Abstract
Skeletal metastases result in significant morbidity and mortality. This is particularly true of cancers with a strong predilection for the bone, such as breast, prostate, and lung cancers. There is currently no reliable cure for skeletal metastasis, and palliative therapy options are limited. The Wnt signaling pathway has been found to play an integral role in the process of skeletal metastasis and may be an important clinical target. Several experimental models of skeletal metastasis have been used to find new biomarkers and test new treatments. In this review, we discuss pathologic process of bone metastasis, the roles of the Wnt signaling, and the available experimental models and treatments.
Keywords: Cancer, skeletal metastasis, therapy, Wnt signaling, mouse models
Skeletal Metastasis
Five-year survival rates for most patients with localized cancers have risen consistently over the past few decades, and cancer metastasis patients are also surviving longer, partially due to advanced treatments, such as treatments with the targeted agent trastuzumab in breast cancer[1]. However, once cancer spreads to the bone, it typically cannot be cured, accounting for significant morbidity and mortality. Skeletal metastasis is common in several of the most prevalent cancers, including prostate, breast, and lung cancers. In fact, 80% of all skeletal metastatic lesions come from one of these primary sites[2]. These three types of cancer account for approximately 43% of all cancer cases in the United States, and they cause approximately 40% of all cancer-related deaths[1]. Recent data support a high rate of hospitalization for patients afflicted by metastatic bone disease, confirming that skeletal-related events also lead to increased use of health care resource[3].
In a healthy person, bone turnover is precisely regulated and occurs on a constant basis. Osteoblasts, which originate from mesenchymal stem cells and eventually differentiate into osteocytes, play a role in bone formation. Osteoblasts and osteocytes also secrete factors that stimulate osteoclastogenesis from hematopoietic precursors; one such factor is receptor activator of nuclear factor kappa B ligand (RANKL) which binds to its receptor RANK, the master osteoclastogenic cytokine[4]. Activated osteoclasts resorb the bones. Osteoprotegerin (OPG) regulates this process and is secreted by osteoblasts and osteocytes. OPG acts as a decoy receptor for RANKL, thereby acting as a competitive inhibitor. In skeletal metastasis, tumor cells disrupt the delicate homeostasis of bone formation and resorption through the secretion of catabolic and/or anabolic factors that uncouple these functions.
The complex process of metastasis consists of several steps[5]. Primary tumor cells become pre-metastatic when they exhibit unlimited proliferation, evasion of apoptosis, and have access to a new supply of blood vessels (angiogenesis)[6]. These cells also display physical changes, such as dysplasia and karyomegaly. Tumor cells must then acquire a metastatic phenotype, generally through mutation or alternative activation of proinvasive molecular pathways[7]. This process is usually referred to as an epithelial-to-mesenchymal transition (EMT): epithelial-like cells, which are well-organized, fully differentiated, and immobile develop into mesenchymal-like cells, which do not organize, are undifferentiated, and are mobile. Tumor cells then intravasate into surrounding blood vessels. Once in the bloodstream, tumor cells that survive anchorage independence and immune surveillance reach new organs, where they extravasate from blood vessels and initiate a metastatic lesion. Angiogenesis is important for the growth and maintenance of the tumor in a new microenvironment. Depending on the type of primary tumor, metastatic lesions often occur in specific organs; bone metastasis occurs most commonly from breast, prostate, and lung cancers.
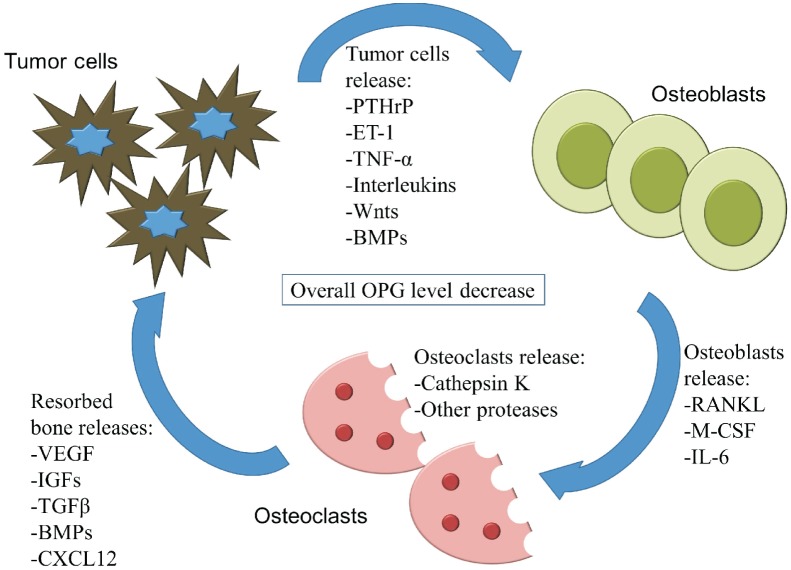
Metastasis to the bones involves tumor cells (the “seed”) invading the bone cavity (the “soil”[8]) and causing a so-called vicious cycle[9]. Upon invasion into the bone, tumor cells secrete factors that affect both osteoblasts and osteoclasts, disrupting normal homeostasis (Figure 1). In many cases of skeletal metastasis, particularly from breast and lung cancers, the lesions are prone to be osteolytic, resorbing more bone tissue than that is being formed. Breast cancer skeletal metastatic cells have up-regulated expression of matrix metallopeptidase-1 (MMP-1), interleukin-11 (IL-11), and C-X-C chemokine receptor 4 (CXCR4), which favor osteoclastogenesis and increase bone resorption[10]. This results in lower bone mass, pain, increased risk of fracture, and other symptoms. In other cases, particularly in most prostate cancer metastases, lesions are prone to be osteoblastic, forming more bone tissue than that is being resorbed. Such lesions—also known as osteosclerotic or osteoblastic lesions—contain poorly organized collagen fibers that have a woven appearance and weak structure, which also results in pain and increased fracture risk[11].
Figure 1. The “vicious cycle” takes place within the tumor-bone microenvironment.
Tumor cells secrete factors that stimulate osteoblast activation and bone formation. Osteoblasts release RANK ligand and other factors that stimulate osteoclast activation and resorption of the bones. This allows for the release of growth factors that stimulate tumor growth and maintenance. These factors and their concentrations can change throughout the process of metastasis and can differ from cancer to cancer, which results in a variable balance of bone formation and resorption. PTHrP, parathyroid hormone-related protein; ET-1, endothelin-1; TNF-α, tumor necrosis factor alpha; BMP, bone morphogenetic protein; RANKL, receptor activator of nuclear kappa-B ligand; M-CSF, macrophage colony-stimulating factor; IL-6, interleukin-6; VEGF, vascular endothelial growth factor; IGF, insulin-like growth factor; TGFβ, transforming growth factor beta; CXCL12, chemokine C-X-C motif ligand 12; OPG, osteoprotegerin.
Prostate cancer cells generally up-regulate the Wnt/β-catenin pathway, bone morphogenetic proteins (BMPs), platelet-derived growth factor (PDGF), and endothelin-1 (ET-1), which results in a more pro-osteoblastic phenotype[7]. It is becoming more widely recognized that most cases of skeletal metastasis have both osteolytic and osteoblastic components and that osteolysis is likely required for advancement of osteoblastic disease[9],[11]. The bone resorption marker N-telopeptide type I collagen is increased in all skeletal metastases, regardless of whether they appear to be osteoblastic or osteolytic, indicating that osteoclastogenesis and bone resorption occur in all skeletal metastasis[12]. Much more work remains to be done to elucidate the complexity of skeletal metastasis and to identify molecular targets that can retain normal bone homeostasis, while simultaneously killing the invading tumor cells.
The affinity that certain cancers have for the bone is still an area under considerable investigation. Studies suggest that tumor cells have up-regulated expression of cell surface proteins such as CXCR4 or certain integrins, and therefore have a higher preference to home to the bone[13],[14]. Cell adhesion molecules regulate circulating tumor cell interactions with endothelial cells in the blood vessels of specific organs[15]. Resorbed bone also releases a wealth of growth factors that induce circulating tumor cell homing, tumor growth, angiogenesis, and maintenance. Additionally, endocrine factors such as heparanase, osteopontin, and matrix metalloproteases are produced by primary tumors and go into circulation before actual metastasis occurs, setting up a “pre-metastatic niche”[16]–[19]. Alternatively, tumor cells may use strategies similar to those of hematopoietic stem cells to home to the bone; recent studies have shown that prostate tumor cells compete for the niche of such stem cells in the bones[20].
In prostate cancer cases, most men present with non-metastatic disease; only about 3% of men are initially diagnosed with metastatic disease, but that number jumps to 12% upon further follow-up[21]. However, skeletal metastases are found in approximately 90% of prostate cancer patients who succumbed to their disease[2],[22]. In breast cancer, skeletal metastases are similarly found in a small proportion of initially diagnosed patients, but approximately 43% developed metastasis upon follow-up[23]. Upon autopsy, skeletal metastases were found in 71% of breast cancer patients and 41% of lung cancer patients[24],[25]. Five-year survival rates decrease dramatically in patients with metastatic disease when compared with patients with local disease (Table 1). In prostate and breast cancers, skeletal metastasis is so prevalent that it is probable that the majority of the tumor burden of patients who succumb to the disease will reside in the bone[9]. Skeletal-related events are defined as pathologic fractures, spinal cord compression, or bone pain requiring palliative radiotherapy and orthopedic surgery[23]. Pain is especially common in the load-bearing bones such as the femoral neck, pelvis, and vertebrae. Approximately 20% of prostate cancer patients with bone metastasis are treated for pathologic fractures[11]. Skeletal metastasis can cause compression syndromes of nerve roots and the spinal cord, inducing systemic and head pain. Osteolytic bone lesions induce hypercalcemia by overwhelming the calcium homeostatic mechanisms in the body; thus, extracellular fluid calcium is filtered out and excreted by the kidneys[9]. Phosphate and other metabolic imbalances can occur[9]. All these symptoms require treatment and palliative care, adding to the burden of the patient and increasing the cost of health care dramatically.
Table 1. Five-year survival rates by stage at diagnosis, 1999-2006.
| Cancer type | Local diseasea | Metastatic diseaseb | Typical sites of metastasis |
| Prostate cancer | 100% | 30% | Bone |
| Breast cancer | 98% | 23% | Bone, lungs, liver, brain |
| Lung cancer | 53% | 4% | Bone, brain, liver, adrenal gland |
aLocal disease refers to invasive malignant cancer that is entirely confined to the primary organ.
bMetastatic disease refers to a malignant cancer that has spread to any part of the body outside of the primary tumor.
Statistics were taken from the American Cancer Society[1].
Current and Potential Therapies for Skeletal Metastasis
Several treatment options exist for patients afflicted with skeletal metastasis. Surgical treatment with curative intent is recommended for certain oligometastatic presentations of skeletal metastasis, but few actual cures are achieved. Traditional treatment methods include chemotherapy, anti-resorptive medications, radiotherapy, and surgical stabilization. Other palliative options include narcotic medications and activity restriction.
Traditional chemotherapy, such as docetaxel, is less effective against bone metastases. A relatively new strategy for treating bone metastasis is targeting the bone microenvironment itself. Table 2 contains an extensive list of potential and currently available treatments for skeletal metastasis. Many of these treatments can target both the bone microenvironment and tumor cells. The most commonly applied strategies for treating bone metastasis are surgery, radiotherapy, hormone ablation, anti-resorptive strategies, and targeted therapies.
