Abstract
Substantial evidence shows that the hypophyseal–pituitary–adrenal (HPA) axis and corticosteroids are involved in the process of addiction to a variety of agents, and the adrenal cortex has a key role. In general, plasma concentrations of cortisol (or corticosterone in rats or mice) increase on drug withdrawal in a manner that suggests correlation with the behavioural and symptomatic sequelae both in man and in experimental animals. Corticosteroid levels fall back to normal values in resumption of drug intake. The possible interactions between brain corticotrophin releasing hormone (CRH) and proopiomelanocortin (POMC) products and the systemic HPA, and additionally with the local CRH–POMC system in the adrenal gland itself, are complex. Nevertheless, the evidence increasingly suggests that all may be interlinked and that CRH in the brain and brain POMC products interact with the blood-borne HPA directly or indirectly. Corticosteroids themselves are known to affect mood profoundly and may themselves be addictive. Additionally, there is a heightened susceptibility for addicted subjects to relapse in conditions that are associated with change in HPA activity, such as in stress, or at different times of the day. Recent studies give compelling evidence that a significant part of the array of addictive symptoms is directly attributable to the secretory activity of the adrenal cortex and the actions of corticosteroids. Additionally, sex differences in addiction may also be attributable to adrenocortical function: in humans, males may be protected through higher secretion of DHEA (and DHEAS), and in rats, females may be more susceptible because of higher corticosterone secretion.
Keywords: behaviour, corticosteroids, HPA axis, glucocorticoid
Introduction
The purpose of this review is to demonstrate the critical role of the adrenal cortex in addiction and additionally to propose that sex differences in adrenocortical function may contribute to sex differences in addiction. Where it is clear, the sex of experimental animals or of human subjects in the cited studies is stated, although in most cases sex differences were not emphasized.
There is a long history of associating addiction with the adrenal. Indeed, it was well before the adrenocortical hormones were even characterized that morphine toxicity was linked to the adrenal gland. Thus, Lewis (1) and Mackay & Mackay (2) showed that adrenalectomy increased morphine sensitivity in female rats, and chronic treatment with morphine in males or methadone in either sex produces adrenocortical hypertrophy (3, 4). Consequently, there has been interest in the actions of the hormones of the adrenal as possible agents in addiction from the time of their discovery. Treatment with cortisone (the therapeutic corticosteroid of choice at the time) was soon applied in the management of meperidine and morphine withdrawal symptoms in men (5), apparently with beneficial effects, while Lovell associated alcoholism and drug addiction with hypoadrenocorticism (6).
More systematic study then discounted corticosteroids along with other novel ‘cures’ for withdrawal symptoms, and Fraser & Isbell (7) were the first to suggest that in fact withdrawal symptoms (from morphine) in men were associated with eosinopaenia, a measure used at that time to reflect high levels of circulating corticosteroids (8). Eosinophil counts swiftly normalized when morphine was restored. These authors also found that treatment with either cortisone or ACTH shortened the period for development of withdrawal symptoms in men, and therefore, they themselves could be considered a cause (7, 9, 10, 11). Indeed, chronic treatment with corticosteroid can itself lead to later withdrawal symptoms (12).
So there are fundamental questions on the role of corticosteroids in addiction. Is the lower adrenocortical activity in sustained morphine administration, and its elevation when administration ceases, a cause or an effect of addictive responses? Could the drive to addictive drugs actually represent a drive to lower cortisol, with its sequelae? Or is the heightened secretion of corticosteroids in drug withdrawal simply a response to stress? We here argue that the adrenal cortex has a critical role in the acquisition of addiction and also in protection against it.
The hypophyseal–pituitary–adrenal axis in the brain and addiction
In relation to addiction, far more attention has been paid to hypophyseal–pituitary–adrenal (HPA) components in the brain than to the systemic (i.e. blood-borne) HPA axis. All the components are present in the brain, and, in relation to the hypothesis that the adrenal itself is crucial to addiction, it is important to unravel the relationship between brain and systemic HPA function. This section examines the evidence for brain HPA function in addiction and shows that it is not autonomous, and its function is closely regulated by and linked to the systemic HPA.
Corticotrophin releasing hormone
Corticotrophin releasing hormone (CRH) is produced in various parts of the brain (13). First, CRH exerts its systemic effects following its release at the median eminence by neuronal tracts that originate in the paraventricular nucleus (PVN) of the hypothalamus. CRH is transported to the corticotrophs of the anterior pituitary via the hypophyseal portal system and then stimulates the secretion of ACTH. ACTH is in turn carried in the general circulation and stimulates the secretion of corticosteroids in the adrenal cortex.
