The stereochemistry of the iridoid plumeridoid C, C15H18O7, was established by X-ray single-crystal structure analysis, giving (2′R,3R,4R,4aS,7aR)-methyl 3-hydroxy-4′-[(S)-1-hydroxyethyl]-5′-oxo-3,4,4a,7a-tetrahydro-1H,5′H-spiro[cyclopenta[c]-pyran-7,2′-furan]-4-carboxylate. The absolute structure of the title compound was determined on the basis of the Flack x parameter and Bayesian statistics on Bijvoet differences. The hydrogen-bond donor and acceptor functions of the two hydroxy groups are employed in the formation of O—H⋯O-bonded helical chains.
Comment
As part of our search for bioactive natural products, we have investigated chemical compounds contained in the bark material of the Amazonian tree Himatanthus sucuuba (spruce) Woodson (Apocynaceae). In folk medicine, the bark and latex of this plant species are used for the treatment of tumours and inflammatory diseases. Pharmacological studies have shown that extracts and constituents of Himatanthus sucuuba possess therapeutic potential (Amaral et al., 2007).
Eleven compounds, denoted (I) to (XI) (see Supplementary materials), were isolated and purified from the bark material (1.9 kg) of Himatanthus sucuuba using extraction, liquid–liquid partition, various chromatographic techniques and crystallization from solvents. The isolated compounds were identified as the iridoids plumeridoid C [(I), 60 mg] (Kuigoua et al., 2010), plumericin [(II), 90 mg] (Elsässer et al., 2005), plumieridin [(III), 8 mg] (Yamauchi et al., 1981) and allamandicin [(IV), 7 mg] (Abe et al., 1984), the flavonoids biochanin A [(V), 8 mg] (Jha et al., 1980; Talukdar et al., 2000), dihydrobiochanin A [(VI), 9 mg] (Osawa et al., 1992), dalbergioidin [(VII), 0.3 mg] (Osawa et al., 1992), naringenin [(VIII), 9 mg] (Hou et al., 2001), ferreirin [(IX), 2 mg] (Osawa et al., 1992) and dihydrocajanin [(X), 8 mg] (Osawa et al., 1992), and the lignan pinoresinol [(XI), 9 mg] (Xie et al., 2003) by means of mass spectrometry, one- and two-dimensional NMR experiments, optical rotation and comparison with data from the literature. Except for (II) and (XI), the isolation of these compounds from Himatanthus sucuuba is reported here for the first time.
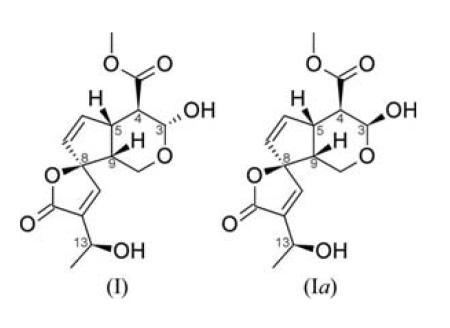
A recent paper by Kuigoua et al. (2010) contains the first report of the existence of the iridoid plumeridoid C, (I). In order to complete the full characterization of (I) and to elucidate its absolute configuration, its crystal structure has been determined.
