Abstract
Depression in students is a major public health problem. Although several risk factors associated with depression have been identified, the cause of depression is still not clear. Several studies have demonstrated that physical activity and nutrient intake, such as increased levels of B vitamins in serum, decrease symptoms of depression. The aim of this study was to investigate the association between physical activity and dietary intake of vitamins B6, B9, and B12 and symptoms of depression among postgraduate students. The results of this study suggest that intake of vitamin B9 may modulate the total score of Center for Epidemiological Studies Depression Scale (CES-D) and two subscales of the CES-D including depressive affect and interpersonal difficulties. This study also showed that moderate/high levels of physical activity were inversely and significantly associated with symptoms of depression (total scores) and three subscales of the CES-D including depressive affect, positive affect, and somatic complaints.
1. Background
Depression has increasingly become a public health problem in both developed [1] and developing countries [2]. Several risk factors cluster together and increase the risk of depressive disorders. Nonmodifiable, modifiable, and contextual risk factors are causes of these disorders. Advancing age, gender [3], and ethnicity [4] are examples of nonmodifiable risk factors of depression. Inflammation [5], cigarette smoking, physical inactivity, poor nutrition, and consumption of alcohol are modifiable risk factors of depression [6]. Sociodemographic variables such as education, income, health insurance, and poverty are contextual risk factors of depression. Accumulations of one or more risk factors in individuals may lead to depressive disorders and will become a public health problem when these people become unable to conduct normal activities of daily living or cause distress to other healthy people in the society.
Depressive disorders have been shown to be associated with the higher rates of mortality compared with individuals without depressive disorders [7]. These disorders have negative effects on the quality of life and decreased life expectancy, especially among the severely mentally ill [8]. It has been shown that depression has a negative impact on many diseases such as cancer [9], cardiovascular disease [2], and diabetes mellitus [10]. A significant elevation in morbidity and mortality has been shown in these diseases because of depression [2, 9, 10].
Symptoms of depression among college students are a growing public health concern [11–13]. One out of seven students may experience depression, which usually presents as feelings of fatigue, guilt, sadness, and hopelessness. Depressed students are prone to low academic performance, withdraw from university, increased smoking, acute infection illnesses, self-injurious behavior, and suicide [14]. Depression may interfere with interpersonal relationships and performance of daily tasks, which may lead to suicidal thoughts and attempts of suicide [15].
Depression in college students may be due to several factors, including vulnerable age, demands of college life, personal issues, adaptation to a new environment, tendency toward perfectionism, and conflict between traditional and modern values [15, 16]. There is increasing interest in the role of physical activity in the prevention and treatment of depression [17]. Over the last 40 years, several studies have investigated the relationship between physical activity and improvements in mental health symptoms among various populations. Many of the studies have indicated that physical activity is able to reduce the risk of several mental health conditions, especially depressive symptoms [18–20]. Numerous gaps remain in each of these individual research areas. For example, these studies have been limited by lack of data on important factors such as levels of physical activity, because although previous studies have reported that physical activity successfully decreases depression, it is not clear which levels of physical activity were related to depression [21]. Furthermore, the role of physical activity as a predictor of depression was not constant [22].
An association between depressive symptoms and polyunsaturated fatty acids or minerals has been found among university students [2, 12, 23, 24]. Also, a few studies among other populations have demonstrated that dietary intake of vitamins B may decrease symptoms of depression [25–27]. However, these studies have been limited in middle age and over, and the association between dietary intake of vitamin B such as vitamin B9 has not received as much attention in special populations such as university students. Also, studies have investigated the association between physical activity and depression [28, 29]. These studies failed to control nutrients intake such as vitamins B, which may reduce symptoms of depression, and this can confound the association between physical activity and depression. In addition, several previous studies did not investigate the relationship between the levels of physical activity with depression. The current study excluded several other confounders such as hyperthyroidism, hypertension, diabetes, cancer, or heart disease; these diseases can affect the associations between vitamins B and both depression and physical activity; these confounders cannot be controlled statistically utterly. Most importantly, there is no any data (locally or universally) on the subject of vitamins B and depression and psychological dimensions of depression in university students. Taking everything into consideration, the association between vitamins B/physical activity and depressive symptoms remains unclear among university students. Therefore, the aim of this study was to investigate the association between physical activity and dietary intake of vitamins B6, B9, and B12 and symptoms of depression and psychological dimensions of depression as measured by the CESD subscales (depressive affect, somatic complaints, positive affect, and interpersonal difficulties).
2. Materials and Methods
A cross-sectional study, designed in 2011, was performed on a convenience sample of 425 Iranian students who were studying in Malaysia, aged 32.54 ± 6.19 years. Based on the study design, students with serious diseases such as hyperthyroidism, hypertension, diabetes, or heart disease were excluded since such conditions may affect lifestyle (e.g., physical activity) or alter the risk factors for depression. Students who had a history of mental illness or those taking psychiatric drugs were also excluded. Consequently, 23 individuals were dropped from the study; data analyses were performed on the sample of 402 participants that remained. This study was approved by The Scientific Counselor and Director of Iranian Students Affairs in South East Asia in Malaysia, and informed consent was obtained from all participants before enrollment.
2.1. Depression Questionnaire
Symptoms of depression in our population were assessed with the Center for Epidemiologic Studies (CES-D) questionnaire [30]. Symptoms calculated with the CES-D included feelings of loneliness, appetite loss, sadness, sleep disorders, fear, and crying. This questionnaire contained twenty self-administered items, using a 4-point Likert-type scale that ranged from 0 (rarely or none of the time; less than 1 day) to 3 (most or all of the time; 5–7 days). Scores on the CES-D could range from 0 to 60. A cut-off score of 16 or greater indicated symptoms of depression. This questionnaire has been categorized in four dimensions including depressive affect, positive affect, somatic complaints, and interpersonal difficulties [30].
2.2. Assessment of Dietary B Vitamins
Dietary intake of B vitamins, including B6, B9, and B12, was assessed with a semiquantitative food frequency questionnaire (FFQ) [31]. The FFQ has been validated in several multiethnic populations [32, 33] and is used to assess food consumption over the previous 12 months. Nutritionist IV software, version 3.5.2, was used to measure the amount of B vitamins consumed. Univariate analyses were performed with SPSS software to analyze the relationships between the levels of B6, B9, and B12 calculated from the FFQ and the socioeconomic status and lifestyle factors of the participants.
