Abstract
Nanotechnology has been applied to dental materials as an innovative concept for the development of materials with better properties and anticaries potential. In this review we discuss the current progress and future applications of functional nanoparticles incorporated in dental restorative materials as useful strategies to dental caries management. We also overview proposed antimicrobial and remineralizing mechanisms. Nanomaterials have great potential to decrease biofilm accumulation, inhibit the demineralization process, to be used for remineralizing tooth structure, and to combat caries-related bacteria. These results are encouraging and open the doors to future clinical studies that will allow the therapeutic value of nanotechnology-based restorative materials to be established.
Keywords: nanoparticles, dental materials, nanotechnology, dental caries
The problem of combating caries
Dental caries (see Glossary) remains the most common and widespread biofilm-dependent oral disease, resulting in the destruction of tooth structure by acidic attack from cariogenic bacteria, such as Streptococcus mutans, Streptococcus sobrinus, and Lactobacillus spp.. These bacteria are present in aggregates of microorganism cells attached to each other and to a tooth surface (i.e., oral biofilm or dental plaque) [1,2]. Thus, caries is a site-specific and dynamic disease resulting from the imbalance in the physiologic equilibrium between mineral ions present in the tooth structure and dental plaque fluid represented by demineralization and remineralization processes [1]. Dips in the pH (<5.5) of the oral biofilm due to the action of bacterial acids can cause the tooth to lose calcium and phosphate (from enamel and dentin) causing tooth demineralization. In the remineralization process calcium and phosphate lost by the enamel may be deposited into the tooth from dental plaque fluid or by direct action of salivary calcium and phosphate soon after the biofilm is removed by toothbrushing [3]. However, the amount of ions gained is lower than the amount lost and the net result is a small mineral loss. If biofilm accumulation and/or acid production are not inhibited, enamel mineral loss will continue by repeated events of mineral dissolution (demineralization). This will eventually exceed the capacity of oral fluids to repair mineral loss, and the disease will show its clinical signs, named caries or carious lesions [4]. These lesions range from white spot lesions (early caries lesions with the appearance of white chalky areas on enamel) to cavities indentin. Consequently, the controlof dental cariesdiseases is traditionally centered on mechanical or nonspecific control of the dental plaque, because this is the causative factor.
Significant progress has been made in reducing and controlling dental caries using fluoride [4]. Fluoride can inhibit demineralization and promote remineralization of hard dental tissues. Nevertheless, the limited penetration of fluoride in dental plaque may restrict its inhibitory effects in residual plaque deposits in inaccessible stagnation sites [5]. Consequently, prevention and management of caries lesions at proximal surfaces (areas where the surface of one tooth touches the surface of another) and around restorations are still challenges for dental caries research [6].
Recurrent caries are lesions at the margin of existing restorations that become carious. The clinical diagnosis of recurrent caries invariably leads to replacement of the affected restoration. The replacement of the failed restorations [7] accounts for around 60% of all restorations performed in the USA each year at an annual cost of over US$5 billion [8]. Prevention of recurrent caries, as with other types of dental caries, usually requires: (i) interference with the metabolism of caries-related bacteria and/or inhibition of the growth of bacteria in dental plaque around restorations; or (ii) the reduction of demineralization and/or increase of remineralization of the dental hard tissues. Thus, to date, scientists have focused on adding anticaries substances in restorative materials.
Functional materials or structures at the nanometer scale (0.1–100.0 nm) can be used to control the formation of cariogenic oral biofilms: nanoparticles can deliver antibiotics and bioactive compounds [9]. Dental plaque contains a vacuum and channels that occasionally extend completely through the biofilm biomass to the underlying dental surface, influencing the transfer of particles through bio-films. Within this context, the nanoparticles are potentially useful because their surface charge, degree of hydrophobicity, the ratio of surface area to biofilm mass, and the ability of the particles to adsorb or be collected on the surface of the biofilm can be changed [9].
Composite is the most widely used and versatile dental material used for restoring dental cavities, especially because of its capacity to mimic natural tooth appearance. It is a multiphase substance composed of four major components: resin (organic polymer matrix); filler (inorganic) particles; coupling agent (silane); and the initiator-accelerator of polymerization [10]. Despite its significant improvement over the years, drawbacks related to mechanical properties (low strength, fracture toughness, and wear), microleakage (bacterial penetration along the tooth-restoration interface), and shrinkage during the process of reacting monomer molecules to form polymer chains (polymerization) still remain. These conditions are closely related to the primary reasons given for replacement of dental composites: recurrent caries and fracture [10].
Composite resins that incorporate nanoparticles and nanoclusters with a broad particle distribution provide a higher filler load, desirable handling characteristics, and better optical and/or physical properties than conventional hybrid composites [11]. Nanofilled composites have better polishing characteristics because nanoparticles may result in wear to surfaces with lower defects over time [12], reflecting on changes in dental plaque formed over restorations, because surface roughness impacts plaque accumulation [13]. However, most growth of dental plaque occurs by cell division within the biofilm, instead of co-aggregation on the surface where the biofilm grows, thus restricting the anticaries effects of nonbioactive nanoparticles. To the best of our knowledge, no study has showed increased caries inhibition around restorations carried out with nanofilled composites without bioactive particles when compared to nanohybrid composites.
Nanotechnology has helped to address negative effects on curing reaction kinetics, conversion and mechanical strength properties, poor functional performance and aesthetics, unsatisfactory biocompatibility, and difficult workability, which comprise difficulties in incorporations of antibacterial and/or remineralizing agents in conventional size in direct restorative materials [14]. Nanomaterials provide superior antimicrobial activity and display comparable physical properties when compared with conventional materials, this is probably because of the small size and high surface area of the nanoparticles, capable of releasing high levels of ions at a low filler level, thereby enabling the incorporation of reinforcing (but nonreleasing) fillers in the same material.