Table 2. Current and potential targeted therapies for skeletal metastasis.
| Mechanism of action | Example therapeutic(s) | Stage of clinical availability | Reference(s) |
| Osteoclast apoptosis | Bisphosphonates | Available | [55] |
| RANKL inhibition | Denosumab | Available | [63] |
| Microtubule inhibition | Docetaxel | Available | [177] |
| Cabazitaxel | Available | [178] | |
| EGFR/ERRB2 inhibitor | Lapatinib | Available | [179] |
| Cancer immunotherapy | Sipuleucel-T / ipilimumab | Available | [180] |
| DNA synthesis inhibition | Gemcitabine / satraplatin / pemetrexed disodium | Available | [181]–[183] |
| VEGF inhibition | Bevacizumab / sorafenib / sunitinib | Available | [184]–[186] |
| CYP17 inhibition | Abiraterone acetate | Available | [44] |
| Competitive binding to RANK | Recombinant osteoprotegrin | Available | [187],[188] |
| Kinase inhibition | Crizontinib | Clinical trials | [189] |
| MET/VEGFR2 inhibition | Cabazantinib | Clinical trials | [95]–[97] |
| Src inhibition | Dasatinib / saracatinib / bosutinib | Clinical trials | [99] |
| Vitamin D analogues | Calcitriol / EB1089 | Clinical trials | [190],[191] |
| Proteasome inhibition | Bortezomib | Clinical trials | [192],[193] |
| Radiopharmaceuticals | Radium-223 / strontium-89 | Clinical trials | [194] |
| Endothelin A inhibition | ZD4054 (Zibotentan) | Clinical trials | [195] |
| DKK-1 inhibition | DKK-1 antibodies | Clinical trials | [158] |
| PARP inhibition | Veliparib | Discovery | [196] |
| Integrin inhibition | Cilengitide | Discovery | [197] |
| PTHrP inhibition | PTHrP antibodies | Discovery | [81],[82] |
| Cathepsin K inhibition | Balicatib / odanacatib | Discovery | [72]–[74] |
| Cathepsin B inhibition | CA-074 | Discovery | [75] |
| TGFβ inhibition | LY2109761 / Ad.sTβRFc / Ki26894 | Discovery | [90],[91],[93] |
| mTOR inhibition | Rapamycin | Discovery | [198],[199] |
| MMP inhibition | Batimastat | Discovery | [200],[201] |
| Organic compounds | Plumbagin | Discovery | [202] |
| IL-6 inhibition | Tocilizumab / elsilimobab | Discovery | [203],[204] |
| Activin-A inhibition | ActRIIA.muFc | Discovery | [205] |
| Platelet inhibition | XV454 | Discovery | [206],[207] |
| Wnt inhibition | Lrp6 antibodies / IWP-2 / C59 | Discovery | [154],[208]–[210] |
RANKL, receptor activator of nuclear factor kappa B ligand; EGFR/ERRB2, epidermal growth factor receptor/estrogen related receptor beta; VEGF, vascular endothelial growth factor; CYP17, cytochrome P450 17α; RANK, receptor activator of nuclear factor; VEGFR, VEGF receptor; DKK, dickkopf; PARP, poly (ADP-ribose) polymerase; PHTrP, parathyroid hormone-related protein; TGFβ, transforming growth factor beta; mTOR, mammalian target of rapamycin; MMP, matrix metallopeptidase; IL-6, interleukin-6.
Surgery
In many cases of bone metastasis, surgery is used to treat impending or displaced pathologic fractures. Pathologic fractures cause significant morbidity and often interrupt ongoing treatment efforts. To this end, the primary focus of the orthopedic surgeons performing these surgeries is to stabilize the bones in the extremities, such as the pelvis, spine, or femur. Stabilization is accomplished with prostheses, arthroplasty, plates, or intramedullary fixation devices[26]. Surgery is effective in improving pain control and mobility, and facilitates subsequent efforts to employ radiation and chemotherapy[27]. The survival rate of patients with skeletal metastasis treated with surgery was approximately 40% after 1 year, 30% after 2 years, and 20% after 3 years[28]. In most cases of skeletal metastases arising from breast cancer, prostate cancer, lung cancer, or melanoma, surgery is not performed with the intent to cure, but an indirect survival benefit may be derived from enhanced function and performance status[29],[30]. Surgery has also been reported to prolong survival for patients with oligometastatic presentation of renal cell carcinoma, thyroid carcinoma, and myeloma[31],[32].
Radiotherapy
Radiotherapy is an effective treatment for skeletal metastasis in terms of preventing impending fractures, controlling pain, and restoring function[33],[34]. In rare cases, radiotherapy has been shown to have a curative effect on bone lesions. Radiation is often used as a stand-alone treatment for radiosensitive cancer types, and as an adjuvant to surgical stabilization of pathologic fractures[35]. External beam radiation therapy has been the mainstay in past years of radiotherapy, meaning that tumor cells and normal cells receive the same amount of radiation in the region being treated. Thus, effort has been placed into more efficiently targeting tumor cells with radiation. In particular, one study showed that image-guided high-dose radiation was an effective treatment, in the absence of surgery, and that patients were typically cancer-free 3 years after treatment[36]. In addition, spine stereotactic body radiotherapy allows for delivery of a high dose to metastatic regions without inducing severe adverse effects on adjacent spinal cord[37]. A promising new treatment is alpha-pharmaceuticals, radionuclides that emit alpha particles, which deliver a more intense radiation than beta-emitting radiopharmaceuticals, in a targeted fashion[38]. Radium-223 is the first alpha-emitting radiopharmaceutical to reach clinical trials and has been shown in phases I and II trials to improve survival in patients with skeletal metastases from prostate cancer[38].
Hormone ablation
Hormone ablation is an efficient strategy for shrinking breast and prostate cancers; both cancers depend on hormones for tumor growth and maintenance. For breast cancer, tamoxifen (Nolvadex), which blocks estrogen binding to the estrogen receptor[39], is the standard of care following chemotherapy. Alternatively, aromatase inhibitors (arimidex, aromasin, and femara are currently approved) inhibit estrogen synthesis, thereby decreasing the concentration of estrogen in the body and inhibiting the primary tumor's ability to grow and metastasize. Aromatase inhibitors are recommended for breast cancer treatment either together with tamoxifen or as an extended therapy after tamoxifen[39]. These estrogen-inhibiting strategies have increased the life span of breast cancer patients and have reduced recurrence[39]. No difference in survival has been reported between tamoxifen and aromatase inhibitor treatment[39].
In prostate cancer, lutenizing hormone-releasing hormone (LHRH) agonists inhibit testosterone production, which has been shown to shrink prostate cancer and increase life span[40]. However, most patients become castration-resistant, and many of these patients have increased skeletal-related events[41]. Deferred androgen deprivation therapy may be ideal; the time at which to initiate androgen deprivation therapy is still controversial[42].
One promising drug approved by the Food and Drug Ad-ministration (FDA) in 2011 for treatment of metastatic castration-resistant prostate cancer is abiraterone (Zytiga). Abiraterone inhibits 17 α-hydroxylase/C17,20 lyase (CYP17A1), which is re-sponsible for the conversion of progesterone and pregnenolone to testosterone precursors. This inhibition of alternate sources of testosterone results in an overall decrease in the amount of circulating testosterone. In the first clinical trial in Britain in 2004, abiraterone induced sustained suppression of testosterone levels[43]. A report in 2011 showed that abiraterone increased overall survival by approximately 4 months in men with metastatic castration-resistant prostate cancer who had previously undergone chemotherapy[44]. A later study showed that it reduced hormone levels not only in the tumor but also in metastatic bone lesions[45].
Although hormone ablation can benefit patients by shrinking their primary tumors, this treatment has been suggested to increase the frequency of skeletal-related events and may in fact promote osteoclastogenesis, thereby “putting fuel on the fire” and increasing bone turnover[46]. Estrogen can inhibit osteoclastogenesis, which may explain why postmenopausal women more commonly suffer from osteoporosis[47]. Also, both estrogen and androgen ablation therapies decrease overall bone mass density, leading to increased risk of fracture independent of metastasis[48]–[50]. However, studies on the selective estrogen response modulators raloxifene and tamoxifen have shown that there is no increased risk of invasive breast cancer, and in fact the risk is decreased in women with postmenopausal osteoporosis[51],[52]. Combined, these data suggest that while hormonal ablation is an important and effective treatment for primary breast and prostate cancers, it may be risky in more aggressive cancers. This topic needs further investigation and clarification.
Anti-resorptive strategies
The theory behind anti-resorptive strategies is that by halting osteoclast-mediated bone resorption, bone turnover is halted, stopping osteolysis and decreasing the size of skeletal metastases. Bisphosphonates are widely used to treat skeletal metastasis and other diseases associated with bone loss, such as osteoporosis and Paget's disease. Bisphosphonates can inhibit bone turnover by causing osteoclast apoptosis in two ways: by acting through toxic metabolites, preventing isoprenylation of small GTP-binding proteins[53]; or by inhibiting farnesyl pyrophosphate synthase, which is essential for stability of the osteoclast cytoskeleton[54],[55]. Additionally, bisphosphonates can directly induce cancer cell apoptosis and inhibit pro-osteoclastic gene expression[56]. The most serious adverse effects of bisphosphonates are renal failure and anaphylaxis[57]; two other rare adverse effects are osteonecrosis of the jaw and atypical femur fractures[58]. Table 3 contains a list of available bisphosphonates.
Table 3. Currently available bisphosphonates.
| Bisphosphonate | Commercial name | Dosage route | Reference |
| Zoledronic acid | Zometa/ Reclast | Intravenous | [57] |
| Pamidronate disodium | Aredia | Intravenous | [211] |
| Alendronate sodium | Fosamax | Oral | [212] |
| Etidronate disodium | Didronel | Oral | [213] |
| Ibandronate sodium | Boniva | Oral | [214] |
| Risedronate sodium | Actonel | Oral | [215] |
| Tiludronate disodium | Skelid | Oral | [216] |
Zoledronic acid (ZA; also known as zolendronate or Zometa) is the most commonly used and most potent bisphosphonate on the market[59]. It can be used at a lower dose (4 mg) than pamidronate (90 mg), another approved bisphosphonate[57]. Treatment with bisphosphonates decreased the number of skeletal-related events and lengthened the time-to-first-skeletal-related event in breast and prostate cancers, as well as in multiple myeloma[57]. Despite their wide use, bisphosphonates have no overall survival or disease-free survival advantage and do not fully inhibit skeletal metastasis or its morbidity. The lack of survival advantage could be because metastatic tumors have already caused debilitating damage in the bone before bisphosphonates are even used for treatment. ZA is now undergoing clinical trials with various other adjuvant therapies[11]. Another interesting use of bisphosphonates was highlighted in a recent study that used alendronate, conjugated to a peptidomimetic ligand against activated integrin α4β1, to serve as a bone-seeking agent to direct the therapeutic compound to the bone[60].
Targeted anti-RANKL therapy is evolving as an anti-resorptive strategy. Denosumab (also known as Xgeva) is a monoclonal antibody that competitively binds RANKL, resulting in potent inhibition of bone resorption[61]. Although mechanistically similar to bisphosphonates, denosumab may be more potent in preventing skeletal-related events in the setting of skeletal metastasis[62]. Denosumab has been shown to decrease the rate of skeletal-related events from 18% (ZA) to 7%[63]. Treatment with denosumab decreased the risk of renal toxicity as compared with bisphosphonates. More data are needed to determine whether anti-RANKL therapy is more efficacious than other anti-resorptive strategies in skeletal metastasis and to determine its relative cost-effectiveness[64]. Denosumab is in clinical trials to determine its effectiveness in combination with other chemotherapies. However, long-term adverse effects could be important; recent studies have shown that patients treated with bisphosphonates for more than 5 years had a higher risk of suffering atypical femur fractures, possibly caused by suppressed, bone turnover-related microdamage[65]. While denosumab has been approved by the FDA for use in non-metastatic prostate cancer patients to reduce the risk of fracture, it was recently rejected for treatment of bone metastasis in prostate cancer patients because it did not significantly increase overall survival[66].
Other RANKL inhibitors, such as RANK-Fc and OPG-Fc, have been developed for treatment of skeletal metastasis. Several animal models that mimic advanced prostate cancer, breast cancer, or non-small cell lung cancer, representing both osteolytic and osteoblastic skeletal lesions, were used to determine whether these RANKL inhibitors were practical and effective in inhibiting bone metastases[67]–[69]. In a murine model of human A431 epidermoid carcinoma bone metastasis, OPG-Fc inhibited RANKL, suppressed tumor-induced osteolysis and ultimately inhibited tumor cell growth in the bone, but there was no effect on the subcutaneous growth of the tumor[70]. The bone metastasis suppression effect of these RANKL inhibitors seems to be more prominent if combined with therapeutics that also target the tumor itself[71].