2.3. Physical Activity Questionnaire
In the present study, physical activity was defined as leisure-time physical activity [34]. This questionnaire was used to investigate the activities the students performed during the last year, including transport to and from work during the last year. The questionnaire has a 4-point interview format or self-report items that measure physical activity. These items include 1 for almost completely inactive or low physical activity less than 2 h per week, 2 for low physical activity performed for 2–4 h per week, 3 for low physical activity intended for more than 4 h per week or more vigorous activity in favor of 2–4 h per week, and 4 for additional vigorous physical activity for over 4 h per week or regular heavy exercise or competitive sports many times per week. The final decision on physical activity was graded as follows: category 1 for low level of physical activity, category 2 for moderate level of physical activity, and categories 3 and 4 for high level of physical activity [34].
2.4. Statistical Analysis
Independent t-test was used to examine the associations between participant characteristics and symptoms of depression. To assess the association between depression and dietary intake of B vitamins, we generated a multiple linear regression model. In this model, depressive symptom was the dependent variable whereas vitamins B intake and other variables were the independent factors.
3. Results
Table 1 shows the characteristics of study participants based on total depression score (CES-D) and vitamins B. Symptoms of depression were significantly associated with decreased dietary reference intake (DRI) of vitamin B9 and age. Female gender was associated with higher score of depressive symptoms and more consumption of vitamin B6 and vitamin B9 compared to males. Less education was also associated with depressive symptoms and decreased dietary intake of vitamin B9. Marital status, current smoking, former smoking, body mass index (BMI), and number of close friends were not associated with symptoms of depression or vitamins B. Monthly expenses were significantly associated with less consumption of vitamins B but not with depressive symptoms. Living in campus was not associated with depressive symptoms or the intake of vitamins B. A high level of depressive symptom was associated with low levels of physical activity, independent of vitamins B.
Table 1.
Characteristics of the study subjects based on overall CES-D score and vitamins B.
| Variables | Depression score (Mean ± SD) |
P | *Vitamin B6
(Mean ± SD) |
P | **Vitamin B9
(Mean ± SD) |
P | **Vitamin B12
(Mean ± SD) |
P |
|---|---|---|---|---|---|---|---|---|
| Age groups | 0.010 | 0.309 | 0.030 | 0.484 | ||||
| ≤35 (n = 284) | 13.85 ± 9.22 | 2.48 ± 1.71 | 436.47 ± 352.53 | 3.62 ± 2.96 | ||||
| >35 (n = 118) | 11.59 ± 7.37 | 2.66 ± 1.25 | 526.52 ± 432.16 | 3.90 ± 5.18 | ||||
| Gender | 0.031 | 0.008 | 0.004 | 0.178 | ||||
| Female (n = 173) | 14.27 ± 9.46 | 2.79 ± 1.91 | 529.98 ± 478.82 | 4.00 ± 4.70 | ||||
| Male (n = 229) | 12.37 ± 8.13 | 2.34 ± 1.32 | 412.23 ± 272.25 | 3.48 ± 2.81 | ||||
| Education | 0.001 | 0.068 | 0.031 | 0.295 | ||||
| <20 y (n = 199) | 14.60 ± 9.23 | 2.38 ± 1.58 | 421.66 ± 344.75 | 3.50 ± 2.32 | ||||
| ≥20 y (n = 203) | 11.80 ± 8.08 | 2.68 ± 1.64 | 503.33 ± 407.21 | 3.89 ± 4.74 | ||||
| Marital status | 0.289 | 0.323 | 0.111 | 0.855 | ||||
| Married (n = 208) | 12.74 ± 7.82 | 2.61 ± 1.38 | 492.04 ± 358.89 | 3.73 ± 4.03 | ||||
| Single (n = 194) | 13.66 ± 9.68 | 2.45 ± 1.83 | 431.66 ± 398.64 | 3.66 ± 3.43 | ||||
| Current smoking | 0.700 | 0.875 | 0.711 | 0.235 | ||||
| Yes (n = 36) | 13.13 ± 8.82 | 2.54 ± 1.64 | 465.10 ± 388.69 | 3.63 ± 3.53 | ||||
| No (n = 366) | 13.72 ± 8.28 | 2.49 ± 1.36 | 440.51 ± 269.16 | 4.41 ± 5.51 | ||||
| Former smoking | 0.338 | 0.356 | 0.327 | 0.860 | ||||
| Yes (n = 16) | 13.27 ± 8.79 | 2.55 ± 1.64 | 466.68 ± 385.22 | 3.71 ± 3.80 | ||||
| No (n = 386) | 11.13 ± 8.26 | 2.17 ± 0.95 | 371.73 ± 168.94 | 3.54 ± 2.17 | ||||
| BMI | 0.084 | 0.347 | 0.919 | 0.635 | ||||
| ≤25 (n = 262) | 13.74 ± 8.96 | 2.48 ± 1.67 | 464.31 ± 424.30 | 3.77 ± 4.42 | ||||
| >25 (n = 140) | 12.15 ± 8.34 | 2.64 ± 1.45 | 460.27 ± 277.56 | 3.58 ± 1.95 | ||||
| Monthly expenses ($) | 0.862 | 0.014 | 0.009 | 0.020 | ||||
| <800 (n = 199) | 13.10 ± 8.85 | 2.33 ± 1.65 | 411.68 ± 349.97 | 3.25 ± 2.28 | ||||
| ≥800 (n = 210) | 13.26 ± 8.71 | 2.72 ± 1.56 | 509.73 ± 399.41 | 4.12 ± 4.69 | ||||
| Close friends | 0.077 | 0.117 | 0.122 | 0.263 | ||||
| <5 (n = 220) | 13.89 ± 9.03 | 2.42 ± 1.67 | 436.25 ± 370.16 | 3.51 ± 3.07 | ||||
| ≥5 (n = 182) | 12.34 ± 8.38 | 2.67 ± 1.54 | 495.12 ± 388.69 | 3.93 ± 4.42 | ||||
| Physical activity | ≤0.001 | 0.485 | 0.969 | 0.206 | ||||
| ≥Moderate (n = 274) | 11.89 ± 8.06 | 2.57 ± 1.71 | 462.40 ± 366.16 | 3.49 ± 2.38 | ||||
| Low (n = 128) | 15.95 ± 9.58 | 2.45 ± 1.39 | 463.97 ± 407.56 | 4.15 ± 5.64 | ||||
| Living in campus | 0.155 | 0.920 | 0.467 | 0.070 | ||||
| Yes (n = 170) | 13.91 ± 9.58 | 2.52 ± 1.62 | 478.99 ± 436.08 | 3.38 ± 2.05 | ||||
| No (n = 232) | 12.65 ± 8.10 | 2.54 ± 1.61 | 451.11 ± 332.14 | 4.14 ± 5.22 |
*mg/day; **μg/day.
