg/mL) (n = 17–35). The ECFP/YPet ratio of each group was normalized by the value of the control suspension group. (D) The average ECFP/YPet ratios of FAK biosensor in the suspended HT1080 cells treated with Col I (40 µg/mL), 12G10 (β1 activating antibody, 10 µg/mL), or K20 (control antibody for 12G10, 10 µg/mL) (n = 14–27). The ECFP/YPet ratio of each group was normalized by the value of the control suspension group. (E) The average ECFP/YPet ratio values of FAK biosensor in the suspended HT1080 cells after the treatment of Col I, with or without the pretreatment of inhibitory antibody for integrin β1 (MAB1965) (n = 25–44). The ECFP/YPet ratio of each group was normalized by the value of control suspension group. (F) The average ECFP/YPet ratio values of FAK biosensor in cells seeded on the 40 kPa gel coated with FN (n = 19). Graphs show mean ± SEM. *Significant difference (P < 0.05). (Scale bar, 10 µm.)
SNAKA51 binds to the calf 1/calf 2 domains of integrin α5, causing a conformational change of the integrin legs to induce or stabilize the active conformation of integrin α5 (26). To further examine whether this SNAKA51 activation effect can be achieved by a simple engagement of integrin α5 with its ligand peptide PHSRN (Pro-His-Ser-Arg-Asn) derived from the FN synergy site, we applied the PHSRN peptide together with RGD motif to the suspended HT1080 cells. A strong FAK activation was observed by simply adding RGD with PHSRN peptide, but not with the control scrambled peptide HPRNS (Fig. 3 A and C). The addition of SNAKA51 did not further increase the ECFP/YPet ratio of the FAK biosensor (Fig. 3C), suggesting that the simple ligation of integrin α5β1 with RGD and synergy peptides is sufficient to activate FAK in suspension. This FAK activation was not inhibited by the pretreatment with ML7, suggesting a tension-independent FAK activation (Fig. 3C). Thus, FAK can be activated by integrin α5β1 independent of tension if both integrin α5 and β1 are engaged, supporting our hypothesis that the role of mechanical tension in FN-induced FAK activation is to unfold the FN molecule and expose its synergy site to be accessed by integrin α5 subunit. In contrast, the Col I-induced FAK activation is tension-independent because the ligand GFOGER motif in Col I is readily accessible to integrin α2. Therefore, our data showed that integrin α subunits play crucial roles in determining the mechanosensitivity of FAK activation, as the adhesion of HT1080 cells to FN or Col I is dependent on integrin α5β1 (15, 28) or α2β1 (29-31), respectively (SI Appendix, Fig. S2 and Fig. 2D). We further revealed that the expression levels of α2 and α5 were comparable (SI Appendix, Fig. S11), and thus the distinct cellular responses to the substrate rigidity on FN- and Col I-coated surfaces should be attributed to the difference in the ligation mechanism of different integrin subtypes, but not to the difference of their expression levels.
Molecular Mechanism of FAK Activation on Integrin Activation.
Although the integrin α subunit determines the mechanosensitivity of FAK activation by different accessibility to its ECM binding motif, the ligation between the α subunit with the ECM proteins eventually can converge at the activation of the integrin β1 subunit, which is directly associated with many signaling proteins including FAK (32). Indeed, direct activation of integrin β1 by an activating antibody 12G10, but not its control antibody K20, was sufficient to cause a full FAK activation (Fig. 3D). A monovalent 12G10 Fab also caused FAK activation (SI Appendix, Fig. S12A), confirming that the activation of integrin β1 is sufficient for the FAK activation, independent of tension. This note is further supported by the observation that cells on soft gels coated with 12G10 antibody displayed a full FAK activation (SI Appendix, Fig. S12B). When an inhibitory antibody for integrin β1 was applied, the FAK activation was inhibited in the suspended cells induced by Col I or in cells on the FN-coated rigid gel (Fig. 3 E and F). Therefore, the ligation of α subunit and the ECM proteins converges at the integrin β1 activation and shares the mechanism of FAK activation. The clustering of integrin β1 by activating antibody AG89 was previously shown to induce the FAK phosphorylation on Tyr861, but not Tyr397, in the suspended HT1080 cells (28). 12G10 recognizes the βA domain of integrin β1 at the ligand-binding region (33), whereas AG89 recognizes the membrane proximal stalk-like regions of integrin β1 (34). The distinct effect of 12G10 and AG89 on FAK activation may thus reflect their different binding epitopes in integrin β1 and the subsequent differential integrin activation status (33, 34).