In this review, we focus on recent approaches in nano-technology to combat caries. Agents such as silver, zinc oxide, calcium phosphate, calcium fluoride, quaternary ammonium polyethylenimine, and nanohydroxyapatite and/or nanofluorohydroxyapatite are incorporated into restorative materials such as composite resins, glass ionomer, and adhesive systems. Dental studies pertinent to key aspects of this review were selected.
Nanotechnology-based strategies for dental caries management
Antibacterial approach
Nanoparticles of metals (i.e., silver and zinc) and antimicrobial polymers have gained significant interest over the years due to their remarkable antimicrobial properties [15]. The excellent antibacterial effect of these nanostructured agents is mainly attributed to the high surface area to volume ratio enabling greater presence of atoms on the surface, which provides maximum contact with the environment. In addition, the small size of these particles makes penetration through cell membranes easier, thus affecting intracellular processes resulting in higher reactivity and antimicrobial activity [16]. This is particularly valuable because microorganisms in biofilms are more resistant to antibacterial agents than planktonic pathogens and much more concentrated biocides may be required for effective treatment [17]. Therefore, antibacterial agents that have broad-spectrum activities and some concerns about dose-related safety issues and toxicity toward host cells have their biologic proprieties re-explored and improved by nano-technology. In the following section, relevant antibacterial agents described in literature data, their proposed mechanisms, and the recent applicability in direct restorative materials were considered.
Silver nanoparticles
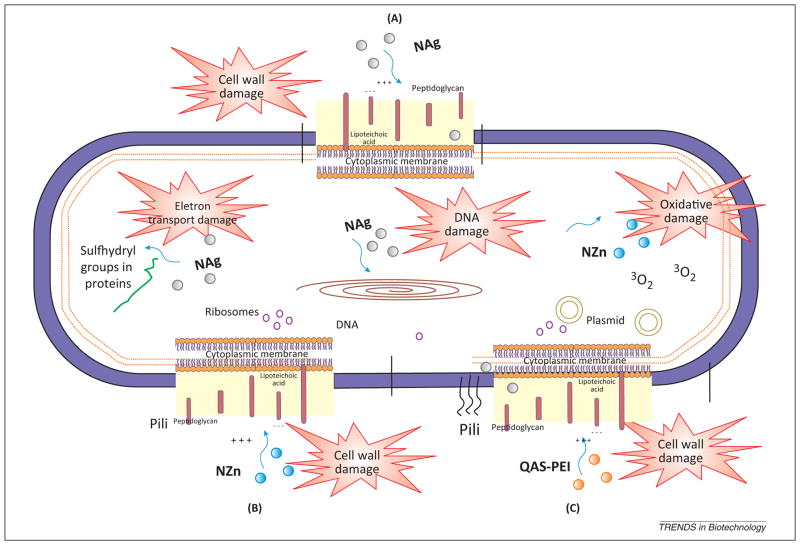
Silver nanoparticles (NAg) have been used in a wide range of antimicrobial applications such as wound dressings, implant coatings, and others [18]; nevertheless their mode of action has not been clearly elucidated. The antimicrobial action of silver may be proportional to the amount of released bioactive silver ions (Ag+) and their interaction with bacterial cell membranes [19]. A larger surface area of the NAg allows a larger amount of atoms to interact with their surroundings, thus the bactericidal effect of NAg is size dependent, where smaller NAg are more potent [20,21]. Silver ions provide the bactericidal effect by interactions with the peptidoglycan cell wall and the plasma membrane [17]. Silver ions prevent bacterial DNA replication [20] by interacting with the exposed sulfhydryl groups in bacterial proteins, especially with the enzymes involved in vital cellular processes such as the electron transport chain (Figure 1) [21].
Figure 1.
Schematic representation of a bacterial cell showing the components targeted by different antibacterial agents incorporated in dental materials. (A) Silver nanoparticles (NAg): NAg have been incorporated in restorative materials to combat the cariogenic bacteria colonization in the marginal gaps and on their surfaces. (B) Zinc oxide nanoparticles (NZn): the primary cause of the antibacterial function of NZn is credited to the disruption of cell membrane activity. (C) Quaternary ammonium polyethylenimine (QAS-PEI) nanoparticles: the mechanism of action may be related to absorption of positively charged polymers onto negatively charged cell surfaces of the bacteria. This process is thought to be responsible for the increase of cell permeability and may disrupt the cell membranes [41].
NAg have been incorporated in dental materials to combat cariogenic bacterial colonization in the marginal gaps and on the material surfaces. NAg exhibit antibacterial effects against a large number of bacterial species, including S. mutans and Lactobacillus spp. [22], when added to adhesive for bonding orthodontics brackets [23] and to a dental composite [24]. In vitro studies on the effects of a composite containing 2.7 nm NAg well-dispersed in the resin matrix (Figure 2) [25,26] showed a high antibacterial efficacy without significantly compromising composite color or mechanical properties. The relative self-diffusion coefficients of NAg in a biofilm decreased exponentially with the square of the radius of the nanoparticle [27]. The effective pore size of biofilms was observed in the nanoparticle size range, strongly suggesting that the mobility of nanoparticles > 50 nm will be greatly reduced with little penetration in the biofilm. Another relevant factor is the charge of the nanoparticles, for a dense bacterial biofilm a greater than predicted decrease in the self-diffusion coefficient was observed for the negatively charged NAg. Previous studies suggested that Ag-containing resin composites had a long-lasting antibacterial activity due to the sustained Ag ion release [28]. A Ag-containing dental composite was shown to inhibit S. mutans growth when tested over a 6-month duration [29].
Figure 2.