Although other drugs against bone metastasis have used the same strategy of inhibiting osteoclasts or bone turnover, none have been as successful in clinical trials as bisphosphonates and denosumab. Cathepsin K is a protease involved in bone resorption, and pre-clinical studies inhibiting cathepsin K have shown promise in ablating skeletal metastasis[72]. Balicatib inhibits cathepsin K and showed potential to inhibit osteolysis and regain bone mass, but it was withdrawn from clinical trials due to safety issues[73]; Odanacatib, another cathepsin K inhibitor, was also withdrawn from clinical trials for breast cancer bone metastasis for safety concerns[11],[74]. Most recently, intraperitoneal injections of the cathepsin B inhibitor CA-074 into mice in the 4T1.2 model (a breast cancer model that exhibits spontaneous bone metastases) reduced bone metastases significantly (P < 0.05), indicating an important role for cathepsin B in late-stage skeletal metastasis[75].
Targeted therapies
A promising strategy for treating cancer as a whole, as well as for treating skeletal metastasis, is to focus on specifically up-regulated oncogenic molecular pathways. The principle of this strategy is to target and inhibit pro-metastatic genes to stop or reduce the ability of cancer cells to metastasize. In this section, we will highlight some hopeful molecular targets in the skeletal metastasis field.
PTHrP
Parathyroid hormone-related protein (PTHrP) has important roles during the development of several organs and is dys-regulated in several types of cancer. The expression of PTHrP correlates with prostate cancer progression[76], and PTHrP can increase prostate cancer cell migration and invasion[77]. EB1089, a potent agonist of the vitamin D signaling, can reverse these effects by down-regulating PTHrP[77]. Furthermore, PTHrP increases osteoblastic cell survival through the vascular endothelial growth factor receptor 2 (VEGFR2) pathway, again indicating its importance in prostate cancer bone metastases[78]. PTHrP has also been linked to breast and lung cancer skeletal metastases[79]–[81]. Humanized monoclonal antibodies that block PTHrP binding to its receptor reduce bone metastasis in a breast cancer model[82] and in a lung cancer model[81].
TGFβ
Transforming growth factor beta (TGFβ) is important during development, and its up-regulation has long been known as a crucial factor in promoting cancer metastasis[83],[84]. TGFβ is the most abundant cytokine in the bone matrix[85],[86]. Its release during bone resorption helps induce bone marrow stromal cells, serving to couple bone resorption and formation during development and bone remodeling[87],[88]. Because TGFβ can induce EMT in tumor cells, it likely has a major role in metastasis, and as such represents a major target for treatment[89]. Ki26894, a novel inhibitor of TGFβ, inhibited the ability of breast tumor cells to secrete PTHrP, reduced the frequency of bone metastases, and increased the life span of mice injected with breast cancer cells[90]. When the dominant-negative mutant TβRIIΔcyt, which is unresponsive to TGFβ, was overexpressed in breast cancer cells injected into mice, skeletal metastasis and bone destruction were reduced, and overall survival increased[91]. Interestingly, this phenotype was shown to be due to a decrease in PTHrP expression[91]. The TGFβ signaling is a biomarker of poor prognosis in prostate cancer[92]. The TGFβ receptor type I (TGFβRI) inhibitor LY2109761 not only decreased tumor growth in the bone but increased bone mass[93]. Finally, oncolytic viruses expressing TGFβ receptor type II (TGFβRII) fused to human Fc (Ad.sTβRFc) reduced skeletal metastasis and associated hypercalcemia, indicating a therapeutic potential[94].
MET/VEGF
Cabozantinib (XL 184) has shown promise in clinical trials. It inhibits the activity of several receptor kinases, most potently hepatocyte growth factor receptor (MET) and vascular endothelial growth factor receptor (VEGFR), which are functionally linked[95],[96]. Studies have suggested that bone formation and angiogenesis are coupled, indicating that VEGF inhibition would be an important strategy for halting skeletal metastasis[83]. In one clinical trial in which 62 patients underwent cabozantinib treatment, 85% achieved complete or partial resolution of metastatic lesions upon bone scan[97]. Cabozantinib is now in phase III clinical trials for castration-resistant prostate cancer and will be used in at least 12 other clinical trials for other tumor types.
There are other molecular targets that also have promise in this field. Notably, the inhibition of Src kinase and mammalian target of rapamycin (mTOR) has each been shown to have positive effects on reducing bone metastasis[98],[99]. These and other targets can be found in recent reviews[41],[100]–[103].
Summary
In each of these pre-clinical models or clinical cases, investigators have reported that inhibition of a specific molecular pathway reduced the frequency of skeletal metastasis, increased patient survival, or both. However, there is still no standard treatment for patients suffering from painful and lethal skeletal metastasis. This suggests that skeletal metastasis is more complex than originally imagined and that we as a research community have not yet identified appropriate targets or combinations of therapies to successfully manage and cure this disease. In the past ten or more years, there have been 8 negative phase III clinical trials for increasing the overall survival of men with skeletal metastatic prostate cancer. This has continued with the failure of denosumab to increase life span. Strategies that target only one cell type or microenvironment, such as the tumor or the bone niche, are not likely to succeed. Accordingly, the need for better experimental models, novel clinical targets, and better use of the knowledge we have is glaringly apparent.
The Wnt Signaling in Skeletal Metastasis
The roles of the Wnt/β-catenin signaling (Figure 2) in tumorigenesis, bone development, and skeletal metastasis have been extensively studied. The Wnt signaling is important in prostate cancer tumorigenesis[104]–[109] and is positively correlated with the progression of prostate cancer[110]–[114]. While breast cancer tumorigenesis and poor prognosis have been associated with increased Wnt signaling[115]–[119], there is less evidence to suggest that active Wnt signaling induces skeletal metastasis[120]. However, recent studies have shown an association between increased β-catenin-independent Wnt5a expression and brain metastasis[121]. There is also strong evidence that the Wnt signaling is crucial in the development of the breast and prostate glands[122]. Lung tumorigenesis is also associated with up-regulated Wnt signaling[123]–[126]. Cigarette smoke, which increases the risk for lung cancer substantially, may even activate the Wnt signaling[127].
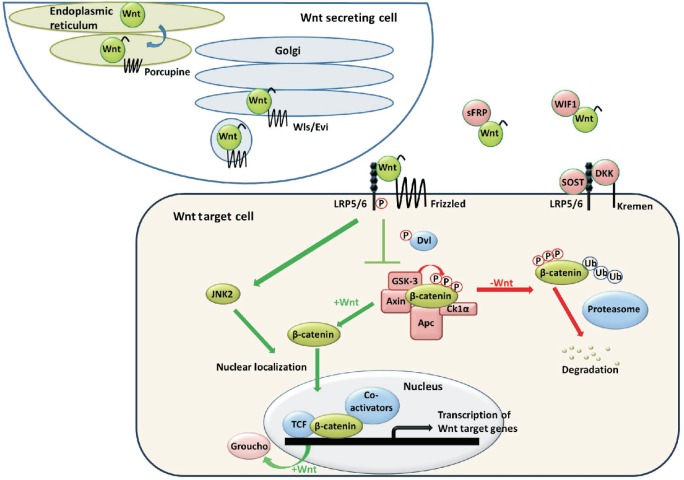
Figure 2. The Wnt/β-catenin signaling pathway.
Wnt is secreted, binds to receptors, and causes β-catenin to accumulate in the cytosol and subsequently migrate to the nucleus to activate transcription of Wnt target genes. Wls/Evi, Wntless/evenness interrupted; sFRP, secreted frizzled-related protein; WIF1, Wnt inhibitory factor 1; SOST, sclerostin; DKK1, Dickkopf 1; LRP5/6, low-density lipoprotein receptor-related protein 5/6; Dvl, disheveled; GSK3, glycogen synthase kinase 3; Apc, adenomatous polyposis coli; CK1α, casein kinase 1 alpha; JNK2, c-Jun N-terminal kinase 2; TCF, T-cell factor. The authors have published this figure previously in "Wnt/β-catenin signaling in normal and cancer stem cells" [Cancer, 2011,3:2050-2079] and have got the permission to republish this figure.
One of the well-established roles of the Wnt signaling is the activation of the osteoblast lineage from mesenchymal stem cells and the maintenance of bone homeostasis[128]. Based on studies using genetically engineered mice, the downstream effector molecules in the canonical Wnt pathway (such as β-catenin) and the endogenous inhibitors of the Wnt signaling [such as Dickkopf-1 (Dkk1) and sclerostin] play profound regulatory roles by direct action on osteoblasts or indirect action on osteoclasts[129]–[132]. In addition, Wnt proteins seem to have a role in regeneration during bone healing[133]. These data suggest that the Wnt signaling is crucial for bone development and turnover and, as such, is implicated in the interruption of bone homeostasis during skeletal metastasis and could be an important player in the development and progression of these lesions.
In prostate cancer, the Wnt signaling is involved in osteoblastic bone metastasis. Wnt1 and β-catenin expression levels are low in normal prostate cells but high in prostate cancer cells; high levels are seen in 85% of human skeletal metastases[110]. Two studies in prostate cancer cell lines have shown that inhibition of the Wnt signaling (via overexpression of the endogenous Wnt inhibitory factor WIF1 or knockdown of β-catenin itself) causes a reversal of EMT and the metastatic phenotype[134],[135]. Hall et al.[112] showed that knockdown of the Wnt inhibitor Dkk1 in osteolytic PC3 xenografts induced osteoblastic activity. Alternatively, when Dkk1 was overexpressed in osteoblastic C42B4 xenografts, the tumors became highly osteolytic. Taken together, this indicates that induction of the Wnt signaling from prostate cancer tumor cells contributes to osteoblastic skeletal metastases[112].
Active Wnt signaling induces bone morphogenetic protein activity, and Wnt can induce osteoblastic activity in culture both in BMP-dependent and BMP-independent fashions[136]. Additionally, loss of Dkk1 promotes osteoblastic activity and the expression of BMP4 and BMP6[136]. A study using a canine prostate cancer model showed that Dkk1 overexpression potently inhibited bone growth in a xenograft model, but it also induced primary tumor growth and increased the rate of metastasis[137]. When osteoblast cultures from Lrp5-knockout mice were treated with medium from a prostate tumor cell line capable of inducing osteoblastic metastases in vivo, there was no effect, suggesting that at least in this cell line (MDA PCa 2b), prostate cancer-induced osteoblastic activity is mediated by the Wnt receptor Lrp5[138]. These data strongly indicate that the Wnt signaling is active and potentially causative in prostate cancer skeletal metastasis. This idea may contrast with data from genetically engineered mouse models in which activation of the Wnt signaling by knockout of adenomatous polyposis coli (Apc)[107] or direct activation of β-catenin[108] induced carcinoma or prostate intraepithelial neoplasia (PIN), respectively, but did not produce skeletal metastasis. Induction of the Wnt signaling at an early stage may cause non-metastatic prostate cancer, and induction of the Wnt signaling at a late stage may cause osteoblastic metastases.
Breast and lung metastases are often osteolytic, and dys-regulation of the Wnt signaling has a role in bone metastasis in these cancers. Dkk1 expression is up-regulated in osteolytic breast cancer bone metastases, resulting in repression of the Wnt signaling[139],[140]. Breast cancer cells treated with Wnt3a-conditioned media caused osteoblastic metastases and RANKL reduction, and this process was blocked by Dkk1-conditioned media from typically osteolytic breast cancer cells[139]. These data indicate that, in contrast to prostate cancer, breast cancer operates via repression of the Wnt signaling rather than induction, thereby producing osteolytic lesions. On the other hand, the Wnt signaling stimulates the motility of breast cancer cells, and the expression of the Wnt inhibitor secreted frizzled-related protein (sFRP1) can inhibit this motility[141]. More work is needed to determine the role of the Wnt signaling in the processes of primary tumorigenesis, EMT, and skeletal metastasis of breast cancer cells.
In lung cancer, the Wnt/TCF4 pathway signature in a cohort of 368 primary tumors was significantly correlated with relapse (P = 0.006)[123]. Additionally, a specific Wnt-responsive gene set was associated with lung cancer metastasis, and expression of a dominant negative version of the Wnt transcription factor TCF4 in a xenograft mouse model resulted in significantly fewer bone metastases[123]. Another group has shown that activation of β-catenin in lung epithelial cells induces EMT; interestingly, this phenotype was reversed after knockdown of Jun N-terminal kinase (JNK1)[142]. Finally, Wnt2 was overexpressed in lung cancer tissue, and when Wnt2 was inhibited with a monoclonal antibody or siRNA, apoptosis occurred in lung cancer cells[143]. These data indicate that aberrant Wnt signaling plays an important role in lung cancer cell survival and skeletal metastasis.