Although the mechanosensitivity of FAK activation is determined by different ECM accessibility to the integrin α subunit, we further investigated the intracellular molecular mechanism of FAK activation after integrin β1 activation to construct an integrated mechanistic model. It has been well established that integrins can be clustered on ECM engagement (35) as a result of the homophilic association between transmembrane domains (36). FAK can be recruited to the clustered integrins through its FAT domain (37), which then induces the transphosphorylation of Y397 between neighboring FAK molecules and the subsequent FAK activation (38). Consistently, a FAK mutant FRNK, which blocks the FAK recruitment/clustering at FAs (19), inhibited the FAK activation (Figs. 1 F and G and 2D). The inhibition of the Y397 phosphorylation by the FAK Y397F mutant, the constitutively active FA-targeted Shp2, an SH2-containing protein tyrosine phosphatase, which is capable of dephosphorylating FAK at Y397 (39), also suppressed the FAK activation (SI Appendix, Fig. S13 A and B). These results support the notion that the transphosphorylation at Y397 by integrin-induced FAK recruitment/clustering via its FAT domain is crucial for the FAK activation.
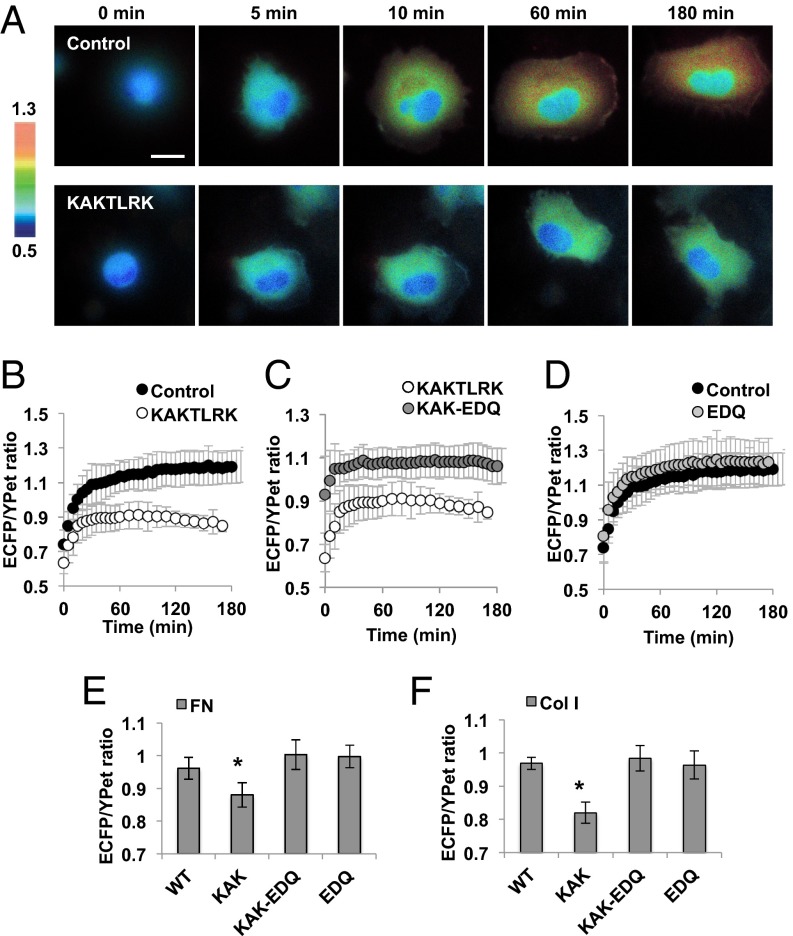
In addition to the FAT domain function, the basic patch in the N-terminal protein 4.1/ezrin/redixin/moesin (FERM) F2 domain and its interaction with acidic molecules [e.g., phosphatidylinositol 4,5-biphosphate (PIP2)] have been suggested to regulate the FAK activation (40, 41). In fact, mutations on the FERM basic patch, K216A/K218A/R221A/K222A (KAKTLRK), or PIP2 inhibitor neomycin (42), inhibited the FAK activation on cell adhesion (Figs. 4 A and B and SI Appendix, Fig. S13C). Interestingly, in suspended cells, FAK kinase dead (FAK KD) or FAK Y397F mutant, but not this KAKTLRK mutant, blocked the FAK activation (SI Appendix, Fig. S13B). We hypothesized that the FAK activation may be sufficiently initiated by transphosphorylation via its FAT domain-mediated localization/clustering (38), however, an intact FERM domain may be additionally required to protect and maintain the FAK activation from inhibitory molecules emerging during cell adhesion. Indeed, the KAKTLRK mutant allowed a normal initiation of FAK activation but had an early termination of this activation phase during cell adhesion on FN-coated surface when compared with the control wild-type group (Fig. 4 A and B).