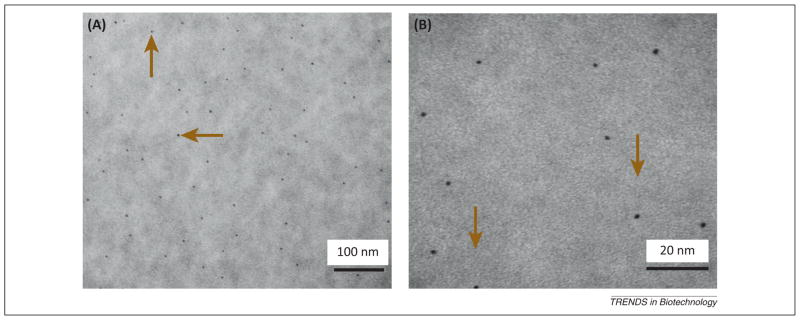
Representative transmission electron microscopy (TEM) micrographs representing the size and dispersion of silver nanoparticles (NAg) in a resin matrix: (A) lower and (B) higher magnifications. Silver 2-ethylhexanoate salt was dissolved in 2-(tert-butylamino) ethyl methacrylate and incorporated into a matrix resin at a silver salt mass fraction of 0.08% in the resin. The NAg were formed in the resin by simultaneous reduction of the silver salt and photopolymerization of the dimethacrylates. The average size was 2.7 ±0.6 nm. Arrows indicate the silver nanoparticles, which were well dispersed in the resin with minimal appearance of nanoparticle aggregates. Adapted, with permission, from [25].
The concurrent reduction of Ag ions and polymerization of dimethacrylate-based polymers is a promising method for incorporating NAg into dental materials. In this process, Ag salt is previously dissolved as a monomer and then mixed with a composite or adhesive system. The reduction of Ag salt to Ag nanoparticles in the resin in situ avoided the difficulty of mixing preformed Ag nanoparticles that could cause agglomeration [25]. This incorporation was performed in composites and adhesive systems rendering antibacterial effects on oral biofilms without impacting their main mechanical proprieties [26,30] and bond strength to dental structures [30]. Besides bonding to enamel, dental adhesive systems need to bond to dentin, which is the major component of teeth that consists of microscopic channels, called dentinal tubules, that lead to and contact the pulp. These tubules contain fluid and cellular structures and often suffer invasion by pathogenic bacteria. Here, small NAg could flow into dentinal tubules to kill residual bacteria inside the tubules [31].
NAg incorporation in dental materials can cause cosmetic changes of tooth-colored materials; however, 0.5–1.0% concentrations present the antibacterial properties of silver and have a less detrimental effect on the color of composite resins. Further understanding of the complex interactions between biofilm and NAg as well as those between NAg and the polymerization process may hold the key to more effective application of this antibacterial agent in restorative materials. Further studies are needed to investigate the Ag ion release and long-term properties of the new NAg-containing dental materials (Table 1).
Table 1.
Nanotechnology-based strategies for combating oral biofilms and dental caries
| Nanotechnology-based agent | Action | Benefiting restorative material | Refs |
|---|---|---|---|
| Silver nanoparticles (NAg) | Antimicrobial | Composite resin; Dental primer; Dental adhesive |
[25] [26] [30] |
| Zinc oxide nanoparticles (NZn) | Antimicrobial | Composite resin | [38] |
| Quaternary ammonium polyethylenimine (QAS-PEI) | Antimicrobial | Composite resin; Glass ionomer cement |
[45] [46] |
| Calcium phosphate (CaPO4) nanoparticles | Remineralizing | Composite resin; Dental adhesive; Glass ionomer cement |
[50] [53] |
| Calcium fluoride (CaF2) nanoparticles | Remineralizing | Composite resin | [63] [64] |
| Nanohydroxyapatite (nano-HA) or nanofluorohydroxyapatite (nano-FHA) | Remineralizing | Resin-modified glass ionomer cement | [71] [74] |
Zinc oxide nanoparticles
Similar to silver, zinc oxide (ZnO) has demonstrated antibacterial effects against several types of bacteria, including S. mutans [32]. Nano-ZnO particles (NZn) have been found to be more effective than conventional particles against Gram negative and Gram positive bacteria. The antibacterial mechanism of NZn is credited to modified cell membrane activity and oxidative stress; these generate active oxygen species such as H2O2 that inhibit growth of planktonic microbes [33] (Figure 1). The transcription levels of oxidative and general stress genes were increased from 3- to 52-fold in Gram negative bacteria by ZnO [33]. These NZn have selective toxicity to bacteria with minimal effects on human cells [34].
Another potential antimicrobial mechanism of NZn is the leaching of Zn2+ into the growth media decreasing biofilm formation by inhibiting the active transport and metabolism of sugars as well as disrupting enzyme systems by displacing magnesium ions essential for enzymatic activity of the of dental biofilms [35]. NZn showed antibacterial effects on S. mutans strains, although a higher concentration than that used for NAg was required for efficacy [36]. Dental composites containing 10% NZn moderately reduce bacterial counts and biofilm growth. However, the antibacterial efficacy was lower when compared with that found for NAg-containing composite [37]. Recently, properties of different NAg (0–5%) resin composites were investigated [38]. Growth of S. mutans was analyzed by direct contact test and was significantly decreased with the increase of weight (%) of ZnO; however, no expressive antimicrobial action was reached after 24 h. Most physical and mechanical properties analyzed remained unchanged by the NZn incorporation [38]. Future research should address suitable rates of nanofiller fraction that can be incorporated in dental composites to show antibacterial activity without sacrificing mechanical properties.
Quaternary ammonium polyethylenimine nanoparticles
Polymers containing quaternary ammonium (QAS) salts have been incorporated into dental materials [39]. QAS have the advantage that the antibacterial agent is copolymerized with the resin by forming a covalent bond with the polymer network, and therefore is immobilized in the composite and not released or lost over time [40]. This method imparts a durable and permanent antibacterial capability to the dental material without significantly affecting the biologic balance in the oral cavity [41]. Adhesive systems containing QAS presented similar antibiofilm properties after 6 months of water aging [42]. Conversely, simply adding organic fluoride salts to dental monomers produces dental composites that tend to form ion pairs that may leach out. Leaching leads to increased water sorption and solubility and decreased mechanical properties with time, decreasing the clinical longevity of these materials [43].