Table 2 presents the associations between the participants' characteristics, DRI of vitamins B6, B9, and B12 with the psychological dimensions of depression including depressive affect, somatic complaints, positive affect, and interpersonal difficulties. Less consumption of vitamin B9 was significantly associated with depressive affect and interpersonal difficulties compared with participants who consumed more vitamin B9. The psychological dimensions of depression were not related to current, past smoking, marital status, monthly expenses, and DRI of vitamins B6 and B12. Postgraduate students with lower education had a significant higher score of depressive affect and interpersonal difficulties. Female gender was significantly associated with depressive affect than males. Higher score of depressive affect was significantly associated with participant who had lower age. Lower BMI was also associated with higher score of depressive affect. Participant with less than five close friends had higher score of depressive affect and interpersonal difficulties compared to those with more close friends. Higher score of depressive affect, somatic complaints, and positive affect was associated with low level of physical activity. Living in campus was significantly associated with interpersonal difficulties compared to those who were living out of campus. Figures 1, 2, 3, and 4 show the association between vitamins B6, B9, and B12 and physical activity with psychological dimensions of depression.
Table 2.
The association between vitamins B and characteristics of the subjects and psychological dimensions of depression.
| Variables | Depressive affect (Mean ± SD) |
P | Disturbed positive affect (Mean ± SD) |
P | Somatic complaints (Mean ± SD) |
P | Interpersonal difficulties (Mean ± SD) |
P |
|---|---|---|---|---|---|---|---|---|
| DRI of vitamin B6 | 0.208 | 0.788 | 0.081 | 0.408 | ||||
| ≥DRI (n = 338) | 3.41 ± 3.56 | 3.80 ± 2.89 | 5.08 ± 3.14 | 0.63 ± 1.05 | ||||
| <DRI (n = 64) | 4.03 ± 3.85 | 3.90 ± 3.09 | 5.86 ± 3.73 | 0.75 ± 1.10 | ||||
| DRI of vitamin B9 | 0.002 | 0.121 | 0.024 | ≤0.001 | ||||
| ≥DRI (n = 183) | 2.91 ± 2.99 | 3.57 ± 2.58 | 4.80 ± 2.86 | 0.44 ± 0.89 | ||||
| <DRI (n = 219) | 4.00 ± 3.99 | 4.02 ± 2.97 | 5.54 ± 3.51 | 0.82 ± 1.16 | ||||
| DRI of vitamin B12 | 0.300 | 0.230 | 0.184 | 0.968 | ||||
| ≥DRI (n = 264) | 3.37 ± 3.67 | 3.69 ± 2.90 | 5.05 ± 3.15 | 0.65 ± 1.13 | ||||
| <DRI (n = 138) | 3.77 ± 3.47 | 4.06 ± 2.97 | 5.51 ± 3.42 | 0.65 ± 0.93 | ||||
| Age groups | 0.001 | 0.178 | 0.065 | 0.375 | ||||
| ≤35 (n = 284) | 3.83 ± 3.84 | 3.94 ± 2.96 | 5.38 ± 3.41 | 0.68 ± 1.11 | ||||
| >35 (n = 118) | 2.72 ± 2.83 | 3.52 ± 2.82 | 4.78 ± 2.80 | 0.58 ± 0.95 | ||||
| Gender | ≤0.001 | 0.092 | 0.996 | 0.311 | ||||
| Male (n = 229) | 2.95 ± 3.21 | 3.60 ± 2.82 | 5.21 ± 3.16 | 0.60 ± 1.02 | ||||
| Female (n = 173) | 4.25 ± 3.26 | 4.10 ± 3.04 | 5.21 ± 3.38 | 0.71 ± 1.11 | ||||
| Education | ≤0.001 | 0.053 | 0.051 | 0.007 | ||||
| <20 y (n = 199) | 4.18 ± 3.76 | 4.10 ± 2.88 | 5.53 ± 3.94 | 0.79 ± 1.18 | ||||
| ≥20 y (n = 203) | 2.86 ± 3.34 | 3.54 ± 2.95 | 4.90 ± 3.08 | 0.51 ± 0.91 | ||||
| Marital status | 0.051 | 0.651 | 0.553 | 0.134 | ||||
| Married (n = 208) | 3.17 ± 3.17 | 3.88 ± 2.85 | 5.12 ± 2.96 | 0.57 ± 0.94 | ||||
| Single (n = 194) | 3.88 ± 4.00 | 3.74 ± 3.00 | 5.31 ± 3.54 | 0.73 ± 1.17 | ||||
| Current smoking | 0.676 | 0.607 | 0.980 | 0.789 | ||||
| Yes (n = 36) | 3.75 ± 2.72 | 4.06 ± 3.10 | 5.22 ± 3.23 | 0.69 ± 1.14 | ||||
| No (n = 366) | 3.49 ± 3.69 | 3.79 ± 2.91 | 5.21 ± 3.26 | 0.64 ± 1.05 | ||||
| Former smoking | 0.225 | 0.255 | 0.979 | 0.567 | ||||
| Yes (n = 16) | 2.44 ± 3.24 | 3.00 ± 3.31 | 5.19 ± 2.37 | 0.50 ± 1.10 | ||||
| No (n = 386) | 3.55 ± 3.62 | 3.85 ± 2.91 | 5.21 ± 3.28 | 0.66 ± 1.06 | ||||
| BMI | 0.006 | 0.469 | 0.455 | 0.437 | ||||
| ≤25 (n = 262) | 3.87 ± 3.82 | 3.89 ± 2.97 | 5.30 ± 3.40 | 0.68 ± 1.07 | ||||
| >25 (n = 140) | 2.84 ± 3.30 | 3.67 ± 3.02 | 5.05 ± 2.95 | 0.59 ± 1.04 | ||||
| Monthly expenses | 0.780 | 0.211 | 0.445 | 0.196 | ||||
| <800 (n = 199) | 3.56 ± 3.62 | 3.63 ± 2.75 | 5.34 ± 3.31 | 0.58 ± 0.97 | ||||
| ≥800 (n = 210) | 3.46 ± 3.60 | 3.99 ± 3.07 | 5.10 ± 3.19 | 0.71 ± 1.14 | ||||
| Close friends | 0.020 | 0.187 | 0.853 | 0.009 | ||||
| <5 (n = 220) | 3.89 ± 3.81 | 4.00 ± 3.03 | 5.24 ± 3.31 | 0.77 ± 1.15 | ||||
| ≥5 (n = 182) | 3.05 ± 3.29 | 3.60 ± 2.78 | 5.18 ± 3.19 | 0.50 ± 0.93 | ||||
| Physical activity | ≤0.001 | 0.006 | <0.001 | 0.056 | ||||
| ≥Moderate (n = 274) | 2.97 ± 3.31 | 3.54 ± 2.81 | 4.79 ± 3.06 | 0.58 ± 1.01 | ||||
| Low (n = 128) | 4.66 ± 3.95 | 4.40 ± 3.08 | 6.10 ± 3.47 | 0.80 ± 1.15 | ||||
| Living in campus | 0.340 | 0.964 | 0.052 | 0.026 | ||||
| Yes (n = 170) | 3.72 ± 4.05 | 3.82 ± 2.74 | 5.58 ± 3.49 | 0.79 ± 1.24 | ||||
| No (n = 232) | 3.36 ± 4.24 | 3.81 ± 3.05 | 4.94 ± 3.04 | 0.54 ± 0.89 |
DRI for vitamin B6: 19–50 y = 1.3 mg/d, >50 y = 1.7 mg/d; DRI for vitamin B9: 400 μg/d; DRI for vitamin B12: 2.4 μ/d (DRI 1998).