Fig. 4.
FERM basic patch is required to protect the FAK activation during cell adhesion from the inhibitory effect of nearby acidic sites. (A and B) The representative images (A) and the average ECFP/YPet ratio values (B) of FAK biosensor in HT1080 cells expressing the control (black) or KAKTLRK mutant (white) during cell adhesion on the FN-coated glass surface. (C) The average ECFP/YPet ratio values of FAK biosensor in cells expressing KAKTLRK mutant (white) or KAK-EDQ mutant (gray). (D) The average ECFP/YPet ratio values of FAK biosensor in cells with (gray) or without WT-EDQ mutant (black) (n = 10–15). Graphs show mean ± SD. (E and F) The average ECFP/YPet ratios of FAK biosensor in cells expressing different FAK mutants after adhesion on (E) FN (n = 29–44) or (F) Col I (n = 27–65). Graphs show mean ± SEM. *Significant difference (P < 0.05). (Scale bar, 10 µm.)
It has been recently reported that the interaction of FERM with myosin negatively regulates FAK activity by promoting the autoinhibited FAK conformation (43). This recent study provided strong evidence of direct interaction between myosin heavy chain and the FERM domain of FAK by extensive biochemical assays including GST pull-down assays as well as coimmunoprecipitation experiment (43). The key residues of FERM F2 domain for myosin binding, E158/D161/Q162 (EDQ) (43), are positioned proximal to the FERM F2 basic patch KAKTLRK (SI Appendix, Fig. S14A). In addition, the EDQ sites contain several acidic amino acids that can bind to the basic residues of coiled-coil myosin (43), whereas KAKTLRK basic patch region binds to acidic molecules (e.g., PIP2) (40). We hypothesized that PIP2 and the inhibitory myosin may compete for the binding of FERM domain through the closely positioned KAKTLRK and EDQ residues, respectively. KAKTLRK mutation may reduce the FERM interaction with PIP2 to result in an enhanced association between FAK and the inhibitory myosin, which leads to FAK suppression (SI Appendix, Fig. S14B). Additional EDQ mutation to the KAKTLRK mutant in the FERM domain should then rescue the FAK activation by releasing inhibitory myosin binding during cell adhesion (SI Appendix, Fig. S14C). Indeed, the defect of FAK activation of KAKTLRK mutant during cell adhesion was fully recovered by these additional EDQ mutations (KAK-EDQ) (Fig. 4C). EDQ mutations in wild-type FAK (EDQ), however, did not have significant enhancing effect on the FAK activation on adhesion (Fig. 4D), suggesting that the wild-type FAK may mainly bind to PIP2 via the FERM basic patch, and hence be protected from the inhibitory myosin binding. Therefore, our results suggest that during cell adhesion on FN, the balance of myosin/PIP2 binding is crucial for the proper FAK activation (Fig. 4E). When cells were applied to the Col I-coated surface, FAK activation was also inhibited in the KAKTLKR mutant but completely rescued by additional EDQ mutations (KAK-EDQ; Fig. 4F), suggesting similar roles of the myosin/PIP2 balance in FAK activation mechanism under both Col I and FN conditions. These results suggest that although distinct FAK mechanoactivation on different ECM is determined by different accessibility of the integrin α subunit to its ECM binding motif (Fig. 5A), intracellular FAK activation can be achieved and maintained in a similar manner through the common integrin β1 subunit and myosin/PIP2 balance (Fig. 5B). Therefore, our study provides unique insights on the biophysical and molecular mechanisms on how different ECM proteins and specific integrin subtypes perceive mechanical forces to regulate intracellular FAK activation in an integrated model.
Fig. 5.
Proposed model of FAK mechanoactivation mechanisms via different ECM and integrin subtypes during cell adhesion process. (A) Integrin α5β1 can be fully activated in the tensioned state where both RGD peptide (yellow circle) and synergy site (red circle) bind to α5 and β1 subunits, respectively. Because FN synergy site is exposed only in the high-tensional state, the FAK activation via integrin α5β1 is dependent on the mechanical environment. In contrast, integrin α2β1 can directly bind to the constitutively exposed GFOGER motif (orange circle) in Col I, thus causing the activation of integrin α2β1 and FAK independent of mechanical tension. (B) Integrin activation can recruit and induce the transphosphorylation of FAK. This leads to the FAK activation, which is maintained by the interaction between the FERM basic patch (blue oval) and PIP2 to prevent the inhibitory interaction of myosin II with FERM acidic sites (red oval).