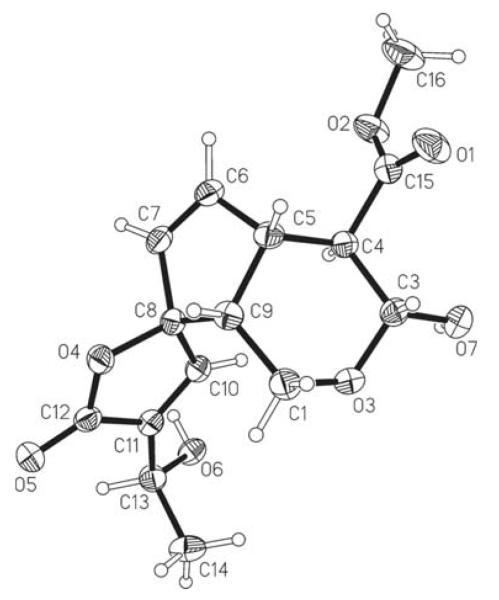
The asymmetric unit of (I) consists of one formula unit (Fig. 1). As the compound consists only of O, C and H atoms, Cu Kα radiation was used to enable the determination of the absolute configuration, and 1210 Friedel pairs were measured. This yielded a refined Flack (1983) x parameter and standard uncertainty (s.u.) of −0.01 (13). We note that the obtained s.u. value is slightly above the suggested upper confidence limit of 0.10 for the determination of the absolute structure of an enantiopure compound (Flack & Bernardinelli, 2000). However, additional confirmation was obtained from the examination of Bayesian statistics on 1210 Bijvoet pairs (Hooft et al., 2008) carried out using the program PLATON (Spek, 2009). The calculated Hooft y parameter was 0.07 (6) with G = 0.9 (1). The calculated probability values P3(true), P3(twin) and P3(wrong) were 1.000, 0.000 and 0.000, respectively. This confirmed the absolute configuration of the six stereocentres as 3R, 4R, 5S, 8R, 9R and 13S (see Scheme). Moreover, these results are consistent with the relative configuration of (I) that was proposed by Kuigoua et al. (2010) on the basis of NMR data.
Figure 1.
The molecular structure of (I), with displacement ellipsoids drawn at the 50% probability level.
Inspection of a plot of the Bijvoet pairs δ(Fo2) against δ(Fc2) generated with PLATON (Spek, 2009) does not reveal a clear trend. All quadrants of the plot are populated by a significant number of points. This contrasts with the observation that all of the numerical indicators (x, y, P2, P3) give a very clear indication of the absolute structure and are consistent between themselves. We note that the error bars on the plot indicate that many of the Bijvoet differences are small compared with experimental error. However, even with this limitation, the data give clear indications in terms of the numerical parameters that have been developed to distinguish absolute structures.
The cyclopentene ring of (I) displays a C9 envelope conformation, with atom C9 displaced by 0.420 (2) Å from the plane defined by atoms C5/C6/C7/C8. As indicated by its Cremer–Pople ring-puckering parameters [Q = 0.544 (2) Å, θ = 163.4 (2)° and φ = 139.7 (6)°; Cremer & Pople, 1975], the geometry of the tetrahydropyran ring (O3/C1/C9/C5/C4/C3) is best described as a slightly distorted chair. The mean plane of the furan ring and the plane defined by atoms C7/C8/C9 form an angle of 88.11 (8)°. The mean plane of the –CC( O)OC fragment C4/C15/O1/O2/C16 is almost perpendicular to the plane defined by atoms C3/C4/C5, with which it forms an angle of 81.27 (10)°.
O)OC fragment C4/C15/O1/O2/C16 is almost perpendicular to the plane defined by atoms C3/C4/C5, with which it forms an angle of 81.27 (10)°.
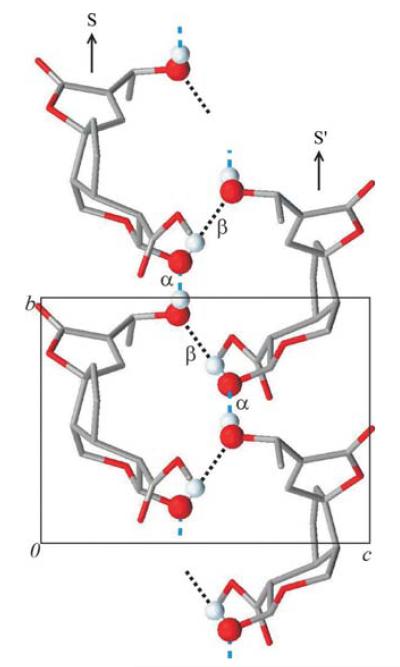
The molecule of (I) contains two OH groups, O6 in the hydroxyethyl fragment and the O7 group on the tetrahydropyran ring. The hydrogen-bond donor and acceptor functions of both are utilized to give a one-dimensional helical chain that propagates along [010] (Table 1 and Fig. 2). This hydrogen-bonded chain consists of two strands of O6—H6O⋯O7(x, y + 1, z)-bonded molecules, neighbouring molecules of which are related to one another by translational symmetry. The two strands of a chain are related by a 21 screw axis and linked to one another via O7—H7O⋯O6(−x + 2, , −z + 1) interactions. Hence, every molecule of (I) is O—H⋯O-bonded to four neighbouring molecules. Using the graph-set notation proposed by Etter et al. (1990) and Bernstein et al. (1995), the hydrogen-bonded molecules of (I) are linked together via fused (15) rings. The crystal packing of these O—H⋯O-bonded chains generates two notable short inter-chain contacts, viz. C1—H1B⋯O5(−x + 2, , −z) and C5—H5⋯O4(−x + 1, , −z), with H⋯O distances of 2.37 and 2.47 Å, respectively (Table 1). The first interchain contact is observed between the CH2 group of the tetrahydropyran ring and the carbonyl group on the furan ring of a neighbouring molecule, while the second contact involves the chiral centre C5 of the fused cyclopentene and tetrahydropyran rings of one molecule and the furan O atom of another.