Figure 1.
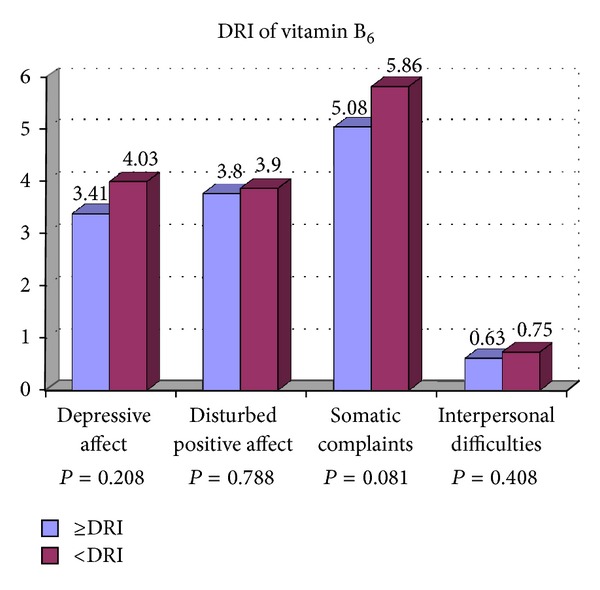
The association between vitamin B6 and psychological dimensions of depression.
Figure 2.
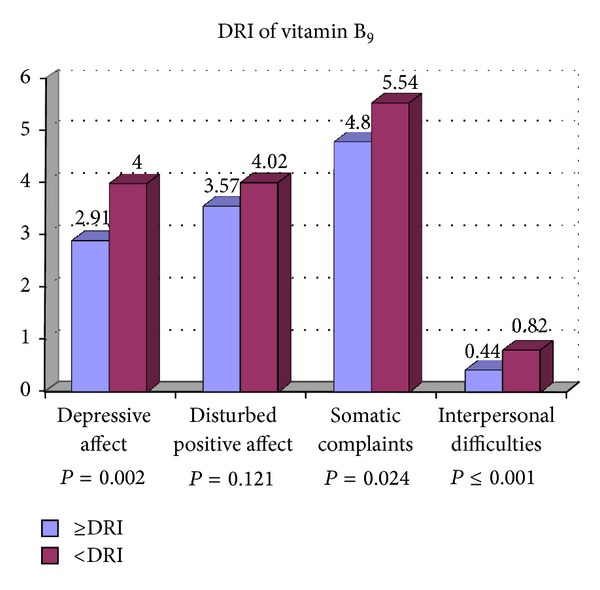
The association between vitamin B9 and psychological dimensions of depression.
Figure 3.
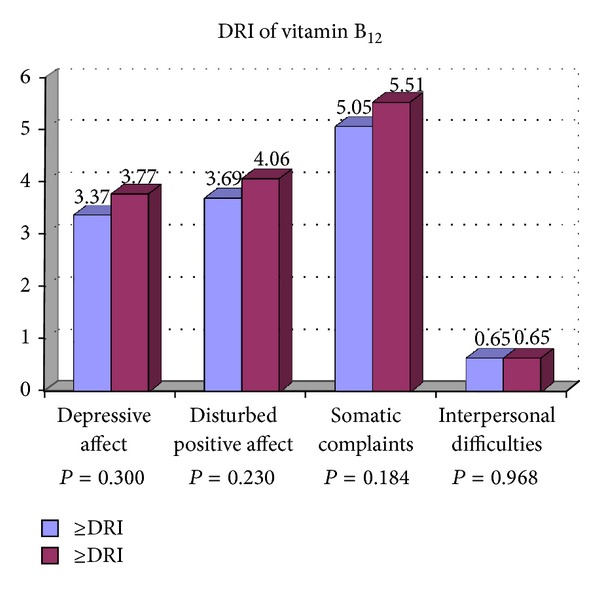
The association between vitamin B12 and psychological dimensions of depression.
Figure 4.
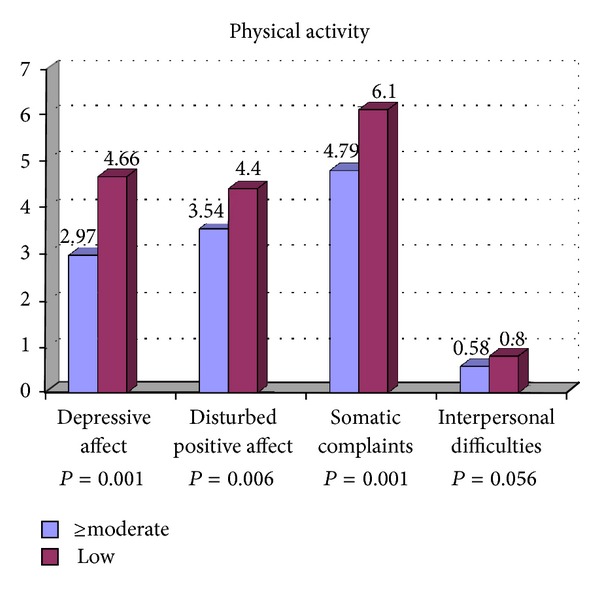
The association between physical activity and psychological dimensions of depression.
Total score of the CES-D was not associated with DRI of vitamins B6 and B12. The score of CES-D was higher in participants who consumed fewer vitamin B9 compared with participants who consumed more vitamin B9 (Table 3). This relationship total score of the CES-D and physical activity and vitamin B9 remained even after accounting for potential confounding variables such as age and sex (Table 4).
Table 3.
Association between vitamins B and overall CES-D score.
| Variables | DRI of vitamin B6 | P | DRI of vitamin B9 | P | DRI of vitamin B12 | P | |||
|---|---|---|---|---|---|---|---|---|---|
| ≥DRI (N = 338) |
<DRI (N = 64) |
≥DRI (N = 183) |
<DRI (N = 219) |
≥DRI (N = 264) |
<DRI (N = 138) |
||||
| The score of CES-D (Mean ± SD) | 12.93 ± 8.56 | 14.55 ± 9.76 | 0.175 | 11.73 ± 7.24 | 14.40 ± 9.72 | 0.002 | 12.77 ± 8.75 | 13.96 ± 8.77 | 0.186 |
Table 4.