The detailed antimicrobial mechanism of QAS is yet to be established. QAS materials appear to cause bacterial lysis by binding to the cell membrane and causing cytoplasmic leakage [39] (Figure 1) as a result of highly active polycationic agents. These agents cause absorption of positively charged polymers onto the negatively charged cell surfaces of the bacteria. This approach requires sufficient contact with the bacteria, sufficient cationic charge to promote adhesion to the bacterial cell envelope, and a hydrophobic moiety that will attach onto or integrate into the cellular membrane. Further investigations on this interaction should be conducted. Several new monomers containing QAS are being investigated to improve the anticaries application of these materials further. This investigation includes the design of new antibacterial monomers and the use of novel strategies to direct toward the ongoing challenges associated with conversion degree, cross-linked networks, and stress of these monomers within the polymer matrix of composites and adhesive systems [42,44].
Quaternary ammonium polyethylenimine (QAS-PEI) nanoparticles were incorporated in restorative materials to improve antibacterial activity and reduce adverse effects on mechanical properties further [44]. The incorporation of 1% QAS-PEI nanoparticles in dental composite resin exhibited an immediate and strong antibacterial effect against S. mutans sustained over 1 month without leaching out and with no alteration of the original mechanical properties of the composite [45]. Comparable improvement was reached when QAS-PEI nanoparticles were incorporated in conventional glass ionomer and tested on S. mutans and Lactobacillus casei [46]. Future studies should focus on long-term antibacterial and mechanical durability.
Remineralizing approach
The induction and modulation of biologic activity are the functions proposed for anticaries restorative materials such as fluoride or other agents affecting the de-/re-mineralization balance [47].
Calcium phosphate nanoparticles
Nanoparticles of the more soluble calcium phosphate phases, such as monocalcium phosphate monohydrate (MCPM), dicalcium phosphate anhydrous (DCPA), tetracalcium phosphate (TTCP), and amorphous calcium phosphate (ACP), have been developed to release calcium (Ca) and phosphate (PO4) ions, increasing the mineral content in the caries lesions [48–50]. These nanoparticles have better ion-release profiles than microparticles: a small particle can release Ca and PO4 ions at higher concentrations [51]. ACP is easily transformed into crystalline phases such as octacalcium phosphate and apatite as a result of microcrystalline growth [52]. The presence of ACP nanofillers (NACP) (Figure 3) in dental composite resins is an approach to release calcium and phosphate ions continuously into the oral environment. These ions can diffuse out of the interior of the pre-saturated resin to create a high local concentration at the surface, thus stimulating precipitation and deposition into tooth structures as apatite mineral [50] (Figure 4).
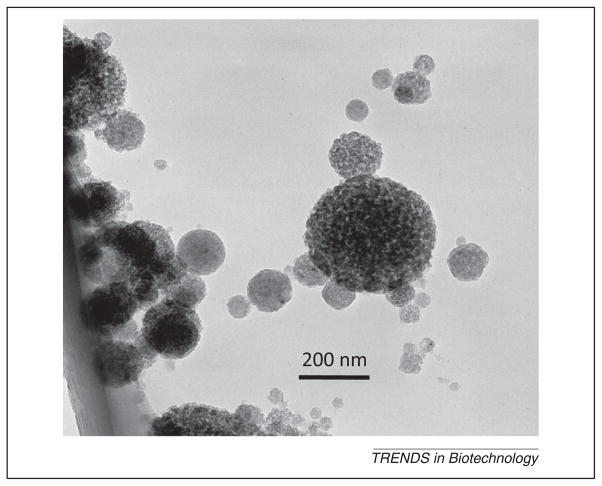
Figure 3.
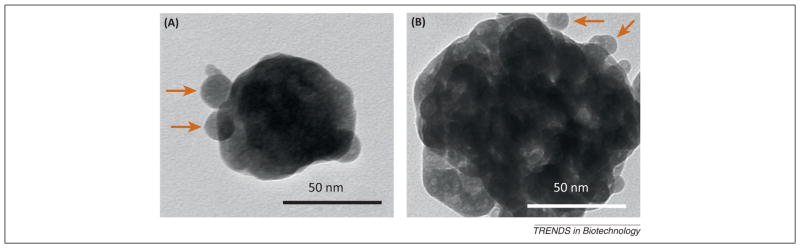
Transmission electron microscopy (TEM) micrographs of the amorphous calcium phosphate (ACP) nanoparticles. (A) Small ACP nanoparticles. (B) ACP cluster. Adapted, with permission, from [51].
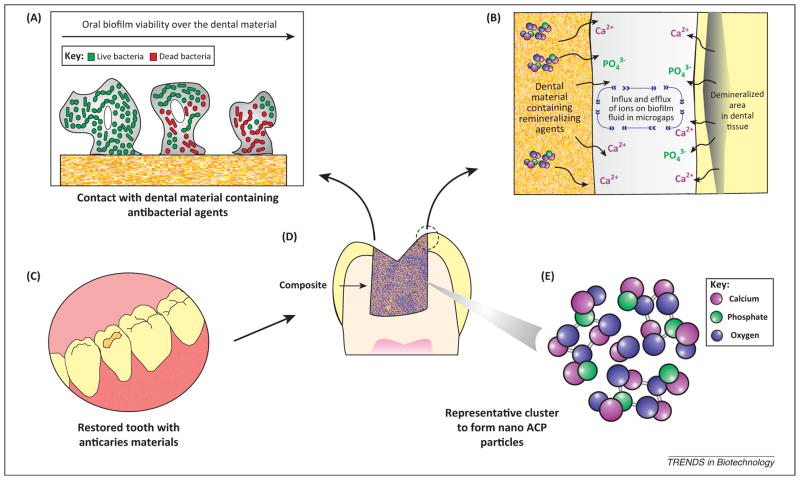
Figure 4.