Table 1.
Geometry of hydrogen bonds and other contacts (Å, °).
| D—H⋯A | D—H | H⋯A | D ⋯ A | D—H⋯A |
|---|---|---|---|---|
| O6—H6O⋯O7i | 0.83 (2) | 1.99 (2) | 2.8153 (15) | 174 (2) |
| O7—H7O⋯O6ii | 0.80 (2) | 1.92 (2) | 2.7218 (15) | 176 (2) |
| C1—H1B⋯O5iii | 0.99 | 2.37 | 3.2969 (18) | 156 |
| C5—H5⋯O4iv | 1.00 | 2.47 | 3.3243 (16) | 143 |
Symmetry codes: x, y + 1, z;
−x + 2, , −z + 1;
−x + 2, , −z;
−x + 1, , −z.
Figure 2.
A single O—H⋯O-bonded helical chain in the crystal structure of (I), viewed along [100]. The two strands of the chain are denoted S and S’, with the hydrogen bonds α = O6—H6O⋯O7(x, y + 1, z) and β = O7—H7O⋯O6(−x + 2, , z + 1).
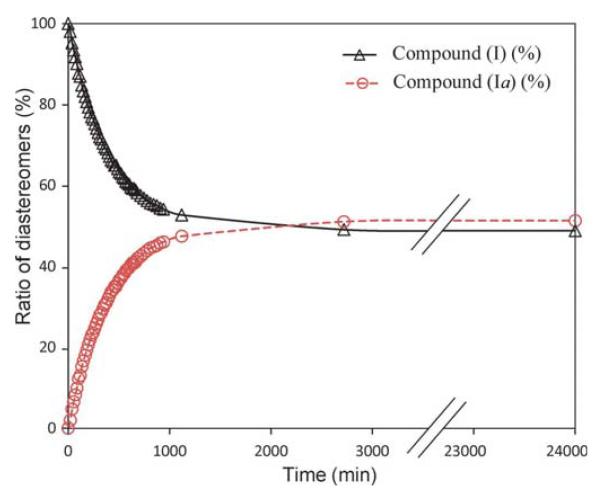
We have found that, in methanol solution, diastereomerization of (I) occurs at position 3, yielding the epiplumeridoid C [(Ia); Kuigoua et al., 2010]. This phenomenon was studied in a time-dependent 1H NMR experiment. Compound (I) (1 mg) was dissolved in CD3OD and isochronous 1H NMR measurements of the (I):(Ia) ratio were started instantaneously. This experiment confirmed the diastereomerization at position 3, giving a chemical equilibrium of 1:1.05 between (I) and (Ia) [(I): 3R, 4R, 5S, 8R, 9R, 13S; (Ia): 3S, 4R, 5S, 8R, 9R, 13S] after approximately two days (Fig. 3). These results suggest that crystallization from a methanol solution yields compound (I) exclusively, even though diastereomerization takes place in a methanol solution so that the epimers (I) and (Ia) are both present. Moreover, NMR experiments have shown that both (I) and (Ia) are also contained in a deuterated pyridine solution.
Figure 3.
Time-dependent ratio of diastereomers (I) and (Ia) in a CD3OD solution, analysed by 1H NMR. Compound (I) was dissolved in CD3OD at zero time.