Materials and Methods
We have provided detailed information of materials and methods in SI Appendix. These materials and methods include the DNA Plasmids, Cell Culture and Reagents, Antibodies and Peptides, Preparation of Polyacrylamide Gels with Coupled ECM Proteins, Traction Force Measurement, Bead Coating, Immunoprecipitation and Immunoblotting, and Image Acquisition.
Supplementary Material
Acknowledgments
This work is supported in part by grants from the National Institutes of Health [HL098472, HL109142, GM106403, and NS063405; GM072744 (to N.W.); and GM065918 (to A.J.G.)], the National Science Foundation (CBET0846429), and Beckman Laser Institute, Inc. (to Y.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.E.D. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1307405110/-/DCSupplemental.
References
- 1.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: Mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10(1):75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 2.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 3.Trappmann B, et al. Extracellular-matrix tethering regulates stem-cell fate. Nat Mater. 2012;11(7):642–649. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- 4.Huebsch N, et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010;9(6):518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol. 2003;19:677–695. doi: 10.1146/annurev.cellbio.19.111301.153011. [DOI] [PubMed] [Google Scholar]
- 6.Sawada Y, et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127(5):1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.del Rio A, et al. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323(5914):638–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grashoff C, et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 2010;466(7303):263–266. doi: 10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byfield FJ, et al. Absence of filamin A prevents cells from responding to stiffness gradients on gels coated with collagen but not fibronectin. Biophys J. 2009;96(12):5095–5102. doi: 10.1016/j.bpj.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orr AW, Ginsberg MH, Shattil SJ, Deckmyn H, Schwartz MA. Matrix-specific suppression of integrin activation in shear stress signaling. Mol Biol Cell. 2006;17(11):4686–4697. doi: 10.1091/mbc.E06-04-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Indra I, Beningo KA. An in vitro correlation of metastatic capacity, substrate rigidity, and ECM composition. J Cell Biochem. 2011;112(11):3151–3158. doi: 10.1002/jcb.23241. [DOI] [PubMed] [Google Scholar]
- 13.Kim TJ, et al. Substrate rigidity regulates Ca2+ oscillation via RhoA pathway in stem cells. J Cell Physiol. 2009;218(2):285–293. doi: 10.1002/jcp.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seong J, et al. Detection of focal adhesion kinase activation at membrane microdomains by fluorescence resonance energy transfer. Nat Commun. 2011;2:406. doi: 10.1038/ncomms1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls alpha5beta1 function. Science. 2009;323(5914):642–644. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]
- 16.Wang N, et al. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am J Physiol Cell Physiol. 2002;282(3):C606–C616. doi: 10.1152/ajpcell.00269.2001. [DOI] [PubMed] [Google Scholar]
- 17.Na S, et al. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc Natl Acad Sci USA. 2008;105(18):6626–6631. doi: 10.1073/pnas.0711704105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poh YC, et al. Dynamic force-induced direct dissociation of protein complexes in a nuclear body in living cells. Nat Commun. 2012;3:866. doi: 10.1038/ncomms1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heidkamp MC, Bayer AL, Kalina JA, Eble DM, Samarel AM. GFP-FRNK disrupts focal adhesions and induces anoikis in neonatal rat ventricular myocytes. Circ Res. 2002;90(12):1282–1289. doi: 10.1161/01.res.0000023201.41774.ea. [DOI] [PubMed] [Google Scholar]
- 20.Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: Integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol. 2010;11(9):633–643. doi: 10.1038/nrm2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reyes CD, García AJ. Engineering integrin-specific surfaces with a triple-helical collagen-mimetic peptide. J Biomed Mater Res A. 2003;65(4):511–523. doi: 10.1002/jbm.a.10550. [DOI] [PubMed] [Google Scholar]
- 22.Lu S, et al. The spatiotemporal pattern of Src activation at lipid rafts revealed by diffusion-corrected FRET imaging. PLOS Comput Biol. 2008;4(7):e1000127. doi: 10.1371/journal.pcbi.1000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leitinger B. Transmembrane collagen receptors. Annu Rev Cell Dev Biol. 2011;27:265–290. doi: 10.1146/annurev-cellbio-092910-154013. [DOI] [PubMed] [Google Scholar]