Schematic illustration of the proposed anticaries procedures via restorative dental materials: antibacterial and remineralizing (ion-diffusion process) approaches. (A) The antibacterial approach involves release of nanostructured agents as described in the text. The strong antibacterial action is mainly attributed to the high surface:volume ratio that maximizes contact with the environment. These small particles easily penetrate through cell membranes and affect intracellular processes resulting in higher reactivity and antimicrobial activity. (B) In the remineralizing approach dental materials release calcium and phosphate to the dental plaque fluid present in microgaps between the tooth and restoration. The calcium and phosphate may be deposited into the tooth leading to gain of net mineral. (C) Clinical applicability of nanotechnology-based materials for dental caries management for restoring teeth with cavities. (D) Schematic drawing of a longitudinal section of a restored tooth showing the close contact of dental material with dental tissue. (E) Representative cluster to form amorphous calcium phosphate (ACP) nanofillers (NACP) with detail of molecular components.
The enrichment of nanocomposite resin with reinforcement fillers and CaPO4 nanoparticles can promote remineralization without loss of the mechanical characteristics of flexural strength, presenting similar values to microfill composite resins [51]. A composite loaded with NACP and glass particles may present a fourfold increase in capability for enamel remineralization relative to a fluoride-releasing composite [53]. This is because ion-releasing nanocomposites cause remineralization, whereas the glass particles increase the composite strength. A human in situ caries model was also used [54] to study the ability of NACP-containing nanocomposites to prevent demineralization at the restoration–enamel margins. This composite produced lower enamel mineral loss compared with the control composite. Oral biofilm exposed to NACP had higher calcium and phosphorus concentrations than that exposed to the control composite. Another study suggested that the incorporation of CaPO4 nanoparticles and antibacterial agents in adhesive systems might reach a combination of anticaries and antibacterial capabilities in the same composite [55].
Calcium fluoride nanoparticles
Fluoride has documented caries-inhibiting effects and has inspired a continuous search for improvement in the performance of fluoride-releasing restorative materials [56,57]. Unfortunately, current dental materials with high fluoride release generally have poor mechanical properties [58] or release only a small amount of fluoride with low fluoride-recharge capability [59,60]. One approach to tackle this problem has been to design dental composites with calcium fluoride (CaF2) nano-particles. The resulting composite exhibits high fluoride release but still maintains strength and wear resistance [61,62]. Cumulative fluoride release increases with nano-CaF2 content, and composites containing 20–30% CaF2 nanoparticles match the fluoride release rates of traditional and resin-modified glass ionomer materials [63]. This process is credited to a 20-fold higher surface area of nano-CaF2 compared with traditional CaF2 (Figure 5) [63,64]. Previous studies showed higher fluoride release from the CaF2 nano-particles at cariogenic low pH (<5.5). Directing these ions when they would be most needed to inhibit caries was achievedwithoutcompromising long-term mechanical properties [63–65].
Figure 5.
Transmission electron microscopy (TEM) micrograph of the new CaF2 nanopowder, with median particle size of 56 nm. Adapted, with permission, from [61].
Nanohydroxyapatite and nanofluorohydroxyapatite
Synthetic hydroxyapatite (HA) is a biologically compatible material, and is considered a logical compound substitute for the natural mineral constituent of dentin [66]. Its applicability in biomaterials by the addition of HA powders to restorative dental materials for remineralization effects and improvement of mechanical properties has been investigated due to its excellent biocompatibility and bioactivity [67,68]. In order to fabricate restorative materials that imitate human hard tissues, nano-HA particles were incorporated in resin-modified glass ionomer cement [69]. The addition of 10% nano-HA (60–100 nm) to glass ionomer cement resulted in an increased resistance to demineralization and acceptable bonding strength compared with micro-HA added to glass ionomer cement. However, the setting time of nano-HA containing glass ionomer cement exceeded the clinically suitable maximum setting time [70,71]. Nano-HA- and nanofluorohydroxyapatite (nano-FHA)-containing glass ionomer cements exhibited higher compressive, diametral tensile, and biaxial flexural strength compared with the control [72,73]. Besides, the presence of fluoride in FHA means there is the potential to increase the amount of fluoride release from the glass ionomer cements [74]. Several studies proposed bioactive glass nanoparticles for application in the remineralization of human dentin and the potential as a filler component in restorative materials [75,76]. The remineralization rates were higher than with micro bioactive glass particles, highlighting the importance of nanotechnology for future clinical applications [76].
Toxicity and possible biologic impacts
Nanoparticles must selectively kill bacteria without imparting toxicity on mammalian cells. Studies have highlighted the positive correlation between NAg concentration and cytotoxic effects [77,78]. Nevertheless, an absence of cytotoxity against human cells was demonstrated when 0.05–0.70% concentrations of NAg were incorporated in polymers [79]. In experimental investigations, NAg at 0.08–0.10% mass fraction concentrations have been used [26,30]. These are very low concentrations compared with a 10% concentration capable of inducing cytotoxity to human cells. As a limitation, NAg may influence the degree of monomer conversion in dental materials and lead to an increase in elutable residual monomers from the hardened composite [24]. Some monomers eluted from composites are known to cause allergic reactions or may be metabolized to reactive oxygen species.
QAS is a low viscosity monomer, which is miscible with common dimethacrylate monomers present in composite composition, and it is expected to have minimal monomer leachability. The addition of 10% quaternary ammonium dimethacrylate or 0.5% NAg into the bonding agent did not cause significant citotoxic effect on human fibroblast, compared to the commercial nonantibacterial bonding agent [80]. Because the assessment of the toxicity of nanomaterials is a relatively new and evolving field, the elucidation of the risks posed by nanoparticles incorporated in dental materials is necessary to further applications.
Concluding remarks
Different up-to-date strategies have been presented in this review, with the focus on the use of cutting-edge nano-technology incorporated in restorative materials. These results demonstrate a promising future for the development of a new generation of improved biomaterials that could have an impact on current dental caries management. However, to develop anticaries materials better, a knowledge base encompassing modes of action, safety, and development of new properties remains to be achieved (Box 1). Most of the research covered in this review spanned a range of disciplines and focused primarily on laboratory tests. Greater consideration should be given to studying the performance of new anticaries dental materials in the clinic. The bacterial adhesion processes and complex biofilm interation differ considerably elucidating the problem of translation from the laboratory to the clinic. Other aspects such as the long-term benefits and interaction with the harsh oral environment remain to be determined through clinical studies. In general, considerable progress has been made in dental caries research. Thus far, there has been no simple solution to the challenges faced in the field of dental caries. Nanotechnology-based restorative materials may provide distinct benefits to prevent and control dental caries and warrant further investigation.