Experimental
The general experimental conditions used for the isolation and identification of compounds (I) to (XI), including the detailed isolation protocols and chemical structures, the characteristic UV–Vis and FT–IR data for (I) and the NMR spectroscopic data for (I) and (Ia), are available in the Supplementary materials.
1H NMR experiments for the evaluation of the diastereomerization were carried out on a Bruker TXI600 at 298 K in CD3OD (referenced to the residual nondeuterated solvent signals). The values for (I) and for the 1:1.05 mixture of (I) and (Ia) were determined as +70.5 and +82.0, respectively (c = 0.97, methanol).
Simultaneous melting and decomposition of (I) were observed above 454 K. The colourless prisms of (I) used for this study were obtained by crystallization from a saturated methanol solution. The unit-cell parameters of six different crystals were determined and found to be consistent with (I). There was no indication of the presence of crystals of (Ia) in the investigated batch.
| Crystal data |
| C15H18O7 |
| Mr = 310.29 |
| Monoclinic, P21 |
| a = 9.6736 (2) Å |
| b = 7.6203 (1) Å |
| c = 10.6303 (2) Å |
| β = 107.142 (2)° |
| V = 748.81 (2) Å 3 |
| Z = 2 |
| Cu Kα radiation |
| μ = 0.93 mm −1 |
| T = 173 K |
| 0.20 × 0.20 × 0.15 mm |
| Data collection |
| Oxford Xcalibur Ruby Gemini Ultra diffractometer |
| Absorption correction: multi-scan [CrysAlis PRO (Oxford Diffraction, 2003); multi-scan absorption correction using spherical harmonics, imple- mented in SCALE3 ABSPACK scaling algorithm] Tmin = 0.766, Tmax = 1.000 |
| 7011 measured reflections |
| 2651 independent reflections |
| 2615 reflections with I > 2σ(I) |
| Rint = 0.029 |
| Refinement |
| R[F2 > 2σ(F2)] = 0.029 |
| wR(F2) = 0.081 |
| S = 1.09 |
| 2651 reflections |
| 225 parameters |
| 3 restraints |
| H atoms treated by a mixture of independent and constrained refinement |
| Δρmax = 0.16 e Å −3 |
| Δρmin = −0.19 e Å −3 |
| Absolute structure: Flack (1983), with 1210 Friedel pairs |
| Flack parameter: −0.01 (13) |
All H atoms were identified in a difference map. Methyl H atoms were idealized and included as rigid groups that were allowed to rotate but not tip (C—H = 0.98 Å). H atoms bonded to tertiary (C—H = 1.00 Å), secondary (C—H = 0.99 Å) and aromatic C atoms (C—H = 0.95 Å) were positioned geometrically. H atoms attached to O atoms were refined with restrained distances [O—H = 0.82 (2) Å]. The Uiso parameters of all H atoms were refined freely.
The absolute configuration of this structure was confirmed by the Flack (1983) parameter and Bayesian statistics on 1210 Bijvoet pairs (Hooft et al., 2008). The Flack parameter is −0.01 (13) for the reported structure and 0.99 (13) for the inverted structure. Friedif, RA and RD values (Flack & Shmueli, 2007; Flack et al., 2011) were calculated using PLATON (Spek, 2009). Friedif reflects the ability to determine the absolute structure, and Friedifstat = 36 has a low value as only C, H and O atoms are present, while Friedifobs is 1286. The RA and RD values are 0.050 and 0.989, respectively, for the reported structure, and 0.050 and 1.022, respectively, for the inverted structure.
Data collection: CrysAlis CCD (Oxford Diffraction, 2003); cell refinement: CrysAlis RED (Oxford Diffraction, 2003); data reduction: CrysAlis RED; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008); molecular graphics: XP in SHELXTL (Sheldrick, 2008) and Mercury (Macrae et al., 2008); software used to prepare material for publication: publCIF (Westrip, 2010).
Supplementary Material
Acknowledgments
This work was supported by the National Research Network project ‘Drugs from Nature Targeting Inflammation’ (subproject No. S10703) granted by the Austrian Science Fund (FWF). The authors thank Peter Schneider and Professor Ernst P. Ellmerer for the NMR measurements, and Professor Volker Kahlenberg for access to the X-ray facilities used for this study.