The Wnt signaling is crucial for the proper development of numerous cell and tissue types, as well as for maintenance of adult stem cell populations[144]. Because of this role, the Wnt signaling may contribute to metastasis by causing cells to de-differentiate and become more mesenchymal-like or stem-like[145],[146]. The Wnt signaling increases the capacity of mesenchymal stem cells to proliferate and migrate, which indicates that the Wnt signaling is important during tissue regenerative processes, but also suggests that up-regulated Wnt signaling could induce higher migration and invasion of tumor cells having undergone EMT[147]. In addition, the Wnt signaling promotes the self-renewal of prostate stem-like cells and growth of prostaspheres in culture, suggesting that inhibition of the Wnt signaling could have therapeutic value by reducing this stem-like potential[109]. Similar conclusions were drawn from studies of breast and lung cells[120],[148],[149].
Due to the well-established role of the Wnt signaling in cancer, EMT, and skeletal metastasis, the Wnt pathway represents an excellent target for therapy. The pathway is complex and has many components that may represent therapeutic targets[150]. There are also many mechanisms by which the pathway can be inhibited[122]. A new way of inhibiting the Wnt signaling is through porcupine inhibitors[151]. Porcupine is a membrane-bound O-acetyltransferase (MBOAT) that is required for Wnt ligand palmitoylation and secretion[152]. It is unclear whether porcupine inhibitors will have a substantial effect in cancer, but there are already pre-clinical trials to test that[153]. Several excellent reviews provide insight into the many facets of drugging the Wnt pathway in cancer[154]–[156].
Because the Wnt signaling can promote both tumorigenesis and osteoblast-mediated bone formation, it is unclear how the Wnt pathway should be modulated as a treatment in skeletal metastasis. Some studies show that promotion of the Wnt signaling via Dkk1 inhibition increased bone formation in osteolytic lesions and decreased osteolysis-related pain and risk of fracture[139],[157],[158]. Others have shown that inhibition of Dkk1 results in loss of control of the Wnt signaling and promotes proliferation and metastasis of cancer cells[137],[159]. The level of the Wnt signaling taking place in the microenvironment of both the primary tumor and skeletal metastasis may be unique for each individual tumor. Therefore, the most likely use of Wnt inhibition will be against tumors in which the Wnt signaling is highly up-regulated, but all indications suggest that the strength and specificity of Wnt inhibition must be strictly regulated to be of utmost benefit to the patient.
Mouse Models of Skeletal Metastasis
Mouse models have provided many insights into human disease. The ideal mouse model for skeletal metastasis would reproduce all the stages of tumorigenesis and metastasis, including genetic changes, metastatic phenotype of cancer cells, and modifications to the bone microenvironment. Obviously, this is a difficult goal to achieve. Genetically engineered mouse models are generally considered to be an accurate way to mimic human cancer, but despite numerous attempts, there is currently no genetically engineered mouse model that accurately and consistently recapitulates the skeletal metastasis process[146],[160].
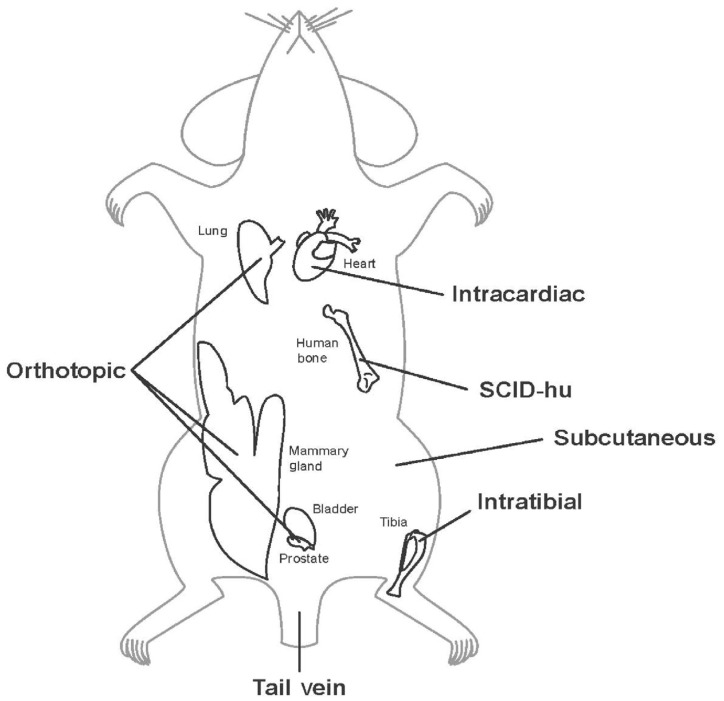
Consequently, the present paradigm for modeling skeletal metastasis is the xenograft mouse model, in which cells from human cancer cell lines or primary tumors are injected into immune-deficient mice. These cells can form primary tumors that metastasize to the bone and other organs. Several immune-deficient mice are available for such models, including athymic nude, severe combined immune deficient (SCID), non-obese diabetic (NOD-SCID), and NOD-SCID-ILR2Gamma-deficient (NSG) mice[146]. Mouse models of skeletal metastasis can be categorized based on the injection site: orthotopic, intraosseous, intracardiac, intravenous, or subcutaneous (Figure 3). The goal of these models is not only to discover the physiological roles of molecular pathways and of the unique interactions between tumor and the bone but also to determine the effects of potential and novel therapeutics.
Figure 3. Experimental models of skeletal metastasis.
Tumor cells can be injected at any of the sites shown to generate a metastasis model. Note that the intracardiac, intravenous, and intratibial models can only establish models of experimental metastasis that do not reflect the entire process of metastasis (see the description of the process of metastasis in the “Skeletal Metastasis” section of this review). However, spontaneous metastasis can be achieved by the orthotopic and subcutaneous models, which generate primary tumors prior to metastasizing.
Orthotopic injections
Orthotopic prostate injections are commonly done into the ventral or dorsal lobes of the murine prostate, as they are the most readily accessible and most anatomically similar to the human prostate[161]. Mammary orthotopic injections are generally done into the 4th mammary fat pad of a 4- to 5-week-old mouse, which has been surgically cleared of mammary epithelium tissue, leaving behind the fat[160]. Lung orthotopic injections are through the chest and directly into the lung[162],[163].
Because skeletal metastases are relatively rare in orthotopic models, such studies are generally centered on the development and growth of primary tumors. This model can be used to study the effect of novel drugs on tumor volume or on the ability of the tumor to invade surrounding stroma or to metastasize to lymph nodes. If skeletal metastasis can be induced using this type of injection, it provides valuable information as to how cancer cells go through the entire process of primary tumor formation through the growth and maintenance of a metastatic lesion. Cancer cells injected into the organ of their origin theoretically behave as they would in the original setting because the microenvironment of the primary tumor host organ is thought to be important for the development of metastasis[164]. However, the microenvironments of the human and the mouse differ, which may explain why so few bone metastases occur in these mouse models.
Another important use of orthotopic models is to select cell lines that are more metastatic or more likely to metastasize to particular organs[165]. For instance, the C4-2 prostate cancer cell lines were derived from the LNCaP cell line that was orthotopically and subcutaneously injected into nude mice. After the mice were castrated and metastasis occurred, metastatic cells were removed from lymph nodes or the bone and immortalized into novel cell lines, called C4-2B (derived from bone metastasis) and C4-2Ln (derived from lymph node metastasis)[166],[167]. C4-2B cells are now commonly used to induce prostate cancer skeletal metastasis in research into the efficacy of novel therapeutics.
Intraosseous injections
Intraosseous injections are generally done in the tibia or femur of the mouse. This model is not a literal metastatic model because the cells do not need to metastasize from the primary tumor to a distant site. However, this model can provide information about how cancer cells grow and maintain tumors in the bone, as well as about the interactions of cancer cells with the bone microenvironment. It is easier to induce tumor growth in the bone using intraosseous injections than any other type of injection[168].
Because of the bone remodeling that takes place in the multitude of mouse models, the pathology of the bones in these models must be extensively characterized. Assays can be conducted to ascertain the osteoblastic or osteoclastic nature of the bones and the lesions in them. Radiographic imaging is commonly used to evaluate the local bone mineral density or bone morphologic changes[136]. Histological tartrate resistant acid phosphatase (TRAP) staining is used to look into the in vivo osteoclastogenesis induced by cancer cells, and it can also be used for in vitro co-culture assays. Alkaline phosphatase staining can be used to test osteoblast activity, and Von Kossa staining or alizarin red staining can be used to assess bone mineralization[112]. In addition, single-photon emission computed tomography (SPECT) is a valuable imaging tool to determine where bone turnover is occurring, and it can be used to calculate specific bone parameters and their changes.
Intracardiac, intravenous, and subcutaneous injections
Compared with direct intraosseous injection (which is mainly for studying tumor-bone interactions), intracardiac, intravenous, or subcutaneous injections better mimic spontaneous bone metastasis in humans. In these models, metastases most commonly form in the lungs because of the direct injection of cells into the blood stream[169], avoiding the steps of invasion and intravasation of tumor cells. These types of injections allow us to study the types of homing that occur during metastasis and to examine the interaction of tumor cells with the microenvironment of the tissue being invaded.
SCID-human models
One model that could incorporate any type of injection described above is the implantation of human adult or fetal bone together with subcutaneous injection of cancer cells into immune-deficient mice[170]–[172]. This model is commonly referred to the SCID-hu model. This model provides a human bone microenvironment on which cancer cells can home, providing a more accurate representation of human skeletal metastasis. In several experiments, the human cancer cells preferentially homed to the human bone over mouse bone[173]. A possible negative to this model is that the fetal bone is mostly woven bone, whereas adult bone consists of mature lamellar bone. If adult human bone is used, there are many biological variables arising from the source of the bone that could complicate the interpretation of results.
Summary
No current experimental model accurately recapitulates bone metastasis, so researchers often choose a model having a specific characteristic relative to the system they are studying. It is unlikely that there will ever be a perfect mouse model of skeletal metastasis, due to the heterogeneous nature of cancer and metastasis, envi-ronmental factors that cannot be mimicked, and the fact that human and mouse genetics and physiology are not identical. Today, however, the mouse model is the mainstay of experimentation in the skeletal metastasis field. There are several excellent reviews that provide additional information on mouse models and their role in skeletal metastasis research[174]–[176].
Conclusion
Metastasis and growth of cancer cells in the bone is a complex problem with no reliable cure. The current clinical treatments do little to prolong the life span of patients. Future treatments will likely target not only the primary tumor but also components of the microenvironment surrounding tumor lesions. The solution likely lies in the synthesis of ideas and technologies from several fields. Because of the role of the Wnt signaling in carcinogenesis and metastasis, the potential for Wnt antagonists as either primary or adjuvant therapeutics is an area of promising study. In humans, aberrant Wnt activation is seen in primary tumors, and increases in osteoblastic metastatic bone lesions. In mice, the Wnt signaling can induce cancer when activated in epithelial cells. So far, it appears that activated Wnt signaling in mice causes carcinoma in situ, but no metastasis has been found in these models. A significant knowledge gap in the field is the reason that activating Wnt in the mouse does not result in metastatic cancer. Several potential explanations exist for this. The Wnt signaling may be a second genetic event that pushes primary tumors toward a metastatic phenotype. In prostate cancer, Wnt activation may increase in response to decreasing levels of circulating androgens during androgen deprivation therapy and then physically bind to the androgen receptor to induce castration-resistant prostate cancer. It is possible that these differences are due to the species-specific differences. However, it is likely that mouse models will continue to provide valuable information for these studies. Improvement in the ability of these models to accurately replicate key aspects of human prostate tumorigenesis will allow assessment of these difficult questions and provide the foundation for the development of improved treatment of cancer patients with skeletal metastasis.