Box 1. Outstanding questions.
How do we define the rates of antibacterial/remineralizing nanoparticles incorporated in dental materials? Is there a suitable approach to evaluate the nanoparticle performance?
How can we control the dose of antibacterial agents released by dental materials in vivo?
Are nanotechnology-based restorative materials for dental caries management a viable long-term option for the clinic?
Acknowledgments
We thank Drs Michael Weir, Lei Cheng, and Ke Zhang for their helpful discussions. M.A.S.M. acknowledges a scholarship from the Coordination for Improvement of Higher Education-CAPES/Fulbright Doctoral Program BEX 0523/11-9. H.H.K.X. acknowledges the support by NIH R01DE17974 and R01DE14190 and a seed fund from the University of Maryland School of Dentistry.
Glossary
- Caries lesion
detectable change in the tooth structure that results from the biofilm–tooth interactions occurring due to the disease caries. It is the clinical manifestation (sign) of the caries process. People have dental caries, teeth have caries lesions
- Demineralization
chemical process mediated by oral biofilm resulting in the loss of calcified material from the structure of the tooth
- Dental caries
the localized destruction of susceptible dental hard tissue by acidic byproducts from bacterial fermentation of dietary carbohydrates. Thus, it is a bacterial driven, generally chronic, site-specific, multifactorial, dynamic disease process that results from the imbalance in the physiologic equilibrium between the tooth mineral and the plaque fluid; that is, when the pH drop results in net mineral loss over time
- Remineralization
net gain of calcified material within the tooth structure, replacing that previously lost through demineralization
- Secondary caries
caries lesions that occur at the margin of, or adjacent to, an existing filling
References
- 1.Fejerskov O, et al., editors. Dental Caries: The Disease and its Clinical Management. Blackwell Munksgaard; 2008. [Google Scholar]
- 2.Gross EL, et al. Beyond Streptococcus mutans: dental caries onset linked to multiple species by 16S rRNA community analysis. PLoS ONE. 2012;7:e47722. doi: 10.1371/journal.pone.0047722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cury JA, Tenuta LM. Enamel remineralization: controlling the caries disease or treating early caries lesions? Braz Oral Res. 2009;23:23–30. doi: 10.1590/s1806-83242009000500005. [DOI] [PubMed] [Google Scholar]
- 4.Fontana M, et al. Defining dental caries for 2010 and beyond. Dent Clin North Am. 2010;54:423–440. doi: 10.1016/j.cden.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Watson PS, et al. Penetration of fluoride into natural plaque biofilms. J Dent Res. 2005;84:451–455. doi: 10.1177/154405910508400510. [DOI] [PubMed] [Google Scholar]
- 6.Malterud MI. Minimally invasive dentistry – A biomimetic approach. Gen Dent. 2012;60:186–187. [PubMed] [Google Scholar]
- 7.Kirkevang LL, et al. Incidence of caries lesions in approximal surfaces: A radiographic study of a general adult Danish population. Caries Res. 2011;45:538–546. doi: 10.1159/000331932. [DOI] [PubMed] [Google Scholar]
- 8.Dental Resin Composites and Caries. NIDCR (National Institute of Dental and Craniofacial Research); Mar 5, 2009. announcement # 13-DE-102. [Google Scholar]
- 9.Allaker RP. The use of nanoparticles to control oral biofilm formation. J Dent Res. 2010;89:1175–1186. doi: 10.1177/0022034510377794. [DOI] [PubMed] [Google Scholar]
- 10.Ferracane JL. Resin composite – state of the art. Dent Mater. 2011;27:29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Mohamed Hamouda I. Current perspectives of nanoparticles in medical and dental biomaterials. J Biomed Res. 2012;26:143–151. doi: 10.7555/JBR.26.20120027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subramani K, Ahmed W, editors. Emerging Nanotechnologies in Dentistry: Processes, Materials and Applications. Elsiever; 2011. [Google Scholar]
- 13.Subramani K, et al., editors. Nanobiomaterials in Clinical Dentistry. Elsiever; 2012. [Google Scholar]
- 14.Allahverdiyev AM, et al. Coping with antibiotic resistance: combining nanoparticles with antibiotics and other antimicrobial agents. Expert Rev Anti Infect Ther. 2011;9:1035–1052. doi: 10.1586/eri.11.121. [DOI] [PubMed] [Google Scholar]
- 15.Hajipour MJ, et al. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30:499–510. doi: 10.1016/j.tibtech.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Park HJ, et al. Biofilm-inactivating activity of silver nanoparticles: A comparison with silver ions. J Ind Eng Chem. 2013;19:614–619. [Google Scholar]
- 17.Chaloupka K, et al. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010;28:580–588. doi: 10.1016/j.tibtech.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Sotiriou GA, Pratsinis SE. Antibacterial activity of nanosilver ions and particles. Environ Sci Technol. 2010;44:5649–5654. doi: 10.1021/es101072s. [DOI] [PubMed] [Google Scholar]
- 19.Peng JJ, et al. Silver compounds used in dentistry for caries management: a review. J Dent. 2012;40:531–541. doi: 10.1016/j.jdent.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Radzig MA, et al. Antibacterial effects of silver nanoparticles on gram-negative bacteria: Influence on the growth and biofilms formation, mechanisms of action. Colloids Surf B: Biointerfaces. 2013;102:300–306. doi: 10.1016/j.colsurfb.2012.07.039. [DOI] [PubMed] [Google Scholar]
- 21.Seth D, et al. Nature-inspired novel drug design paradigm using nanosilver: Efficacy on multi-drug-resistant clinical isolates of tuberculosis. Curr Microbiol. 2011;62:715–726. doi: 10.1007/s00284-010-9770-7. [DOI] [PubMed] [Google Scholar]
- 22.Espinosa-Cristóbal LF. Antimicrobial sensibility of Streptococcus mutans serotypes to silver nanoparticles. Mater Sci Eng C. 2012;32:896–901. [Google Scholar]