Association between vitamins B intake and physical activity and depressive symptoms in a regression model.
| Variables | β | t | p |
|---|---|---|---|
| DRI of vitamin B9 (≥DRI versus <DRI) Physical activity (≥moderate versus low) |
−0.14 −0.21 |
2.48 4.34 |
0.014 ≤0.001 |
Adjusted for sex (male, female), age (continuous), BMI (continuous), monthly expenses (continuous), close friends (continuous), living in campus (yes versus no), smoking (yes versus no), education (continuous), marital status (yes versus no), and vitamins B6 (continuous) and B12 (continuous).
We conducted a multiple linear regression model analysis to identify the association between physical activity and dietary intake of B vitamins and the psychological dimensions of depression including depressive affect, somatic complaints, positive affect, and interpersonal difficulties. In this model, an inverse association between DRI of vitamin B9 and depressive affect, and interpersonal difficulties was found after adjusting for potential confounds, including sex, age, BMI, monthly expenses, close friends, living in campus, smoking, physical inactivity, education, marital status, and vitamins B6 and B12. This study also showed that moderate/high levels of physical activity were associated with symptoms of depression (total scores) and three subscales of the CES-D including depressive affect, positive affect, and somatic complaints (Table 5).
Table 5.
The association between DRI of vitamin B9 and physical activity and psychological dimensions of depression in a regression model.
| Variables | Depressive affect | Disturbed positive affect | Somatic complaints | Interpersonal difficulties | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | t | p | β | t | p | β | t | p | β | t | p | |
| Vitamin B9 | −0.13 | 2.48 | 0.014 | −0.07 | 1.18 | 0.239 | −0.08 | 1.40 | 0.163 | −0.21 | 3.83 | ≤0.001 |
| Physical activity | −0.22 | 4.51 | ≤0.001 | −0.12 | 2.43 | 0.016 | −0.19 | 3.74 | ≤0.001 | −0.10 | 1.95 | 0.052 |
Adjusted for sex (male, female), age (continuous), BMI (continuous), monthly expenses (continuous), close friends (continuous), living in campus (yes versus no), smoking (yes versus no), education (continuous), marital status (yes versus no), vitamin B6 (continuous), vitamin B12 (continuous), vitamin B9 (≥DRI versus <DRI), and physical activity (≥moderate versus low).
4. Discussion
The present cross-sectional study determined that inverse relationships existed between intake of vitamin B9 and the total score of CES-D and two subscales of the CES-D-score including depressive affect and interpersonal difficulties among university students. This study also showed that moderate/high levels of physical activity were inversely and significantly associated with symptoms of depression (total scores) and three subscales of the CES-D including depressive affect, positive affect, and somatic complaints. The associations persisted even after adjusting for sex, age, BMI, monthly expenses, close friends, living in campus, smoking (current and former), education, marital status, and vitamins B6 and B12.
To the best our knowledge this is the first study to show an association between physical activity and vitamin B9 intake and the psychological dimensions of depression among Iranian university students. Our results and methodology used in this cross-sectional study is novel and unique because no study has investigated the association between depression/the psychological dimensions of depression and vitamins B and the levels of physical activity among university students.
An inverse association between depressive symptoms and vitamin B9 among our population can be supported by previous cross-sectional studies. A cross-sectional study documented that low intake of vitamin B9 was inversely associated with depression among currently smoking men and men with low anxiety levels [35]. A second cross-sectional study also showed a significant association between lower depressive symptoms and higher dietary intakes of vitamin B9 in middle-aged (42–60 y) men; the association was not found for other vitamins such as vitamins B6 and B12 [36]. A third cross-sectional study found an inverse association between vitamin B9 intake and prevalence of depression among men as well as an inverse association between depression and vitamin B12 among women; mean age was 41 and 34 years for men and women, respectively [37]. Conversely, the fourth cross-sectional study in men, aged 70–90 years, does not detected a relationship between symptoms of depression and vitamins B6, B9, and B12 [38]. Despite the results described above, it has been documented that vitamins B6 and B12 are involved in the synthesis of monoamines neurotransmitters in the central nervous system, such as dopamine, serotonin, norepinephrine, and epinephrine [39, 40].
The mechanism underlying the relationship between depression and vitamin B9 is unknown, but it can partly explain the role of vitamin B9 in the regulation of homocysteine. It was found that hyperhomocysteinemia is associated with depressive disorders [41]. For instance, research showed that more than 50% of depressed patients have hyperhomocysteinemia [42]. Homocysteine can increase oxidative stress, apoptosis, and DNA strand breakage; it is also directly toxic to neurons and blood vessels [43], and the vascular system of depressed patients may be destroyed by homocysteine. Three vitamins including B6, B9, and B12 are involved in the regulation of homocysteine [43, 44]. However, it has been indicated that hyperhomocysteinemia was more common in patients with vitamin B9 deficiencies [45]. One possible explanation involves dysfunction of metabolic pathways that require S-adenosylmethionine (SAM), which serves as a methyl donor in numerous biochemical processes including those important for neurological function. Under homeostatic conditions, SAM is formed from methionine but is subsequently metabolized to homocysteine after serving as a methyl donor. Vitamin B9 is required to donate one-carbon groups to homocysteine to recycle it to methionine for continued SAM formation. Deficiencies in vitamin B9 lead to accumulation of homocysteine, decreased SAM, and therefore impairment of neurological function [46–49].
Vitamin B9 deficiency may increase symptoms of depression by impairing the synthesis of tetrahydrobiopterin (BH4). BH4 is a compulsory cofactor for the three aromatic amino acids (tryptophan, phenylalanine, and tyrosine) hydroxylase enzymes. The three aromatic amino acids produce several neurotransmitters including serotonin (5-hydroxytryptamine, 5-TH), dopamine, melatonin, norepinephrine, and epinephrine [50]. Deregulation of this reaction may lead to abnormalities in the synthesis of mentioned neurotransmitters [51, 52], as well as depression [50].
The present study found an inverse association between depressive symptoms and moderate/high levels of physical activity among Iranian university students. Previous cross-several studies have shown that less depressive symptoms are associated with physically active individuals [28, 29]. The dose-response correlation between differing levels of physical activity and depression symptoms is not very clear. In only a few studies, individuals were categorized according to their level of physical activity into 2 or more groups to investigate a dose gradient relationship with depressive symptoms. A cross-sectional study conducted on adolescents found that total amount of physical activity was associated with symptoms of depression; however, moderate and vigorous physical activity was not independently related to depressive symptoms [28]. In contrast, a cross-sectional study concluded that high levels of physical activity were associated with less depression as compared to low level of physical activity [53].