References
- 1.Atlanta: American Cancer Society, Inc; 2011. Cancer facts & figures. [Google Scholar]
- 2.Hess KR, Varadhachary GR, Taylor SH, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006;106:1624–1633. doi: 10.1002/cncr.21778. [DOI] [PubMed] [Google Scholar]
- 3.Pockett RD, Castellano D, McEwan P, et al. The hospital burden of disease associated with bone metastases and skeletal-related events in patients with breast cancer, lung cancer, or prostate cancer in spain. Eur J Cancer Care (Engl) 2010;19:755–760. doi: 10.1111/j.1365-2354.2009.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiong J, Onal M, Jilka RL, et al. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen DX, Massague J. Genetic determinants of cancer metastasis. Nat Rev Genet. 2007;8:341–352. doi: 10.1038/nrg2101. [DOI] [PubMed] [Google Scholar]
- 7.Chiang AC, Massague J. Molecular basis of metastasis. N Engl J Med. 2008;359:2814–2823. doi: 10.1056/NEJMra0805239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–573. [PubMed] [Google Scholar]
- 9.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 10.Kang Y, Siegel PM, Shu W, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 11.Sturge J, Caley MP, Waxman J. Bone metastasis in prostate cancer: emerging therapeutic strategies. Nat Rev Clin Oncol. 2011;8:357–368. doi: 10.1038/nrclinonc.2011.67. [DOI] [PubMed] [Google Scholar]
- 12.Demers LM, Costa L, Lipton A. Biochemical markers and skeletal metastases. Cancer. 2000;88:2919–2926. doi: 10.1002/1097-0142(20000615)88:12+<2919::aid-cncr7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 13.Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 14.Sun YX, Fang M, Wang J, et al. Expression and activation of alpha v beta 3 integrins by sdf-1/cxc12 increases the aggressiveness of prostate cancer cells. Prostate. 2007;67:61–73. doi: 10.1002/pros.20500. [DOI] [PubMed] [Google Scholar]
- 15.Pauli BU, Augustin-Voss HG, el-Sabban ME, et al. Organ-preference of metastasis. The role of endothelial cell adhesion molecules. Cancer Metastasis Rev. 1990;9:175–189. doi: 10.1007/BF00046359. [DOI] [PubMed] [Google Scholar]
- 16.Kelly T, Suva LJ, Huang Y, et al. Expression of heparanase by primary breast tumors promotes bone resorption in the absence of detectable bone metastases. Cancer Res. 2005;65:5778–5784. doi: 10.1158/0008-5472.CAN-05-0749. [DOI] [PubMed] [Google Scholar]
- 17.McAllister SS, Gifford AM, Greiner AL, et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell. 2008;133:994–1005. doi: 10.1016/j.cell.2008.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lynch CC, Hikosaka A, Acuff HB, et al. Mmp-7 promotes prostate cancer-induced osteolysis via the solubilization of rankl. Cancer Cell. 2005;7:485–496. doi: 10.1016/j.ccr.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11:411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiozawa Y, Pedersen EA, Havens AM, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011;121:1298–1312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norgaard M, Jensen AO, Jacobsen JB, et al. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in denmark (1999 to 2007) J Urol. 2010;184:162–167. doi: 10.1016/j.juro.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 22.Bubendorf L, Schopfer A, Wagner U, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 23.Jensen AO, Jacobsen JB, Norgaard M, et al. Incidence of bone metastases and skeletal-related events in breast cancer patients: a population-based cohort study in denmark. BMC Cancer. 2011;11:29. doi: 10.1186/1471-2407-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stenbygaard LE, Sorensen JB, Olsen JE. Metastatic pattern at autopsy in non-resectable adenocarcinoma of the lung—a study from a cohort of 259 consecutive patients treated with chemotherapy. Acta Oncol. 1997;36:301–306. doi: 10.3109/02841869709001267. [DOI] [PubMed] [Google Scholar]
- 25.Lee YT. Breast carcinoma: pattern of metastasis at autopsy. J Surg Oncol. 1983;23:175–180. doi: 10.1002/jso.2930230311. [DOI] [PubMed] [Google Scholar]
- 26.Steensma M, Boland PJ, Morris CD, et al. Endoprosthetic treatment is more durable for pathologic proximal femur fractures. Clin Orthop Relat Res. 2012;470:920–926. doi: 10.1007/s11999-011-2047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aboulafia AJ, Levine AM, Schmidt D, et al. Surgical therapy of bone metastases. Semin Oncol. 2007;34:206–214. doi: 10.1053/j.seminoncol.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Hansen BH, Keller J, Laitinen M, et al. The scandinavian sarcoma group skeletal metastasis register. Survival after surgery for bone metastases in the pelvis and extremities. Acta Orthop Scand Suppl. 2004;75:11–15. doi: 10.1080/00016470410001708270. [DOI] [PubMed] [Google Scholar]
- 29.Luketich JD, Martini N, Ginsberg RJ, et al. Successful treatment of solitary extracranial metastases from non-small cell lung cancer. Ann Thorac Surg. 1995;60:1609–1611. doi: 10.1016/0003-4975(95)00760-1. [DOI] [PubMed] [Google Scholar]
- 30.Wedin R, Falkenius J, Weiss RJ, et al. Surgical treatment of skeletal metastases in 31 melanoma patients. Acta Orthop Belg. 2012;78:246–253. [PubMed] [Google Scholar]
- 31.Lin PP, Mirza AN, Lewis VO, et al. Patient survival after surgery for osseous metastases from renal cell carcinoma. J Bone Joint Surg Am. 2007;89:1794–1801. doi: 10.2106/JBJS.F.00603. [DOI] [PubMed] [Google Scholar]
- 32.Martini N, Huvos AG, Burt ME, et al. Predictors of survival in malignant tumors of the sternum. J Thorac Cardiovasc Surg. 1996;111:96–105; discussion 105–106. doi: 10.1016/S0022-5223(96)70405-1. [DOI] [PubMed] [Google Scholar]
- 33.Feyer PC, Steingraeber M. Radiotherapy of bone metastasis in breast cancer patients—current approaches. Breast Care (Basel) 2012;7:108–112. doi: 10.1159/000338724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vakaet LA, Boterberg T. Pain control by ionizing radiation of bone metastasis. Int J Dev Biol. 2004;48:599–606. doi: 10.1387/ijdb.041817lv. [DOI] [PubMed] [Google Scholar]
- 35.Maranzano E, Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: final results from a prospective trial. Int J Radiat Oncol Biol Phys. 1995;32:959–967. doi: 10.1016/0360-3016(95)00572-g. [DOI] [PubMed] [Google Scholar]
- 36.Yamada Y. Treating spinal bone metastasis with image-guided, high-dose radiation. Varian Medical Systems. 2008:1–4. [Google Scholar]
- 37.Koyfman SA, Djemil T, Burdick MJ, et al. Marginal recurrence requiring salvage radiotherapy after stereotactic body radio-therapy for spinal metastases. Int J Radiat Oncol Biol Phys. 2012;83:297–302. doi: 10.1016/j.ijrobp.2011.05.067. [DOI] [PubMed] [Google Scholar]
- 38.Cheetham PJ, Petrylak DP. Alpha particles as radiopharmaceu-ticals in the treatment of bone metastases: Mechanism of action of radium-223 chloride (alpharadin) and radiation protection. Oncology (Williston Park) 2012;26:330–337, 341. [PubMed] [Google Scholar]
- 39.Carlson RW, Allred DC, Anderson BO, et al. Breast cancer. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2009;7:122–192. doi: 10.6004/jnccn.2009.0012. [DOI] [PubMed] [Google Scholar]
- 40.Sternberg CN. Systemic chemotherapy and new experimental approaches in the treatment of metastatic prostate cancer. Ann Oncol. 2008;19(Suppl. 7):vii91–95. doi: 10.1093/annonc/mdn473. [DOI] [PubMed] [Google Scholar]
- 41.Loriot Y, Massard C, Fizazi K. Recent developments in treatments targeting castration-resistant prostate cancer bone metastases. Ann Oncol. 2012;23:1085–1094. doi: 10.1093/annonc/mdr573. [DOI] [PubMed] [Google Scholar]
- 42.Makarov DV, Humphreys EB, Mangold LA, et al. The natural history of men treated with deferred androgen deprivation therapy in whom metastatic prostate cancer developed following radical prostatectomy. J Urol. 2008;179:156–161; discussion 161–152. doi: 10.1016/j.juro.2007.08.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Donnell A, Judson I, Dowsett M, et al. Hormonal impact of the 17alpha-hydroxylase/c(17,20)-lyase inhibitor abiraterone acetate (cb7630) in patients with prostate cancer. Br J Cancer. 2004;90:2317–2325. doi: 10.1038/sj.bjc.6601879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Efstathiou E, Titus M, Tsavachidou D, et al. Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone. J Clin Oncol. 2012;30:637–643. doi: 10.1200/JCO.2010.33.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corey E, Quinn JE, Vessella RL. A novel method of generating prostate cancer metastases from orthotopic implants. Prostate. 2003;56:110–114. doi: 10.1002/pros.10235. [DOI] [PubMed] [Google Scholar]
- 47.Khosla S. Update on estrogens and the skeleton. J Clin Endocrinol Metab. 2010;95:3569–3577. doi: 10.1210/jc.2010-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shahinian VB, Kuo YF, Freeman JL, et al. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 49.Smith MR, Lee WC, Brandman J, et al. Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005;23:7897–7903. doi: 10.1200/JCO.2004.00.6908. [DOI] [PubMed] [Google Scholar]
- 50.Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA. 2004;292:490–495. doi: 10.1001/jama.292.4.490. [DOI] [PubMed] [Google Scholar]
- 51.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 52.Martino S, Cauley JA, Barrett-Connor E, et al. Continuing outcomes relevant to Evista: breast cancer incidence in postmeno pausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751–1761. doi: 10.1093/jnci/djh319. [DOI] [PubMed] [Google Scholar]
- 53.Luckman SP, Hughes DE, Coxon FP, et al. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13:581–589. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 54.Lipton A, Uzzo R, Amato RJ, et al. The science and practice of bone health in oncology: managing bone loss and metastasis in patients with solid tumors. J Natl Compr Canc Netw. 2009;7(Suppl 7):S1–29; quiz S30. doi: 10.6004/jnccn.2009.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032–1045. doi: 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Asahi H, Mizokami A, Miwa S, et al. Bisphosphonate induces apoptosis and inhibits pro-osteoclastic gene expression in prostate cancer cells. Int J Urol. 2006;13:593–600. doi: 10.1111/j.1442-2042.2006.01360.x. [DOI] [PubMed] [Google Scholar]
- 57.Ibrahim A, Scher N, Williams G, et al. Approval summary for zoledronic acid for treatment of multiple myeloma and cancer bone metastases. Clin Cancer Res. 2003;9:2394–2399. [PubMed] [Google Scholar]
- 58.Puhaindran ME, Farooki A, Steensma MR, et al. Atypical subtrochanteric femoral fractures in patients with skeletal malignant involvement treated with intravenous bisphosphonates. J Bone Joint Surg Am. 2011;93:1235–1242. doi: 10.2106/JBJS.J.01199. [DOI] [PubMed] [Google Scholar]
- 59.Li EC, Davis LE. Zoledronic acid: a new parenteral bisphos-phonate. Clin Ther. 2003;25:2669–2708. doi: 10.1016/s0149-2918(03)80327-2. [DOI] [PubMed] [Google Scholar]
- 60.Guan M, Yao W, Liu R, et al. Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass. Nat Med. 2012;18:456–462. doi: 10.1038/nm.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bekker PJ, Holloway DL, Rasmussen AS, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res. 2004;19:1059–1066. doi: 10.1359/JBMR.040305. [DOI] [PubMed] [Google Scholar]
- 62.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fizazi K, Bosserman L, Gao G, et al. Denosumab treatment of prostate cancer with bone metastases and increased urine N-telopeptide levels after therapy with intravenous bisphosphonates: results of a randomized phase ii trial. J Urol. 2009;182:509–515; discussion 515–506. doi: 10.1016/j.juro.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 64.Xie J, Namjoshi M, Wu EQ, et al. Economic evaluation of denosumab compared with zoledronic acid in hormone-refractory prostate cancer patients with bone metastases. J Manag Care Pharm. 2011;17:621–643. doi: 10.18553/jmcp.2011.17.8.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lenart BA, Lorich DG, Lane JM. Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate. N Engl J Med. 2008;358:1304–1306. doi: 10.1056/NEJMc0707493. [DOI] [PubMed] [Google Scholar]
- 66.Mechcatie E. FDA panel rejects denosumab against bone metastasis in prostate cancer. Internal Medicine News. Available at: http://www.internalmedicinenews.com/single-view/fda-panel-rejects-denosumab-against-bone-metastasis-in-prostate-cancer/839e175a56.html. 2012.