- 23.Ahn SJ. Experimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles. Dent Mater. 2009;25:206–213. doi: 10.1016/j.dental.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Durner J, et al. Influence of silver nano-particles on monomer elution from light-cured composites. Dent Mater. 2011;27:631–636. doi: 10.1016/j.dental.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Cheng L, et al. Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dent Mater. 2012;28:561–572. doi: 10.1016/j.dental.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng L, et al. Effect of amorphous calcium phosphate and silver nanocomposites on dental plaque microcosm biofilms. J Biomed Mater Res B: Appl Biomater. 2012;100:1378–1386. doi: 10.1002/jbm.b.32709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peulen TO, Wilkinson KJ. Diffusion of nanoparticles in a biofilm. Environ Sci Technol. 2011;45:3367–3373. doi: 10.1021/es103450g. [DOI] [PubMed] [Google Scholar]
- 28.Damm C, et al. Long-term antimicrobial polyamide 6/silver nanocomposites. J Mater Sci. 2007;42:6067–6073. [Google Scholar]
- 29.Yoshida K, et al. Characterization and inhibitory effect of antibacterial dental resin composites incorporating silver-supported materials. J Biomed Mater Res B: Appl Biomater. 1999;4:516–522. doi: 10.1002/(sici)1097-4636(19991215)47:4<516::aid-jbm7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 30.Zhang K, et al. Effect of quaternary ammonium and silver nanoparticle-containing adhesives on dentin bond strength and dental plaque microcosm biofilms. Dent Mater. 2012;28:842–852. doi: 10.1016/j.dental.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng L, et al. Anti-biofilm dentin primer with quaternary ammonium and silver nanoparticles. J Dent Res. 2012;91:598–604. doi: 10.1177/0022034512444128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones N, et al. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol Lett. 2008;279:71–76. doi: 10.1111/j.1574-6968.2007.01012.x. [DOI] [PubMed] [Google Scholar]
- 33.Xie Y, et al. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl Environ Microbiol. 2011;77:2325–2331. doi: 10.1128/AEM.02149-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma V, et al. DNA damaging potential of zinc oxide nanoparticles in human epidermal cells. Toxicol Lett. 2009;185:211–218. doi: 10.1016/j.toxlet.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Gu H, et al. Effect of ZnCl2 on plaque growth and biofilm vitality. Arch Oral Biol. 2012;57:369–375. doi: 10.1016/j.archoralbio.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Hernández-Sierra JF, et al. The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomed Nanotechnol Biol Med. 2008;4:237–240. doi: 10.1016/j.nano.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Sevinç BA, Hanley L. Antibacterial activity of dental composites containing zinc oxide nanoparticles. J Biomed Mater Res B: Appl Biomater. 2010;94:22–31. doi: 10.1002/jbm.b.31620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tavassoli Hojati S, et al. Antibacterial, physical and mechanical properties of flowable resin composites containing zinc oxide nanoparticles. Dent Mater. 2013;29:495–505. doi: 10.1016/j.dental.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 39.Sun XZ, et al. The antimicrobial activities of a series of bis-quaternary ammonium compounds. Chin Chem Lett. 2011;22:887–890. [Google Scholar]
- 40.Vaidyanathan M, et al. Antimicrobial properties of dentine bonding agents determined using in vitro and ex vivo methods. J Dent. 2009;37:514–521. doi: 10.1016/j.jdent.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 41.Imazato S, et al. Antibacterial resin monomers based on quaternary ammonium and their benefits in restorative dentistry. Jpn Dent Sci Rev. 2012;48:115–125. [Google Scholar]
- 42.Zhang K, et al. Effect of water-ageing on dentine bond strength and anti-biofilm activity of bonding agent containing new monomer dimethylaminododecyl methacrylate. J Dent. 2013;41:504–513. doi: 10.1016/j.jdent.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu X, et al. Formulation and characterization of a novel fluoride-releasing dental composite. Dent Mater. 2006;22:1014–1023. doi: 10.1016/j.dental.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 44.Beyth N, et al. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials. 2006;27:3995–4002. doi: 10.1016/j.biomaterials.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Beyth N, et al. Long-term antibacterial surface properties of composite resin incorporating polyethyleneimine nanoparticles. Quintessence Int. 2010;41:827–835. [PubMed] [Google Scholar]
- 46.Beyth N, et al. Antibacterial activity of dental cements containing quaternary ammonium polyethylenimine nanoparticles. J Nanomater. 2012 doi: 10.1016/j.biomaterials.2006.03.003. Article ID 814763, 6 pages, ( http://dx.doi.org/10.1155/2012/814763) [DOI] [PubMed]
- 47.Ten Cate JM. Novel anticaries and remineralizing agents: prospects for the future. J Dent Res. 2012;91:813–815. doi: 10.1177/0022034512455032. [DOI] [PubMed] [Google Scholar]
- 48.Zhou H, Bhaduri S. Novel microwave synthesis of amorphous calcium phosphate nanospheres. J Biomed Mater Res B: Appl Biomater. 2012;100:1142–1150. doi: 10.1002/jbm.b.32681. [DOI] [PubMed] [Google Scholar]
- 49.Sun L, et al. Preparation and properties of nanoparticles of calcium phosphates with various Ca/P ratios. J Res Natl Inst Stand Technol. 2010;115:243–255. doi: 10.6028/jres.115.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu HH, et al. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dent Mater. 2011;27:762–769. doi: 10.1016/j.dental.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng L, et al. Antibacterial and physical properties of calcium-phosphate and calcium-fluoride nanocomposites with chlorhexidine. Dent Mater. 2012;28:573–583. doi: 10.1016/j.dental.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao J, et al. First detection, characterization, and application of amorphous calcium phosphate in dentistry. J Dent Sci. 2012;7:316–323. [Google Scholar]