- 24.Smith ML, et al. Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLoS Biol. 2007;5(10):e268. doi: 10.1371/journal.pbio.0050268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antia M, Baneyx G, Kubow KE, Vogel V. Fibronectin in aging extracellular matrix fibrils is progressively unfolded by cells and elicits an enhanced rigidity response. Faraday Discuss. 2008;139:229–249. doi: 10.1039/b718714a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark K, et al. A specific alpha5beta1-integrin conformation promotes directional integrin translocation and fibronectin matrix formation. J Cell Sci. 2005;118(Pt 2):291–300. doi: 10.1242/jcs.01623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takagi J, Strokovich K, Springer TA, Walz T. Structure of integrin alpha5beta1 in complex with fibronectin. EMBO J. 2003;22(18):4607–4615. doi: 10.1093/emboj/cdg445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi Q, Boettiger D. A novel mode for integrin-mediated signaling: Tethering is required for phosphorylation of FAK Y397. Mol Biol Cell. 2003;14(10):4306–4315. doi: 10.1091/mbc.E03-01-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knight CG, et al. The collagen-binding A-domains of integrins alpha(1)beta(1) and alpha(2)beta(1) recognize the same specific amino acid sequence, GFOGER, in native (triple-helical) collagens. J Biol Chem. 2000;275(1):35–40. doi: 10.1074/jbc.275.1.35. [DOI] [PubMed] [Google Scholar]
- 30.Ruggiero F, Comte J, Cabañas C, Garrone R. Structural requirements for alpha 1 beta 1 and alpha 2 beta 1 integrin mediated cell adhesion to collagen V. J Cell Sci. 1996;109(Pt 7):1865–1874. doi: 10.1242/jcs.109.7.1865. [DOI] [PubMed] [Google Scholar]
- 31.Carragher NO, et al. Calpain 2 and Src dependence distinguishes mesenchymal and amoeboid modes of tumour cell invasion: A link to integrin function. Oncogene. 2006;25(42):5726–5740. doi: 10.1038/sj.onc.1209582. [DOI] [PubMed] [Google Scholar]
- 32.Calderwood DA, et al. Integrin beta cytoplasmic domain interactions with phosphotyrosine-binding domains: A structural prototype for diversity in integrin signaling. Proc Natl Acad Sci USA. 2003;100(5):2272–2277. doi: 10.1073/pnas.262791999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Humphries JD, et al. Dual functionality of the anti-beta1 integrin antibody, 12G10, exemplifies agonistic signalling from the ligand binding pocket of integrin adhesion receptors. J Biol Chem. 2005;280(11):10234–10243. doi: 10.1074/jbc.M411102200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsuchida J, Ueki S, Saito Y, Takagi J. Classification of ‘activation’ antibodies against integrin beta1 chain. FEBS Lett. 1997;416(2):212–216. doi: 10.1016/s0014-5793(97)01206-4. [DOI] [PubMed] [Google Scholar]
- 35.Yu CH, Luo W, Sheetz MP. Spatial-temporal reorganization of activated integrins. Cell Adhes Migr. 2012;6(3):280–284. doi: 10.4161/cam.20753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li R, et al. Activation of integrin alphaIIbbeta3 by modulation of transmembrane helix associations. Science. 2003;300(5620):795–798. doi: 10.1126/science.1079441. [DOI] [PubMed] [Google Scholar]
- 37.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: In command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6(1):56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 38.Katz BZ, et al. Direct transmembrane clustering and cytoplasmic dimerization of focal adhesion kinase initiates its tyrosine phosphorylation. Biochim Biophys Acta. 2002;1592(2):141–152. doi: 10.1016/s0167-4889(02)00308-7. [DOI] [PubMed] [Google Scholar]
- 39.von Wichert G, Haimovich B, Feng GS, Sheetz MP. Force-dependent integrin-cytoskeleton linkage formation requires downregulation of focal complex dynamics by Shp2. EMBO J. 2003;22(19):5023–5035. doi: 10.1093/emboj/cdg492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dunty JM, et al. FERM domain interaction promotes FAK signaling. Mol Cell Biol. 2004;24(12):5353–5368. doi: 10.1128/MCB.24.12.5353-5368.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cai X, et al. Spatial and temporal regulation of focal adhesion kinase activity in living cells. Mol Cell Biol. 2008;28(1):201–214. doi: 10.1128/MCB.01324-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gabev E, Kasianowicz J, Abbott T, McLaughlin S. Binding of neomycin to phosphatidylinositol 4,5-bisphosphate (PIP2) Biochim Biophys Acta. 1989;979(1):105–112. doi: 10.1016/0005-2736(89)90529-4. [DOI] [PubMed] [Google Scholar]
- 43.Santos AM, et al. FERM domain interaction with myosin negatively regulates FAK in cardiomyocyte hypertrophy. Nat Chem Biol. 2012;8(1):102–110. doi: 10.1038/nchembio.717. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.