There are several proposed mechanisms for the relationship between physical activity and depression symptoms. The effects of physical activity on depression mechanisms can be explained with both psychological and physiological/biochemical theories. It is to be expected that a combination of mechanisms influences the link between physical activity and depression [54]. Several factors related to depressive symptoms such as the sense of enjoyment, fulfillment, and social interactions can be provided by physical activities during leisure time [55]. One of the most frequently proposed mechanisms for the antidepressant effect of physical activity is cognitive-behavioral hypothesis [56]. The hypothesis suggests that negative feelings that may cause depression and activity releases may block these conditions. Physical activity seems to increase skill mastery, self-efficacy, feelings of success, and locus of control, and a lack of these factors is associated with depression symptoms [54]. Also, the time out/distraction hypothesis suggests that physical activity helps individuals overcome their daily worries and thus reduces depression symptoms [54, 56].
The effect of physical activity as an antidepressant can be further explained by the amine hypothesis. Individuals with depression have lower levels of monoamine neurotransmitters, including serotonin [57], dopamine, and norepinephrine [58]. Exercise can increase the level of these neurotransmitters [54]; this hypothesis is supported by antidepressant drugs such as tricyclic monoamine oxidase inhibitors and electroconvulsive therapy, all of which raise the level of amine transmission [56]. Furthermore, according to the endorphin hypothesis, exercise influences depression with an increased discharge of β-endorphins. Endorphins have a positive effect on mood and improve sense of happiness [59].
Physical activity can also modify several risk factors related to depressive symptoms. Many biological risk factors such as glucose intolerance, inflammation, and vascular dysfunction can be improved by physical activity; these factors have shown to trigger possibly important mechanisms leading to depression [60].
Our study found that women have higher levels of depressive symptoms as well as high consumption of vitamins B6 and B9 compared to men. A number of studies have shown that women have higher prevalence of depression versus men [56, 61]; however, the mechanism remains unclear. Researcher are trying to explain these differences through biological, psychological, genetic, and social factors [62, 63]. Higher levels of two psychological factors including interpersonal orientation and rumination among women were shown to be associated with the higher levels of depression [63]. Also, it has been suggested that higher rates of sexual harassment, poverty, chronic strain, and child abuse among females compared to men may cause of higher level of depressive symptoms [63]. In the matter of biological factors, the serotoninergic activity in the brain can be changed with female gonadal hormones, although the mechanism is not clear, but it is possible that gonadal hormones alter the regulation of the level of monoamines such as serotonin [64].
5. Limitation of Study
We controlled for several important variables linked with depression that may confound interpretation of the data, including sex, age, BMI, monthly expenses, close friends, living in campus, smoking habit, education, physical activity, and marital status. However, this study was a cross-sectional design, and therefore causality of dietary intake of vitamin B9 or physical activity and depressive symptoms cannot be determined.
6. Conclusion
The results of this study suggest that physical activity and intake of vitamin B9 may modulate the total score of CES-D and psychological dimensions of depressive symptoms in university students.
Conflict of Interests
The authors declare that they have no conflict interests.
Acknowledgments
The author would like to acknowledge all Iranian students in Malaysia, especially the students who were participants in this study. He also wishes to express his sincerest appreciation to Dr. Siavash Yari for guiding him and for encouraging and supporting him in this study.
References
- 1.Yeung A. Effects of qigong on depression: a systemic review. Evidence-Based Complementary and Alternative Medicine. 2013;2013:8 pages. doi: 10.1155/2013/134737.134737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yary T, Soleimannejad K, Abd Rahim F, et al. Contribution of diet and major depression to incidence of acute myocardial infarction (AMI) Lipids in Health and Disease. 2010;9, article 133 doi: 10.1186/1476-511X-9-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stordal E, Bjartveit Krüger M, Dahl NH, Krüger O, Mykletun A, Dahl AA. Depression in relation to age and gender in the general population: the Nord-Trøndelag health study (HUNT) Acta Psychiatrica Scandinavica. 2001;104(3):210–216. doi: 10.1034/j.1600-0447.2001.00130.x. [DOI] [PubMed] [Google Scholar]
- 4.Riolo SA, Nguyen TA, Greden JF, King CA. Prevalence of depression by race/ethnicity: findings from the national health and nutrition examination survey III. The American Journal of Public Health. 2005;95(6):998–1000. doi: 10.2105/AJPH.2004.047225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stuart MJ, Baune BT. Depression and type 2 diabetes: inflammatory mechanisms of a psychoneuroendocrine co-morbidity. Neuroscience and Biobehavioral Reviews. 2012;36(1):658–676. doi: 10.1016/j.neubiorev.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Parks J, Svendsen D, Singer P, Foti ME, Mauer B. Morbidity and Mortality in People with Serious Mental Illness. Alexandria, VA, USA: National Association of State Mental Health Program Directors Medical Directors Council; 2006. [Google Scholar]
- 7.Angst F, Stassen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow-up over 34–38 years. Journal of Affective Disorders. 2002;68(2-3):167–181. doi: 10.1016/s0165-0327(01)00377-9. [DOI] [PubMed] [Google Scholar]
- 8.Mystakidou K, Tsilika E, Parpa E, Katsouda E, Galanos A, Vlahos L. Assessment of anxiety and depression in advanced cancer patients and their relationship with quality of life. Quality of Life Research. 2005;14(8):1825–1833. doi: 10.1007/s11136-005-4324-3. [DOI] [PubMed] [Google Scholar]
- 9.Giese-Davis J, Collie K, Rancourt KM, Neri E, Kraemer HC, Spiegel D. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: a secondary analysis. Journal of Clinical Oncology. 2011;29(4):413–420. doi: 10.1200/JCO.2010.28.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan A, Lucas M, Sun Q, et al. Increased mortality risk in women with depression and diabetes mellitus. Archives of General Psychiatry. 2011;68(1):42–50. doi: 10.1001/archgenpsychiatry.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young CB, Fang DZ, Zisook S. Depression in Asian-American and Caucasian undergraduate students. Journal of Affective Disorders. 2010;125(1–3):379–382. doi: 10.1016/j.jad.2010.02.124. [DOI] [PubMed] [Google Scholar]