In addition, however, CRH, its receptors CRHR1 and CRHR2, and also CRH binding protein (CRH-BP), which modulates CRH actions, are found in other brain locations, where CRH presumably acts primarily as a neurotransmitter. These sites include the cerebrocortex, limbic system, hippocampus, amygdala, locus coeruleus, olfactory bulb and cerebellum (14, 15, 16, 17, 18, 19, 20). While the involvement of such extra hypophyseal CRH with addiction may be independent of the HPA (18, 20), there are certainly pathways through which it contributes to the multifactorial regulation of hypothalamic CRH (Fig. 1).
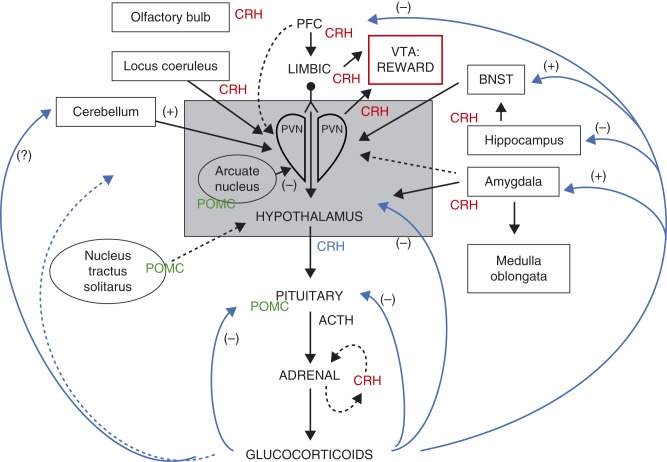
Figure 1.
The expanded HPA axis. From (20, 49, 80, 82, 192, 193) and see text. BNST, bed nucleus of stria terminalis; PFC, pre-frontal cortex; PVN, paraventricular nucleus; VTA, ventral tegumental area (associated with reward responses); CRH, corticotrophin releasing hormone; POMC, proopiomelanocortin; +, stimulatory; −, inhibitory. Solid arrows show proven regulation, and dotted arrows show postulated actions. Secreted CRH is indicated in blue lettering, and sites of CRH and POMC signalling are indicated in red and green respectively: here, arrows indicate regulatory pathways that are unquestionably multifactorial but may include actions of CRH and POMC peptides. The inhibitory effect of neural POMC peptides on PVN CRH is particularly interesting, and, by comparison with other systems, might suggest a negative feedback mechanism; however, there is little evidence for reciprocal feedback of CRH on POMC in the brain. Instead, regulation of neural POMC is multifactorial (e.g. (65, 67), and this is primarily linked to its role in energy balance and nutrition, see text. There is, however, much evidence to show the feedback of glucocorticoids on CRH expression in several brain regions. Mostly, this is negative, except in the amygdala, a key region in addiction (19), where it is positive.
In the brain, CRH binds to both receptor types, CRHR1 and CRHR2. In addition to CRH itself, both these receptors bind ligands of the urotensin family. The two receptors mediate different responses; CRHR1 agonists produce stress-related responses on which CRHR2 may have less effect, while more potently depressing food intake (21, 22, 23, 24).
There is certainly substantial evidence for the role of CRH in addiction (18, 25), and particularly in reinstatement, but the data are not always consistent. For example, cocaine stimulates the HPA axis through a hypothalamic/CRH-mediated mechanism in male rats (26, 27), and although this is not invariably closely linked to corticosterone (28), both Crh mRNA transcription and circulating corticosterone are further increased on cocaine withdrawal (29). In contrast, shock-induced reinstatement of heroin or alcohol seeking clearly depends on CRH, but not on corticosterone, according to some authors (30, 31, 32). Nevertheless, adrenal function is required during cocaine self-administration for subsequent CRH-dependent shock-induced reinstatement to occur (33). The modulator of CRH actions, CRH-BP, is now emerging as an additional factor, although not so widely studied in the addiction field (34, 35). Although both corticosterone and ACTH secretion are increased by acute alcohol exposure, they are inhibited in chronic exposure (36, 37). Neither CRH nor cortisol is implicated in cocaine reinstatement in squirrel monkeys (38).