- 53.Weir MD, et al. Remineralization of demineralized enamel via calcium phosphate nanocomposite. J Dent Res. 2012;91:979–984. doi: 10.1177/0022034512458288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Melo MAS, et al. Novel calcium phosphate nanocomposite with caries-inhibition in a human in situ model. Dent Mater. 2013;29:231–240. doi: 10.1016/j.dental.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melo MAS, et al. Antibacterial dental adhesive containing silver and amorphous calcium phosphate nanoparticles. Dent Mater. 2013;29:199–210. doi: 10.1016/j.dental.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moreau JL, Xu HK. Fluoride releasing restorative materials: Effects of pH on mechanical properties and ion release. Dent Mater. 2010;26:227–235. doi: 10.1016/j.dental.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ling L, et al. Novel F-releasing composite with improved mechanical properties. J Dent Res. 2009;88:83–88. doi: 10.1177/0022034508328254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kirsten GA, et al. Microhardness of dentin underneath fluoride-releasing adhesive systems subjected to cariogenic challenge and fluoride therapy. J Dent. 2010;38:460–468. doi: 10.1016/j.jdent.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 59.Cenci MS, et al. Effect of microleakage and fluoride on enamel-dentine demineralization around restorations. Caries Res. 2008;42:369–379. doi: 10.1159/000151663. [DOI] [PubMed] [Google Scholar]
- 60.Mousavinasab SM, Meyers I. Fluoride release and uptake by glass ionomer cements, compomers and giomers. Res J Bio Sci. 2009;4:609–616. [PMC free article] [PubMed] [Google Scholar]
- 61.Xu HH, et al. Novel CaF(2) nanocomposite with high strength and fluoride ion release. J Dent Res. 2010;89:739–745. doi: 10.1177/0022034510364490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun L, Chow LC. Preparation and properties of nano-sized calcium fluoride for dental applications. Dent Mater. 2008;24:111–116. doi: 10.1016/j.dental.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu HH, et al. Strength and fluoride release characteristics of a calcium fluoride based dental nanocomposite. Biomaterials. 2008;29:4261–4267. doi: 10.1016/j.biomaterials.2008.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu HH, et al. Novel CaF2 nanocomposite with high strength and fluoride ion release. J Dent Res. 2010;89:739–745. doi: 10.1177/0022034510364490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weir MD, et al. Nanocomposite containing CaF2 nanoparticles: thermal cycling, wear and long-term water-aging. Dent Mater. 2012;28:642–652. doi: 10.1016/j.dental.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Besinis A, et al. Infiltration of demineralized dentin with silica and hydroxyapatite nanoparticles. Dent Mater. 2012;28:1012–1023. doi: 10.1016/j.dental.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 67.Moshaverinia A, et al. A review of powder modifications in conventional glass-ionomer dental cements. J Mater Chem. 2011;21:1319–1328. [Google Scholar]
- 68.Zhang H, Darvell BW. Mechanical properties of hydroxyapatite whisker-reinforced bis-GMA-based resin composites. Dent Mater. 2012;28:824–830. doi: 10.1016/j.dental.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 69.Goenka S, et al. Effects of nanocrystalline calcium deficient hydroxyapatite incorporation in glass ionomer cements. J Mech Behav Biomed Mater. 2012;7:69–76. doi: 10.1016/j.jmbbm.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 70.Wang Q-S, et al. Effects of light-initiation agent on mechanical properties of light-cured nano-hydroxyapatite composite for dental restoration. Appl Mech Mater. 2012;138–139:1012–1016. [Google Scholar]
- 71.Lee JJ, et al. Physical properties of resin-reinforced glass ionomer cement modified with micro and nano-hydroxyapatite. J Nanosci Nanotechnol. 2010;10:5270–5276. doi: 10.1166/jnn.2010.2422. [DOI] [PubMed] [Google Scholar]
- 72.Moshaverinia A, et al. Modification of conventional glass-ionomer cements with N-vinylpyrrolidone containing polyacids, nano-hydroxy and fluoroapatite to improve mechanical properties. Dent Mater. 2008;24:1381–1390. doi: 10.1016/j.dental.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 73.Moshaverinia A, et al. Effects of incorporation of hydroxyapatite and fluoroapatite nanobioceramics into conventional glass ionomer cements (GIC) Acta Biomater. 2008;4:432–440. doi: 10.1016/j.actbio.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 74.Lin J, et al. Effects of incorporation of nano-fluorapatite or nano-fluorohydroxyapatite on a resin-modified glass ionomer cement. Acta Biomater. 2011;7:1346–1353. doi: 10.1016/j.actbio.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 75.Aldo R, et al. Polymer/bioactive glass nanocomposites for biomedical applications: A review. Compos Sci Technol. 2010;70:1764–1776. [Google Scholar]
- 76.de Oliveira AA, et al. Synthesis, characterization and cytocompatibility of spherical bioactive glass nanoparticles for potential hard tissue engineering applications. Biomed Mater. 2013;8:025011. doi: 10.1088/1748-6041/8/2/025011. [DOI] [PubMed] [Google Scholar]
- 77.You C, et al. The progress of silver nanoparticles in the antibacterial mechanism, clinical application and cytotoxicity. Mol Biol Rep. 2012;39:9193–9201. doi: 10.1007/s11033-012-1792-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hackenberg S, et al. Silver nanoparticles: evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cells. Toxicol Lett. 2011;201:27–33. doi: 10.1016/j.toxlet.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 79.Martínez-Gutiérrez F, et al. Antimicrobial activity, cytotoxicity and inflammatory response of novel plastics embedded with silver nanoparticles. Future Microbiol. 2013;8:403–411. doi: 10.2217/fmb.13.5. [DOI] [PubMed] [Google Scholar]
- 80.Li F, et al. Comparison of quaternary ammonium-containing with nano-silver-containing adhesive in antibacterial properties and cytotoxicity. Dent Mater. 2013;29:450–461. doi: 10.1016/j.dental.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]