- 67.Armstrong AP, Miller RE, Jones JC, et al. RANKL acts directly on RANK-expressing prostate tumor cells and mediates migration and expression of tumor metastasis genes. Prostate. 2008;68:92–104. doi: 10.1002/pros.20678. [DOI] [PubMed] [Google Scholar]
- 68.Canon JR, Roudier M, Bryant R, et al. Inhibition of RANKL blocks skeletal tumor progression and improves survival in a mouse model of breast cancer bone metastasis. Clin Exp Metastasis. 2008;25:119–129. doi: 10.1007/s10585-007-9127-1. [DOI] [PubMed] [Google Scholar]
- 69.Zhang J, Dai J, Yao Z, et al. Soluble receptor activator of nuclear factor kappaB Fc diminishes prostate cancer progression in bone. Cancer Res. 2003;63:7883–7890. [PubMed] [Google Scholar]
- 70.Canon J, Bryant R, Roudier M, et al. Inhibition of RANKL increases the anti-tumor effect of the EGFR inhibitor panitu-mumab in a murine model of bone metastasis. Bone. 2010;46:1613–1619. doi: 10.1016/j.bone.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 71.Holland PM, Miller R, Jones J, et al. Combined therapy with the RANKL inhibitor RANK-Fc and rhApo2l/TRAIL/dulanermin reduces bone lesions and skeletal tumor burden in a model of breast cancer skeletal metastasis. Cancer Biol Ther. 2010;9:539–550. doi: 10.4161/cbt.9.7.11266. [DOI] [PubMed] [Google Scholar]
- 72.Le Gall C, Bellahcene A, Bonnelye E, et al. A cathepsin K inhibitor reduces breast cancer induced osteolysis and skeletal tumor burden. Cancer Res. 2007;67:9894–9902. doi: 10.1158/0008-5472.CAN-06-3940. [DOI] [PubMed] [Google Scholar]
- 73.Peroni A, Zini A, Braga V, et al. Drug-induced morphea: report of a case induced by balicatib and review of the literature. J Am Acad Dermatol. 2008;59:125–129. doi: 10.1016/j.jaad.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 74.Jensen AB, Wynne C, Ramirez G, et al. The cathepsin K inhibitor odanacatib suppresses bone resorption in women with breast cancer and established bone metastases: results of a 4-week, double-blind, randomized, controlled trial. Clin Breast Cancer. 2010;10:452–458. doi: 10.3816/CBC.2010.n.059. [DOI] [PubMed] [Google Scholar]
- 75.Withana NP, Blum G, Sameni M, et al. Cathepsin B inhibition limits bone metastasis in breast cancer. Cancer Res. 2012;72:1199–1209. doi: 10.1158/0008-5472.CAN-11-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liao J, Li X, Koh AJ, et al. Tumor expressed PTHrP facilitates prostate cancer-induced osteoblastic lesions. Int J Cancer. 2008;123:2267–2278. doi: 10.1002/ijc.23602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bhatia V, Saini MK, Shen X, et al. EB1089 inhibits the parathyroid hormone-related protein-enhanced bone metastasis and xenograft growth of human prostate cancer cells. Mol Cancer Ther. 2009;8:1787–1798. doi: 10.1158/1535-7163.MCT-09-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alonso V, de Gortazar AR, Ardura JA, et al. Parathyroid hormone-related protein (107-139) increases human osteoblastic cell survival by activation of vascular endothelial growth factor receptor-2. J Cell Physiol. 2008;217:717–727. doi: 10.1002/jcp.21547. [DOI] [PubMed] [Google Scholar]
- 79.Li J, Karaplis AC, Huang DC, et al. PTHrP drives breast tumor initiation, progression, and metastasis in mice and is a potential therapy target. J Clin Invest. 2011;121:4655–4669. doi: 10.1172/JCI46134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guise TA, Yin JJ, Taylor SD, et al. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Invest. 1996;98:1544–1549. doi: 10.1172/JCI118947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Iguchi H, Tanaka S, Ozawa Y, et al. An experimental model of bone metastasis by human lung cancer cells: the role of parathyroid hormone-related protein in bone metastasis. Cancer Res. 1996;56:4040–4043. [PubMed] [Google Scholar]
- 82.Saito H, Tsunenari T, Onuma E, et al. Humanized monoclonal antibody against parathyroid hormone-related protein suppresses osteolytic bone metastasis of human breast cancer cells derived from MDA-MB-231. Anticancer Res. 2005;25:3817–3823. [PubMed] [Google Scholar]
- 83.Coleman RE, Lipton A, Roodman GD, et al. Metastasis and bone loss: advancing treatment and prevention. Cancer Treat Rev. 2010;36:615–620. doi: 10.1016/j.ctrv.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Javelaud D, Alexaki VI, Dennler S, et al. TGF-beta/SMAD/GLI2 signaling axis in cancer progression and metastasis. Cancer Res. 2011;71:5606–5610. doi: 10.1158/0008-5472.CAN-11-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bismar H, Kloppinger T, Schuster EM, et al. Transforming growth factor beta (TGF-beta) levels in the conditioned media of human bone cells: relationship to donor age, bone volume, and concentration of TGF-beta in human bone matrix in vivo. Bone. 1999;24:565–569. doi: 10.1016/s8756-3282(99)00082-4. [DOI] [PubMed] [Google Scholar]
- 86.Seyedin SM, Thomas TC, Thompson AY, et al. Purification and characterization of two cartilage-inducing factors from bovine demineralized bone. Proc Natl Acad Sci U S A. 1985;82:2267–2271. doi: 10.1073/pnas.82.8.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tang Y, Wu X, Lei W, et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009;15:757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Janssens K, ten Dijke P, Janssens S, et al. Transforming growth factor-beta1 to the bone. Endocr Rev. 2005;26:743–774. doi: 10.1210/er.2004-0001. [DOI] [PubMed] [Google Scholar]
- 89.Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 90.Ehata S, Hanyu A, Fujime M, et al. Ki26894, a novel transforming growth factor-beta type I receptor kinase inhibitor, inhibits in vitro invasion and in vivo bone metastasis of a human breast cancer cell line. Cancer Sci. 2007;98:127–133. doi: 10.1111/j.1349-7006.2006.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yin JJ, Selander K, Chirgwin JM, et al. TGF-beta signaling blockade inhibits pthrp secretion by breast cancer cells and bone metastases development. J Clin Invest. 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reis ST, Pontes-Junior J, Antunes AA, et al. TGF-beta1 expression as a biomarker of poor prognosis in prostate cancer. Clinics (Sao Paulo) 2011;66:1143–1147. doi: 10.1590/S1807-59322011000700004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wan X, Li ZG, Yingling JM, et al. Effect of transforming growth factor beta (TGF-beta) receptor I kinase inhibitor on prostate cancer bone growth. Bone. 2012;50:695–703. doi: 10.1016/j.bone.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hu Z, Gupta J, Zhang Z, et al. Systemic delivery of oncolytic adenoviruses targeting transforming growth factor-beta inhibits established bone metastasis in a prostate cancer mouse model. Hum Gene Ther. 2012;23:871–882. doi: 10.1089/hum.2012.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10:2298–2308. doi: 10.1158/1535-7163.MCT-11-0264. [DOI] [PubMed] [Google Scholar]
- 96.Sennino B, Ishiguro-Oonuma T, Wei Y, et al. Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov. 2012;2:270–287. doi: 10.1158/2159-8290.CD-11-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Laino C. Prostate cancer: promising results with cabozantinibg, abiraterone, and degarelix. Oncology Times. 2011;8:9–10. [Google Scholar]
- 98.Hussein O, Tiedemann K, Murshed M, et al. Rapamycin inhibits osteolysis and improves survival in a model of experimental bone metastases. Cancer Lett. 2012;314:176–184. doi: 10.1016/j.canlet.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 99.Rucci N, Recchia I, Angelucci A, et al. Inhibition of protein kinase c-Src reduces the incidence of breast cancer metastases and increases survival in mice: implications for therapy. J Pharmacol Exp Ther. 2006;318:161–172. doi: 10.1124/jpet.106.102004. [DOI] [PubMed] [Google Scholar]
- 100.Barton S, Swanton C. Recent developments in treatment stratification for metastatic breast cancer. Drugs. 2011;71:2099–2113. doi: 10.2165/11594480-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 101.Onishi T, Hayashi N, Theriault RL, et al. Future directions of bone-targeted therapy for metastatic breast cancer. Nat Rev Clin Oncol. 2010;7:641–651. doi: 10.1038/nrclinonc.2010.134. [DOI] [PubMed] [Google Scholar]
- 102.Rove KO, Crawford ED. Evolution of treatment options for patients with CRPC and bone metastases: bone-targeted agents that go beyond palliation of symptoms to improve overall survival. Oncology (Williston Park) 2011;25:1362–1370, 1375-1381, 1387. [PubMed] [Google Scholar]
- 103.Tsang RY, Finn RS. Beyond trastuzumab: novel therapeutic strategies in HER2-positive metastatic breast cancer. Br J Cancer. 2012;106:6–13. doi: 10.1038/bjc.2011.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Robinson DR, Zylstra CR, Williams BO. Wnt signaling and prostate cancer. Curr Drug Targets. 2008;9:571–580. doi: 10.2174/138945008784911831. [DOI] [PubMed] [Google Scholar]
- 105.Verras M, Sun Z. Roles and regulation of wnt signaling and beta-catenin in prostate cancer. Cancer Lett. 2006;237:22–32. doi: 10.1016/j.canlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 106.Yardy GW, Brewster SF. Wnt signalling and prostate cancer. Prostate Cancer Prostatic Dis. 2005;8:119–126. doi: 10.1038/sj.pcan.4500794. [DOI] [PubMed] [Google Scholar]
- 107.Bruxvoort KJ, Charbonneau HM, Giambernardi TA, et al. Inactivation of APC in the mouse prostate causes prostate carcinoma. Cancer Res. 2007;67:2490–2496. doi: 10.1158/0008-5472.CAN-06-3028. [DOI] [PubMed] [Google Scholar]
- 108.Yu X, Wang Y, DeGraff DJ, et al. Wnt/beta-catenin activation promotes prostate tumor progression in a mouse model. Oncogene. 2011;30:1868–1879. doi: 10.1038/onc.2010.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bisson I, Prowse DM. Wnt signaling regulates self-renewal and differentiation of prostate cancer cells with stem cell characteristics. Cell Res. 2009;19:683–697. doi: 10.1038/cr.2009.43. [DOI] [PubMed] [Google Scholar]
- 110.Chen G, Shukeir N, Potti A, et al. Up-regulation of Wnt-1 and beta-catenin production in patients with advanced metastatic prostate carcinoma: potential pathogenetic and prognostic implications. Cancer. 2004;101:1345–1356. doi: 10.1002/cncr.20518. [DOI] [PubMed] [Google Scholar]
- 111.Gupta S, Iljin K, Sara H, et al. FZD4 as a mediator of ERG oncogene-induced WNT signaling and epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 2010;70:6735–6745. doi: 10.1158/0008-5472.CAN-10-0244. [DOI] [PubMed] [Google Scholar]
- 112.Hall CL, Bafico A, Dai J, et al. Prostate cancer cells promote osteoblastic bone metastases through wnts. Cancer Res. 2005;65:7554–7560. doi: 10.1158/0008-5472.CAN-05-1317. [DOI] [PubMed] [Google Scholar]
- 113.Hall CL, Daignault SD, Shah RB, et al. Dickkopf-1 expression increases early in prostate cancer development and decreases during progression from primary tumor to metastasis. Prostate. 2008;68:1396–1404. doi: 10.1002/pros.20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Uysal-Onganer P, Kawano Y, Caro M, et al. Wnt-11 promotes neuroendocrine-like differentiation, survival and migration of prostate cancer cells. Mol Cancer. 2010;9:55. doi: 10.1186/1476-4598-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lindvall C, Zylstra CR, Evans N, et al. The Wnt co-receptor Lrp6 is required for normal mouse mammary gland development. PLoS One. 2009;4:e5813. doi: 10.1371/journal.pone.0005813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vaillant F, Asselin-Labat ML, Shackleton M, et al. The mammary progenitor marker CD61/beta3 integrin identifies cancer stem cells in mouse models of mammary tumorigenesis. Cancer Res. 2008;68:7711–7717. doi: 10.1158/0008-5472.CAN-08-1949. [DOI] [PubMed] [Google Scholar]