- 12.Yary T, Aazami S. Dietary intake of zinc was inversely associated with depression. Biological Trace Element Research. 2012;145(3):286–290. doi: 10.1007/s12011-011-9202-y. [DOI] [PubMed] [Google Scholar]
- 13.Furr SR, Westefeld JS, McConnell GN, Jenkins JM. Suicide and depression among college students: a decade later. Professional Psychology. 2001;32(1):97–100. [Google Scholar]
- 14.Buchanan JL. Prevention of depression in the college student population: a review of the literature. Archives of Psychiatric Nursing. 2012;26(1):21–42. doi: 10.1016/j.apnu.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Hovey JD, Kim SE, Seligman LD. The influences of cultural values, ethnic identity, and language use on the mental health of Korean American college students. Journal of Psychology. 2006;140(5):499–511. doi: 10.3200/JRLP.140.5.499-511. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed I, Banu H, Al-Fageer R, Al-Suwaidi R. Cognitive emotions: depression and anxiety in medical students and staff. Journal of Critical Care. 2009;24(3):e1–e7. doi: 10.1016/j.jcrc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Sallis J, Owen N. Physical Activity and Behavioral Medicine. SAGE Publications; 1998. [Google Scholar]
- 18.Dimeo F, Bauer M, Varahram I, Proest G, Halter U. Benefits from aerobic exercise in patients with major depression: a pilot study. The British Journal of Sports Medicine. 2001;35(2):114–117. doi: 10.1136/bjsm.35.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunn AL, Trivedi MH, O’Neal HA. Physical activity dose-response effects on outcomes of depression and anxiety. Medicine and Science in Sports and Exercise. 2001;33(6):S587–S597. doi: 10.1097/00005768-200106001-00027. [DOI] [PubMed] [Google Scholar]
- 20.Wyshak G. Women’s college physical activity and self-reports of physician-diagnosed depression and of current symptoms of psychiatric distress. Journal of Women’s Health and Gender-Based Medicine. 2001;10(4):363–370. doi: 10.1089/152460901750269689. [DOI] [PubMed] [Google Scholar]
- 21.Goodwin RD. Association between physical activity and mental disorders among adults in the United States. Preventive Medicine. 2003;36(6):698–703. doi: 10.1016/s0091-7435(03)00042-2. [DOI] [PubMed] [Google Scholar]
- 22.Teychenne M, Ball K, Salmon J. Physical activity and likelihood of depression in adults: a review. Preventive Medicine. 2008;46(5):397–411. doi: 10.1016/j.ypmed.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Yary T, Aazami S. The association between polyunsaturated fatty acids and depression among Iranian postgraduate students in Malaysia. Lipids in Health and Disease. 2011;10, article 151 doi: 10.1186/1476-511X-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yary T, Aazami S. The association between polyunsaturated fatty acids and depression among Iranian postgraduate students in Malaysia. Lipids in Health and Disease. 2011;10, article 151 doi: 10.1186/1476-511X-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Astorg G, Couthouis A, deCourcy GP. Association of folate intake with the occurrence of depressive episodes in middle-aged French men and women. British Journal of Nutrition. British Journal of Nutrition. 2008;100(1):p. 7. doi: 10.1017/S0007114507873612. [DOI] [PubMed] [Google Scholar]
- 26.Sánchez-Villegas A, Doreste J, Schlatter J, Pla J, Bes-Rastrollo M, Martínez-González MA. Association between folate, vitamin B6 and vitamin B12 intake and depression in the SUN cohort study. Journal of Human Nutrition and Dietetics. 2009;22(2):122–133. doi: 10.1111/j.1365-277X.2008.00931.x. [DOI] [PubMed] [Google Scholar]
- 27.Tolmunen T, Voutilainen S, Hintikka J, et al. Dietary folate and the risk of depression in finnish middle-aged men. The Journal of Nutrition. 2003;133(10):3233–3236. doi: 10.1093/jn/133.10.3233. [DOI] [PubMed] [Google Scholar]
- 28.Wiles NJ, Haase AM, Lawlor DA, Ness A, Lewis G. Physical activity and depression in adolescents: cross-sectional findings from the ALSPAC cohort. Social Psychiatry and Psychiatric Epidemiology. 2012;47:1023–1033. doi: 10.1007/s00127-011-0422-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen LJ, Stevinson C, Ku PW, Chang YK, Chu DC. Relationships of leisure-time and non-leisure-time physical activity with depressive symptoms: a population-based study of Taiwanese older adults. International Journal of Behavioral Nutrition and Physical Activity. 2012;9, article 28 doi: 10.1186/1479-5868-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:p. 385. [Google Scholar]
- 31.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. The American Journal of Epidemiology. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 32.Kimiagar SM, Ghaffarpour M, Houshiar-Rad A, Hormozdyari H, Zellipour L. Food consumption pattern in the Islamic Republic of Iran and its relation to coronary heart disease. Eastern Mediterranean Health Journal. 1998;4(3):539–547. [Google Scholar]
- 33.Romieu I, Parra S, Hernández JF, Madrigal H, Willett W, Hernández M. Questionnaire assessment of antioxidants and retinol intakes in Mexican women. Archives of Medical Research. 1999;30(3):224–239. doi: 10.1016/s0188-0128(99)00017-2. [DOI] [PubMed] [Google Scholar]
- 34.Mikkelsen SS, Tolstrup JS, Flachs EM, Mortensen EL, Schnohr P, Flensborg-Madsen T. A cohort study of leisure time physical activity and depression. Preventive Medicine. 2010;51(6):471–475. doi: 10.1016/j.ypmed.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Sánchez-Villegas A, Doreste J, Schlatter J, Pla J, Bes-Rastrollo M, Martínez-González MA. Association between folate, vitamin B6 and vitamin B12 intake and depression in the SUN cohort study. Journal of Human Nutrition and Dietetics. 2009;22(2):122–133. doi: 10.1111/j.1365-277X.2008.00931.x. [DOI] [PubMed] [Google Scholar]
- 36.Tolmunen T, Hintikka J, Ruusunen A, et al. Dietary folate and the risk of depression in finnish middle-aged men: a prospective follow-up study. Psychotherapy and Psychosomatics. 2004;73(6):334–339. doi: 10.1159/000080385. [DOI] [PubMed] [Google Scholar]
- 37.Sánchez-Villegas A, Henríquez P, Bes-Rastrollo M, Doreste J. Mediterranean diet and depression. Public Health Nutrition. 2006;9:1104–1109. doi: 10.1017/S1368980007668578. [DOI] [PubMed] [Google Scholar]