Footnotes
Supplementary data for this paper are available from the IUCr electronic archives (Reference: FA3259). Services for accessing these data are described at the back of the journal.
References
- Abe F, Mori T, Yamauchi T. Chem. Pharm. Bull. 1984;32:2947–2956. [Google Scholar]
- Amaral ACF, Ferreira JLP, Pinheiro MLB, Silva JRA. Pharmacogn. Rev. 2007;1:305–313. [Google Scholar]
- Bernstein J, Davis RE, Shimoni L, Chang N-L. Angew. Chem. Int. Ed. Engl. 1995;34:1555–1573. [Google Scholar]
- Cremer D, Pople JA. J. Am. Chem. Soc. 1975;97:1354–1358. [Google Scholar]
- Elsässer B, Krohn K, Akhtar MN, Flörke U, Kouam SF, Kuigoua MG, Ngadjui BT, Abegaz BM, Antus S, Kurtan T. Chem. Biodivers. 2005;2:799–808. doi: 10.1002/cbdv.200590058. [DOI] [PubMed] [Google Scholar]
- Etter MC, MacDonald JC, Bernstein J. Acta Cryst. 1990;B46:256–262. doi: 10.1107/s0108768189012929. [DOI] [PubMed] [Google Scholar]
- Flack HD. Acta Cryst. 1983;A39:876–881. [Google Scholar]
- Flack HD, Bernardinelli G. J. Appl. Cryst. 2000;33:1143–1148. [Google Scholar]
- Flack HD, Sadki M, Thompson AL, Watkin DJ. Acta Cryst. 2011;A67:21–34. doi: 10.1107/S010876731004287X. [DOI] [PubMed] [Google Scholar]
- Flack HD, Shmueli U. Acta Cryst. 2007;A63:257–265. doi: 10.1107/S0108767307002802. [DOI] [PubMed] [Google Scholar]
- Hooft RWW, Straver LH, Spek AL. J. Appl. Cryst. 2008;41:96–103. doi: 10.1107/S0021889807059870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou A, Fukai T, Shimazaki M, Sakagami H, Sun H, Nomura T. J. Nat. Prod. 2001;64:65–70. doi: 10.1021/np000406p. [DOI] [PubMed] [Google Scholar]
- Jha HC, Zilliken F, Breitmaier E. Can. J. Chem. 1980;58:1211–1219. [Google Scholar]
- Kuigoua GM, Kouam SF, Ngadjui BT, Schulz B, Green IR, Choudhary MI, Krohn K. Planta Med. 2010;76:620–625. doi: 10.1055/s-0029-1240611. [DOI] [PubMed] [Google Scholar]
- Macrae CF, Bruno IJ, Chisholm JA, Edgington PR, McCabe P, Pidcock E, Rodriguez-Monge L, Taylor R, van de Streek J, Wood PA. J. Appl. Cryst. 2008;41:466–470. [Google Scholar]
- Osawa K, Yasuda H, Maruyama T, Morita H, Takeya K, Itokawa H. Chem. Pharm. Bull. 1992;40:2970–2974. doi: 10.1248/cpb.40.2970. [DOI] [PubMed] [Google Scholar]
- Oxford Diffraction . CrysAlis CCD and CrysAlis RED. Versions 1.171 Oxford Diffraction Ltd; Abingdon, Oxfordshire, England: 2003. [Google Scholar]
- Sheldrick GM. Acta Cryst. 2008;A64:112–122. [Google Scholar]
- Spek AL. Acta Cryst. 2009;D65:148–155. doi: 10.1107/S090744490804362X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar AC, Jain N, De S, Krishnamurty HG. Phytochemistry. 2000;53:155–157. doi: 10.1016/s0031-9422(99)00489-6. [DOI] [PubMed] [Google Scholar]
- Westrip SP. J. Appl. Cryst. 2010;43:920–925. [Google Scholar]
- Xie L-H, Akao T, Hamasaki K, Deyama T, Hattori M. Chem. Pharm. Bull. 2003;51:508–515. doi: 10.1248/cpb.51.508. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Abe F, Taki M. Chem. Pharm. Bull. 1981;29:3051–3055. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.