- 117.Khramtsov AI, Khramtsova GF, Tretiakova M, et al. Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am J Pathol. 2010;176:2911–2920. doi: 10.2353/ajpath.2010.091125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Teissedre B, Pinderhughes A, Incassati A, et al. MMTV-Wnt1 and -DeltaN89beta-catenin induce canonical signaling in distinct progenitors and differentially activate Hedgehog signaling within mammary tumors. PLoS One. 2009;4:e4537. doi: 10.1371/journal.pone.0004537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tsukamoto AS, Grosschedl R, Guzman RC, et al. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell. 1988;55:619–625. doi: 10.1016/0092-8674(88)90220-6. [DOI] [PubMed] [Google Scholar]
- 120.DiMeo TA, Anderson K, Phadke P, et al. A novel lung metastasis signature links Wnt signaling with cancer cell self-renewal and epithelial-mesenchymal transition in basal-like breast cancer. Cancer Res. 2009;69:5364–5373. doi: 10.1158/0008-5472.CAN-08-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Klemm F, Bleckmann A, Siam L, et al. Beta-catenin-independent wnt signaling in basal-like breast cancer and brain metastasis. Carcinogenesis. 2011;32:434–442. doi: 10.1093/carcin/bgq269. [DOI] [PubMed] [Google Scholar]
- 122.Valkenburg KC, Graveel CR, Zylstra-Diegel CR, et al. Wnt/beta-catenin signaling in normal and cancer stem cells. Cancers. 2011;3:2050–2079. doi: 10.3390/cancers3022050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nguyen DX, Chiang AC, Zhang XH, et al. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell. 2009;138:51–62. doi: 10.1016/j.cell.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nakashima N, Liu D, Huang CL, et al. Wnt3 gene expression promotes tumor progression in non-small cell lung cancer. Lung Cancer. 2012;76:228–234. doi: 10.1016/j.lungcan.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 125.Liu YL, Yang HP, Zhou XD, et al. The hypomethylation agent bisdemethoxycurcumin acts on the WIF-1 promoter, inhibits the canonical Wnt pathway and induces apoptosis in human non-small-cell lung cancer. Curr Cancer Drug Targets. 2011;11:1098–1110. doi: 10.2174/156800911798073041. [DOI] [PubMed] [Google Scholar]
- 126.Pacheco-Pinedo EC, Durham AC, Stewart KM, et al. Wnt/beta-catenin signaling accelerates mouse lung tumorigenesis by imposing an embryonic distal progenitor phenotype on lung epithelium. J Clin Invest. 121:1935–1945. doi: 10.1172/JCI44871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xi S, Yang M, Tao Y, et al. Cigarette smoke induces C/EBP-beta-mediated activation of miR-31 in normal human respiratory epithelia and lung cancer cells. PLoS One. 2010;5:e13764. doi: 10.1371/journal.pone.0013764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Goldring SR, Goldring MB. Eating bone or adding it: the Wnt pathway decides. Nat Med. 2007;13:133–134. doi: 10.1038/nm0207-133. [DOI] [PubMed] [Google Scholar]
- 129.Holmen SL, Zylstra CR, Mukherjee A, et al. Essential role of beta-catenin in postnatal bone acquisition. J Biol Chem. 2005;280:21162–21168. doi: 10.1074/jbc.M501900200. [DOI] [PubMed] [Google Scholar]
- 130.Kramer I, Halleux C, Keller H, et al. Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol Cell Biol. 2010;30:3071–3085. doi: 10.1128/MCB.01428-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cui Y, Niziolek PJ, MacDonald BT, et al. Lrp5 functions in bone to regulate bone mass. Nat Med. 2011;17:684–691. doi: 10.1038/nm.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Holmen SL, Giambernardi TA, Zylstra CR, et al. Decreased BMD and limb deformities in mice carrying mutations in both Lrp5 and Lrp6. J Bone Miner Res. 2004;19:2033–2040. doi: 10.1359/JBMR.040907. [DOI] [PubMed] [Google Scholar]
- 133.Minear S, Leucht P, Jiang J, et al. Wnt proteins promote bone regeneration. Sci Transl Med. 2010;2:29ra30. doi: 10.1126/scitranslmed.3000231. [DOI] [PubMed] [Google Scholar]
- 134.Yee DS, Tang Y, Li X, et al. The Wnt inhibitory factor 1 restoration in prostate cancer cells was associated with reduced tumor growth, decreased capacity of cell migration and invasion and a reversal of epithelial to mesenchymal transition. Mol Cancer. 2010;9:162. doi: 10.1186/1476-4598-9-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhao JH, Luo Y, Jiang YG, et al. Knockdown of beta-Catenin through shRNA cause a reversal of EMT and metastatic phenotypes induced by HIF-1alpha. Cancer Invest. 2011;29:377–382. doi: 10.3109/07357907.2010.512595. [DOI] [PubMed] [Google Scholar]
- 136.Dai J, Hall CL, Escara-Wilke J, et al. Prostate cancer induces bone metastasis through Wnt-induced bone morphogenetic protein-dependent and independent mechanisms. Cancer Res. 2008;68:5785–5794. doi: 10.1158/0008-5472.CAN-07-6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Thudi NK, Martin CK, Murahari S, et al. Dickkopf-1 (DKK-1) stimulated prostate cancer growth and metastasis and inhibited bone formation in osteoblastic bone metastases. Prostate. 2011;71:615–625. doi: 10.1002/pros.21277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li ZG, Yang J, Vazquez ES, et al. Low-density lipoprotein receptor-related protein 5 (LRP5) mediates the prostate cancer-induced formation of new bone. Oncogene. 2008;27:596–603. doi: 10.1038/sj.onc.1210694. [DOI] [PubMed] [Google Scholar]
- 139.Bu G, Lu W, Liu CC, et al. Breast cancer-derived dickkopf1 inhibits osteoblast differentiation and osteoprotegerin expression: implication for breast cancer osteolytic bone metastases. Int J Cancer. 2008;123:1034–1042. doi: 10.1002/ijc.23625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Voorzanger-Rousselot N, Goehrig D, Journe F, et al. Increased dickkopf-1 expression in breast cancer bone metastases. Br J Cancer. 2007;97:964–970. doi: 10.1038/sj.bjc.6603959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Matsuda Y, Schlange T, Oakeley EJ, et al. Wnt signaling enhances breast cancer cell motility and blockade of the Wnt pathway by sFRP1 suppresses MDA-MB-231 xenograft growth. Breast Cancer Res. 2009;11:R32. doi: 10.1186/bcr2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.van der Velden JL, Guala AS, Leggett SE, et al. Induction of a mesenchymal expression program in lung epithelial cells by Wnt/beta-Catenin requires the presence of c-Jun N-terminal kinase 1. Am J Respir Cell Mol Biol. 2012;47:306–314. doi: 10.1165/rcmb.2011-0297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.You L, He B, Xu Z, et al. Inhibition of Wnt-2-mediated signaling induces programmed cell death in non-small-cell lung cancer cells. Oncogene. 2004;23:6170–6174. doi: 10.1038/sj.onc.1207844. [DOI] [PubMed] [Google Scholar]
- 144.Nusse R, Varmus H. Three decades of wnts: a personal perspective on how a scientific field developed. EMBO J. 2012;31:2670–2684. doi: 10.1038/emboj.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 146.Valkenburg KC, Williams BO. Mouse models of prostate cancer. Prostate Cancer. 2011;2011:1–22. doi: 10.1155/2011/895238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Neth P, Ciccarella M, Egea V, et al. Wnt signaling regulates the invasion capacity of human mesenchymal stem cells. Stem Cells. 2006;24:1892–1903. doi: 10.1634/stemcells.2005-0503. [DOI] [PubMed] [Google Scholar]
- 148.Badders NM, Goel S, Clark RJ, et al. The Wnt receptor, LRP5, is expressed by mouse mammary stem cells and is required to maintain the basal lineage. PLoS One. 2009;4:e6594. doi: 10.1371/journal.pone.0006594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Teng Y, Wang X, Wang Y, et al. Wnt/beta-Catenin signaling regulates cancer stem cells in lung cancer A549 cells. Biochem Biophys Res Commun. 2010;392:373–379. doi: 10.1016/j.bbrc.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 150.Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 151.Chen B, Dodge ME, Tang W, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.van den Heuvel M, Harryman-Samos C, Klingensmith J, et al. Mutations in the segment polarity genes wingless and porcupine impair secretion of the wingless protein. EMBO J. 1993;12:5293–5302. doi: 10.1002/j.1460-2075.1993.tb06225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fishman MC. Changing the practice of medicine. Novartis. 2010 [Google Scholar]
- 154.Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov. 2006;5:997–1014. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- 155.Moon RT, Kohn AD, De Ferrari GV, et al. Wnt and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 156.Polakis P. Drugging Wnt signalling in cancer. EMBO J. 2012;31:3375. doi: 10.1038/emboj.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fulciniti M, Tassone P, Hideshima T, et al. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood. 2009;114:371–379. doi: 10.1182/blood-2008-11-191577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yaccoby S, Ling W, Zhan F, et al. Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. 2007;109:2106–2111. doi: 10.1182/blood-2006-09-047712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhou XL, Qin XR, Zhang XD, et al. Downregulation of dickkopf-1 is responsible for high proliferation of breast cancer cells via losing control of Wnt/beta-Catenin signaling. Acta Pharmacol Sin. 2010;31:202–210. doi: 10.1038/aps.2009.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Borowsky AD. Choosing a mouse model: experimental biology in context—the utility and limitations of mouse models of breast cancer. Cold Spring Harb Perspect Biol. 2011;3:a009670. doi: 10.1101/cshperspect.a009670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Park SI, Kim SJ, McCauley LK, et al. Pre-clinical mouse models of human prostate cancer and their utility in drug discovery. Curr Protoc Pharmacol. 2010;Chapter 14:Unit 14 15. doi: 10.1002/0471141755.ph1415s51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Onn A, Isobe T, Itasaka S, et al. Development of an orthotopic model to study the biology and therapy of primary human lung cancer in nude mice. Clin Cancer Res. 2003;9:5532–5539. [PubMed] [Google Scholar]
- 163.Howard RB, Mullen JB, Pagura ME, et al. Characterization of a highly metastatic, orthotopic lung cancer model in the nude rat. Clin Exp Metastasis. 1999;17:157–162. doi: 10.1023/a:1006637712294. [DOI] [PubMed] [Google Scholar]
- 164.Chung LW, Hsieh CL, Law A, et al. New targets for therapy in prostate cancer: modulation of stromal-epithelial interactions. Urology. 2003;62:44–54. doi: 10.1016/s0090-4295(03)00796-9. [DOI] [PubMed] [Google Scholar]
- 165.Pettaway CA, Pathak S, Greene G, et al. Selection of highly metastatic variants of different human prostatic carcinomas using orthotopic implantation in nude mice. Clin Cancer Res. 1996;2:1627–1636. [PubMed] [Google Scholar]
- 166.Thalmann GN, Anezinis PE, Chang SM, et al. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994;54:2577–2581. [PubMed] [Google Scholar]
- 167.Thalmann GN, Sikes RA, Wu TT, et al. LNCaP progression model of human prostate cancer: androgen-independence and osseous metastasis. Prostate. 2000;44:91–103. doi: 10.1002/1097-0045(20000701)44:2<91::aid-pros1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 168.Wu TT, Sikes RA, Cui Q, et al. Establishing human prostate cancer cell xenografts in bone: induction of osteoblastic reaction by prostate-specific antigen-producing tumors in athymic and SCID/bg mice using LNCaP and lineage-derived metastatic sublines. Int J Cancer. 1998;77:887–894. doi: 10.1002/(sici)1097-0215(19980911)77:6<887::aid-ijc15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 169.Liepe K, Geidel H, Haase M, et al. New model for the induction of osteoblastic bone metastases in rat. Anticancer Res. 2005;25:1067–1073. [PubMed] [Google Scholar]