- 38.Kamphuis MH, Geerlings MI, Grobbee DE, Kromhout D. Dietary intake of B6-9-12 vitamins, serum homocysteine levels and their association with depressive symptoms: the Zutphen Elderly Study. European Journal of Clinical Nutrition. 2008;62(8):939–945. doi: 10.1038/sj.ejcn.1602804. [DOI] [PubMed] [Google Scholar]
- 39.Baldewicz TT, Goodkin K, Blaney NT, et al. Cobalamin level is related to self-reported and clinically rated mood and to syndromal depression in bereaved HIV-1+ and HIV-1− homosexual men. Journal of Psychosomatic Research. 2000;48(2):177–185. doi: 10.1016/s0022-3999(99)00108-7. [DOI] [PubMed] [Google Scholar]
- 40.Murakami K, Mizoue T, Sasaki S, et al. Dietary intake of folate, other B vitamins, and ω-3 polyunsaturated fatty acids in relation to depressive symptoms in Japanese adults. Nutrition. 2008;24(2):140–147. doi: 10.1016/j.nut.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 41.Tiemeier H, van Tuijl HR, Hofman A, Meijer J, Kiliaan AJ, Breteler MMB. Vitamin B12, folate, and homocysteine in depression: the Rotterdam study. The American Journal of Psychiatry. 2002;159(12):2099–2101. doi: 10.1176/appi.ajp.159.12.2099. [DOI] [PubMed] [Google Scholar]
- 42.Bottiglieri T, Laundy M, Crellin R, Toone BK, Carney MWP, Reynolds EH. Homocysteine, folate, methylation, and monoamine metabolism in depression. Journal of Neurology Neurosurgery and Psychiatry. 2000;69(2):228–232. doi: 10.1136/jnnp.69.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Folstein M, Liu T, Peter I, et al. The homocysteine hypothesis of depression. The American Journal of Psychiatry. 2007;164(6):861–867. doi: 10.1176/ajp.2007.164.6.861. [DOI] [PubMed] [Google Scholar]
- 44.Bottiglieri T. Homocysteine and folate metabolism in depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2005;29(7):1103–1112. doi: 10.1016/j.pnpbp.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 45.Stanger O, Herrmann W, Pietrzik K, et al. DACH-LIGA homocystein (German, Austrian and Swiss homocysteine society): consensus paper on the rational clinical use of homocysteine, folic acid and B-vitamins in cardiovascular and thrombotic diseases: Guidelines and recommendations. Clinical Chemistry and Laboratory Medicine. 2003;41(11):1392–1403. doi: 10.1515/CCLM.2003.214. [DOI] [PubMed] [Google Scholar]
- 46.Abou-Saleh MT, Coppen A. Folic acid and the treatment of depression. Journal of Psychosomatic Research. 2006;61(3):285–287. doi: 10.1016/j.jpsychores.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Coppen A, Bolander-Gouaille C. Treatment of depression: time to consider folic acid and vitamin B12 . Journal of Psychopharmacology. 2005;19(1):59–65. doi: 10.1177/0269881105048899. [DOI] [PubMed] [Google Scholar]
- 48.Miller AL. The methylation, neurotransmitter, and antioxidant connections between folate and depression. Alternative Medicine Review. 2008;13(3):216–226. [PubMed] [Google Scholar]
- 49.Zhao G, Ford ES, Li C, Greenlund KJ, Croft JB, Balluz LS. Use of folic acid and vitamin supplementation among adults with depression and anxiety: a cross-sectional, population-based survey. Nutrition Journal. 2011;10(1, article 102) doi: 10.1186/1475-2891-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller AL. The methylation, neurotransmitter, and antioxidant connections between folate and depression. Alternative Medicine Review. 2008;13(3):216–226. [PubMed] [Google Scholar]
- 51.Coppen A, Swade C, Jones SA, Armstrong RA, Blair JA, Leeming RJ. Depression and tetrahydrobiopterin: the folate connection. Journal of Affective Disorders. 1989;16(2-3):103–107. doi: 10.1016/0165-0327(89)90062-1. [DOI] [PubMed] [Google Scholar]
- 52.Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: a randomised, placebo controlled trial. Journal of Affective Disorders. 2000;60(2):121–130. doi: 10.1016/s0165-0327(00)00153-1. [DOI] [PubMed] [Google Scholar]
- 53.Ruuskanen JM, Ruoppila I. Physical activity and psychological well-being among people aged 65 to 84 years. Age and Ageing. 1995;24(4):292–296. doi: 10.1093/ageing/24.4.292. [DOI] [PubMed] [Google Scholar]
- 54.Craft LL, Perna FM. The benefits of exercise for the clinically depressed. Primary Care Companion to the Journal of Clinical Psychiatry. 2004;6:104–111. doi: 10.4088/pcc.v06n0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ku PW, Fox KR, Chen LJ, Chou P. Physical activity and depressive symptoms in older adults: 11-year follow-up. The American Journal of Preventive Medicine. 2012;42(4):355–362. doi: 10.1016/j.amepre.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 56.Kendler KS, Thornton LM, Prescott CA. Gender differences in the rates of exposure to stressful life events and sensitivity to their depressogenic effects. The American Journal of Psychiatry. 2001;158(4):587–593. doi: 10.1176/appi.ajp.158.4.587. [DOI] [PubMed] [Google Scholar]
- 57.Robinson OJ, Cools R, Crockett MJ, Sahakian BJ. Mood state moderates the role of serotonin in cognitive biases. Journal of Psychopharmacology. 2010;24(4):573–583. doi: 10.1177/0269881108100257. [DOI] [PubMed] [Google Scholar]
- 58.Meyer JH, Krüger S, Wilson AA, et al. Lower dopamine transporter binding potential in striatum during depression. NeuroReport. 2001;12(18):4121–4125. doi: 10.1097/00001756-200112210-00052. [DOI] [PubMed] [Google Scholar]
- 59.Daley AJ, Winter H, Grimmett C, McGuinness M, McManus R, MacArthur C. Feasibility of an exercise intervention for women with postnatal depression: a pilot randomised controlled trial. The British Journal of General Practice. 2008;58(548):178–183. doi: 10.3399/bjgp08X277195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamer M, Molloy GJ, de Oliveira C, Demakakos P. Leisure time physical activity, risk of depressive symptoms, and inflammatory mediators: the English Longitudinal Study of Ageing. Psychoneuroendocrinology. 2009;34(7):1050–1055. doi: 10.1016/j.psyneuen.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 61.Cyranowski JM, Frank E, Young E, Shear MK. Adolescent onset of the gender difference in lifetime rates of major depression: a theoretical model. Archives of General Psychiatry. 2000;57(1):21–27. doi: 10.1001/archpsyc.57.1.21. [DOI] [PubMed] [Google Scholar]
- 62.Veijola J, Puukka P, Lehtinen V, Moring J, Lindholm T, Väisänen E. Sex differences in the association between childhood experiences and adult depression. Psychological Medicine. 1998;28(1):21–27. doi: 10.1017/s0033291797006089. [DOI] [PubMed] [Google Scholar]
- 63.Goodwin RD, Gotlib IH. Gender differences in depression: the role of personality factors. Psychiatry Research. 2004;126(2):135–142. doi: 10.1016/j.psychres.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 64.Yary T, Aazami S, Soleimannejad K. Dietary intake of magnesium may modulate depression. Biological Trace Element Research. 2013;151:324–329. doi: 10.1007/s12011-012-9568-5. [DOI] [PubMed] [Google Scholar]