- 170.Nemeth JA, Harb JF, Barroso U, Jr, et al. Severe combined immunodeficient-hu model of human prostate cancer metastasis to human bone. Cancer Res. 1999;59:1987–1993. [PubMed] [Google Scholar]
- 171.Yonou H, Yokose T, Kamijo T, et al. Establishment of a novel species- and tissue-specific metastasis model of human prostate cancer in humanized non-obese diabetic/severe combined immunodeficient mice engrafted with human adult lung and bone. Cancer Res. 2001;61:2177–2182. [PubMed] [Google Scholar]
- 172.Shtivelman E, Namikawa R. Species-specific metastasis of human tumor cells in the severe combined immunodeficiency mouse engrafted with human tissue. Proc Natl Acad Sci U S A. 1995;92:4661–4665. doi: 10.1073/pnas.92.10.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tsingotjidou AS, Zotalis G, Jackson KR, et al. Development of an animal model for prostate cancer cell metastasis to adult human bone. Anticancer Res. 2001;21:971–978. [PubMed] [Google Scholar]
- 174.Bos PD, Nguyen DX, Massague J. Modeling metastasis in the mouse. Curr Opin Pharmacol. 2010;10:571–577. doi: 10.1016/j.coph.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rosol TJ, Tannehill-Gregg SH, LeRoy BE, et al. Animal models of bone metastasis. Cancer. 2003;97:748–757. doi: 10.1002/cncr.11150. [DOI] [PubMed] [Google Scholar]
- 176.Singh AS, Figg WD. In vivo models of prostate cancer metastasis to bone. J Urol. 2005;174:820–826. doi: 10.1097/01.ju.0000169133.82167.aa. [DOI] [PubMed] [Google Scholar]
- 177.Dagher R, Li N, Abraham S, et al. Approval summary: docetaxel in combination with prednisone for the treatment of androgen-independent hormone-refractory prostate cancer. Clin Cancer Res. 2004;10:8147–8151. doi: 10.1158/1078-0432.CCR-04-1402. [DOI] [PubMed] [Google Scholar]
- 178.Galsky MD, Dritselis A, Kirkpatrick P, et al. Cabazitaxel. Nat Rev Drug Discov. 2010;9:677–678. doi: 10.1038/nrd3254. [DOI] [PubMed] [Google Scholar]
- 179.Blackwell KL, Burstein HJ, Storniolo AM, et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 study. J Clin Oncol. 2012;31:2585–2592. doi: 10.1200/JCO.2011.35.6725. [DOI] [PubMed] [Google Scholar]
- 180.Paller CJ, Antonarakis E. Sipuleucel-T for the treatment of metastatic prostate cancer: promise and challenges. Hum Vaccin Immunother. 2012;8:509–519. doi: 10.4161/hv.18860. [DOI] [PubMed] [Google Scholar]
- 181.Di Lauro V, Torrisi E, Bidoli E, et al. Trastuzumab and gemcitabine in pretreated HER2 overexpressing metastatic breast cancer patients: retrospective analysis of our series. J Oncol. 2012;2012:198412. doi: 10.1155/2012/198412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sternberg CN, Petrylak DP, Sartor O, et al. Multinational, double-blind, phase III study of prednisone and either satraplatin or placebo in patients with castrate-refractory prostate cancer progressing after prior chemotherapy: the sparc trial. J Clin Oncol. 2009;27:5431–5438. doi: 10.1200/JCO.2008.20.1228. [DOI] [PubMed] [Google Scholar]
- 183.Dittrich C, Solska E, Manikhas A, et al. A phase II multicenter study of two different dosages of pemetrexed given in combination with cyclophosphamide as first-line treatment in patients with locally advanced or metastatic breast cancer. Cancer Invest. 2012;30:309–316. doi: 10.3109/07357907.2012.658938. [DOI] [PubMed] [Google Scholar]
- 184.Hardy-Bessard AC, Delva R, Pivot X, et al. Safety and efficacy of bevacizumab combined with taxanes in the first-line treatment of metastatic breast cancer: ATHENA study-France. Bull Cancer. 2012;99:609–618. doi: 10.1684/bdc.2012.1586. [DOI] [PubMed] [Google Scholar]
- 185.Moreno-Aspitia A, Morton RF, Hillman DW, et al. Phase II trial of sorafenib in patients with metastatic breast cancer previously exposed to anthracyclines or taxanes: North Central Cancer Treatment Group and Mayo Clinic Trial N0336. J Clin Oncol. 2009;27:11–15. doi: 10.1200/JCO.2007.15.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Castellano D, Gonzalez-Larriba JL, Anton-Aparicio LM, et al. Experience in the use of sunitinib given as a single agent in metastatic chemoresistant and castration-resistant prostate cancer patients. Expert Opin Pharmacother. 2011;12:2433–2439. doi: 10.1517/14656566.2011.590132. [DOI] [PubMed] [Google Scholar]
- 187.Miller RE, Roudier M, Jones J, et al. Rank ligand inhibition plus docetaxel improves survival and reduces tumor burden in a murine model of prostate cancer bone metastasis. Mol Cancer Ther. 2008;7:2160–2169. doi: 10.1158/1535-7163.MCT-08-0046. [DOI] [PubMed] [Google Scholar]
- 188.Vanderkerken K, De Leenheer E, Shipman C, et al. Recombinant osteoprotegerin decreases tumor burden and increases survival in a murine model of multiple myeloma. Cancer Res. 2003;63:287–289. [PubMed] [Google Scholar]
- 189.Curran MP. Crizotinib: in locally advanced or metastatic non-small cell lung cancer. Drugs. 2012;72:99–107. doi: 10.2165/11207680-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 190.Dunlap N, Schwartz GG, Eads D, et al. 1alpha,25-dihydroxyvitamin D(3) (calcitriol) and its analogue, 19-nor-1alpha,25(OH)(2)D(2), potentiate the effects of ionising radiation on human prostate cancer cells. Br J Cancer. 2003;89:746–753. doi: 10.1038/sj.bjc.6601161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sundaram S, Sea A, Feldman S, et al. The combination of a potent vitamin D3 analog, EB 1089, with ionizing radiation reduces tumor growth and induces apoptosis of MCF-7 breast tumor xenografts in nude mice. Clin Cancer Res. 2003;9:2350–2356. [PubMed] [Google Scholar]
- 192.Jones MD, Liu JC, Barthel TK, et al. A proteasome inhibitor, bortezomib, inhibits breast cancer growth and reduces osteolysis by downregulating metastatic genes. Clin Cancer Res. 2010;16:4978–4989. doi: 10.1158/1078-0432.CCR-09-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Qiang YW, Hu B, Chen Y, et al. Bortezomib induces osteoblast differentiation via Wnt-independent activation of beta-catenin/TCF signaling. Blood. 2009;113:4319–4330. doi: 10.1182/blood-2008-08-174300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nilsson S, Franzen L, Parker C, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: A randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 2007;8:587–594. doi: 10.1016/S1470-2045(07)70147-X. [DOI] [PubMed] [Google Scholar]
- 195.Yu EY, Miller K, Nelson J, et al. Detection of previously unidentified metastatic disease as a leading cause of screening failure in a phase III trial of zibotentan versus placebo in patients with nonmetastatic, castration resistant prostate cancer. J Urol. 2012;188:103–109. doi: 10.1016/j.juro.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Palma JP, Wang YC, Rodriguez LE, et al. ABT-888 confers broad in vivo activity in combination with temozolomide in diverse tumors. Clin Cancer Res. 2009;15:7277–7290. doi: 10.1158/1078-0432.CCR-09-1245. [DOI] [PubMed] [Google Scholar]
- 197.Bretschi M, Merz M, Komljenovic D, et al. Cilengitide inhibits metastatic bone colonization in a nude rat model. Oncol Rep. 2011;26:843–851. doi: 10.3892/or.2011.1373. [DOI] [PubMed] [Google Scholar]
- 198.Boffa DJ, Luan F, Thomas D, et al. Rapamycin inhibits the growth and metastatic progression of non-small cell lung cancer. Clin Cancer Res. 2004;10:293–300. doi: 10.1158/1078-0432.ccr-0629-3. [DOI] [PubMed] [Google Scholar]
- 199.Zhou H, Huang S. Mtor signaling in cancer cell motility and tumor metastasis. Crit Rev Eukaryot Gene Expr. 2010;20:1–16. doi: 10.1615/critreveukargeneexpr.v20.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lynch CC. Matrix metalloproteinases as master regulators of the vicious cycle of bone metastasis. Bone. 2011;48:44–53. doi: 10.1016/j.bone.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 201.Lee J, Weber M, Mejia S, et al. A matrix metalloproteinase inhibitor, batimastat, retards the development of osteolytic bone metastases by MDA-MB-231 human breast cancer cells in Balb C nu/nu mice. Eur J Cancer. 2001;37:106–113. doi: 10.1016/s0959-8049(00)00363-4. [DOI] [PubMed] [Google Scholar]
- 202.Sung B, Oyajobi BO, Aggarwal BB. Plumbagin inhibits osteoclastogenesis and reduces human breast cancer-induced osteolytic bone metastasis in mice through suppression of RANKL signaling. Mol Cancer Ther. 2011;11:350–359. doi: 10.1158/1535-7163.MCT-11-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tawara K, Oxford JT, Jorcyk CL. Clinical significance of interleukin (IL)-6 in cancer metastasis to bone: potential of anti-IL-6 therapies. Cancer Manag Res. 2011;3:177–189. doi: 10.2147/CMR.S18101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ara T, Declerck YA. Interleukin-6 in bone metastasis and cancer progression. Eur J Cancer. 2010;46:1223–1231. doi: 10.1016/j.ejca.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chantry AD, Heath D, Mulivor AW, et al. Inhibiting activin-a signaling stimulates bone formation and prevents cancer-induced bone destruction in vivo. J Bone Miner Res. 2010;25:2633–2646. doi: 10.1002/jbmr.142. [DOI] [PubMed] [Google Scholar]
- 206.Amirkhosravi A, Mousa SA, Amaya M, et al. Inhibition of tumor cell-induced platelet aggregation and lung metastasis by the oral GpIIb/IIIa antagonist XV454. Thromb Haemost. 2003;90:549–554. doi: 10.1160/TH03-02-0102. [DOI] [PubMed] [Google Scholar]
- 207.Gupta GP, Massague J. Platelets and metastasis revisited: a novel fatty link. J Clin Invest. 2004;114:1691–1693. doi: 10.1172/JCI23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rey JP, Ellies DL. Wnt modulators in the biotech pipeline. Dev Dyn. 2010;239:102–114. doi: 10.1002/dvdy.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yeh JR, Peterson RT. Novel Wnt antagonists target porcupine and axin. Nat Chem Biol. 2009;5:74–75. doi: 10.1038/nchembio0209-74. [DOI] [PubMed] [Google Scholar]
- 210.Ettenberg SA, Charlat O, Daley MP, et al. Inhibition of tumorigenesis driven by different wnt proteins requires blockade of distinct ligand-binding regions by Lrp6 antibodies. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1007428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Fitton A, McTavish D. Pamidronate. A review of its pharma-cological properties and therapeutic efficacy in resorptive bone disease. Drugs. 1991;41:289–318. doi: 10.2165/00003495-199141020-00009. [DOI] [PubMed] [Google Scholar]
- 212.Adami S, Passeri M, Ortolani S, et al. Effects of oral alendronate and intranasal salmon calcitonin on bone mass and biochemical markers of bone turnover in postmenopausal women with osteoporosis. Bone. 1995;17:383–390. doi: 10.1016/s8756-3282(95)00262-6. [DOI] [PubMed] [Google Scholar]
- 213.Khairi MR, Altman RD, DeRosa GP, et al. Sodium etidronate in the treatment of paget's disease of bone. A study of long-term results. Ann Intern Med. 1977;87:656–663. doi: 10.7326/0003-4819-87-6-656. [DOI] [PubMed] [Google Scholar]
- 214.Schimmer RC, Bauss F. Effect of daily and intermittent use of ibandronate on bone mass and bone turnover in postmenopausal osteoporosis: a review of three phase II studies. Clin Ther. 2003;25:19–34. doi: 10.1016/s0149-2918(03)90005-1. [DOI] [PubMed] [Google Scholar]
- 215.Goa KL, Balfour JA. Risedronate. Drugs Aging. 1998;13:83–91; discussion 92. doi: 10.2165/00002512-199813010-00008. [DOI] [PubMed] [Google Scholar]
- 216.Murakami H, Takahashi N, Tanaka S, et al. Tiludronate inhibits protein tyrosine phosphatase activity in osteoclasts. Bone. 1997;20:399–404. doi: 10.1016/s8756-3282(97)00025-2. [DOI] [PubMed] [Google Scholar]