With specific regard to morphine and the opioids, it is clear that reduced circulating corticosteroid concentrations may be a consequence of opioid inhibition of CRH secretion, acting through μ- and κ-type opioid receptors in the male rat hypothalamus (39, 40, 41). In humans, opioids directly inhibit CRH secretion and the HPA axis, resulting in decreased circulating cortisol. In male rats, the effect is biphasic, with early enhancement of CRH (and the HPA) followed by inhibition after a few days of treatment (41, 42); such responses are affected by stress in male rats (43). Indeed, the evidence suggests that opioidergic mechanisms may at least partially underlie both the behavioural effects of CRH in male rats (44) and also the increase in CRH secretion under conditions of stress. This may not be true in other situations such as the increased HPA activity in adrenalectomized animals (45). This double effect in rats may be because opioids have differential effects on different cell types: they certainly inhibit CRH secretion that is promoted by neurotransmitters (46). The possibly critical involvement of opioids in alcohol addiction in humans (47) has also been shown to be exerted via other than HPA pathways (48).
There are clear differences between the actions of different addictive drugs on Crh mRNA transcription in the hypothalamus, and although alcohol acts directly on the PVN, other drugs, including cocaine, nicotine and cannabinoids, activate Crh transcription in other brain sites (49). Adrenocortical activity may still be critical, for example in reinstatement of cocaine addiction in male rats (33). Timing of exposure is also significant; early exposure can affect subsequent responses (50), and in male rats, adolescent exposure to alcohol vapour blunts subsequent adult Crh transcription response to acute alcohol (51).
The development of specific CRHR1 antagonists has provided more information. CRHR1 blockade inhibits further alcohol drinking in male rats habituated to a high intake (52), and, in conjunction with additional studies using Crh1 knockout animals, it has been shown that CRHR1 signalling pathways are essential for sensitization to alcohol addiction in male mice (53); a common expression of neuroadaptations induced by repeated exposure to addictive drugs is a persistent sensitized behavioural response to their stimulant properties. These authors also show that acquisition and sensitization are differentially regulated. Acquisition involves the HPA axis and is inhibited by the glucocorticoid blocker mifepristone as well as by CRHR1 blockade, whereas sensitization is unaffected by mifepristone. Pastor et al. (53) propose that this suggests a non-hypothalamic CRHR1-linked pathway in sensitization. Different effects were seen in methamphetamine (MA) responses, in which behavioural sensitization measured as increased drug-induced locomotor activity was unaffected in Crh1 knockouts or by the antagonist CP 154 526 in DBA/2J mice, whereas deletion of Crh2 attenuated MA-induced behavioural sensitization. Here, an action of endogenous urocortins was suggested, focused in the basolateral and central nuclei of the amygdala (54).
Proopiomelanocortin
Proopiomelanocortin (POMC) provides, in ACTH and α-melanocyte stimulating hormone (α-MSH), the other components of the HPA axis, and in this context, its primary site of expression and processing is the anterior pituitary and (in rodents) the pars intermedia. POMC is also expressed in brain sites, primarily in projections from the arcuate nucleus of the hypothalamus and from the nucleus tractus solitarius of the brainstem (55, 56, 57). Its primary role in the brain is the generation of α-MSH, which participates in the regulation of food intake and in the production of β-endorphin, pain control. α-MSH acts through two of the melanocortin receptor (MCR) series, MC3R and MC4R, and the latter may also regulate aspects of pain recognition (25, 58).
POMC expression and processing suggests that although ACTH and other POMC products such as β-endorphin can be found in non-hypothalamic regions of the brain or cerebrospinal fluid (59, 60), some may be transported to the brain from the blood (60, 61). From early development, the major adrenocortical-related POMC product in the brain is α-MSH (62), presumably associated with the distribution of the prohormone convertases PC1 and PC2 (63, 64). By far, the major focus of attention in this regard is the role of α-MSH with leptin, ghrelin and agouti protein in the regulation of food intake and energy balance (56, 62, 65, 66, 67, 68).
In addition to its role in energy balance, α-MSH also plays a part in the physiology of addiction, and MC4R, like CRH receptors, respond to morphine (69, 70, 71), and the behavioural effects of morphine or cocaine are modulated by selective MC4R inhibition (72, 73). Additionally, acute alcohol treatment reduced α-MSH expression in hypothalamic and other brain locations in rats, but chronic treatment enhanced it (74).
Of course, POMC processing in relation to addiction cannot be considered purely in terms of its HPA-linked functions. The production of β-endorphin leads inevitably to direct effects on addiction pathways. Its main action is mediated by μ-receptors as are the opiates morphine, heroin and methadone, and in humans, the endogenous opiates are similarly inhibitory on HPA function, although both stimulatory and inhibitory in rats (49, 75).
What has not been clear hitherto is whether the term ‘HPA axis’ can in reality be extended to these components in the brain. In other words, it has been unclear whether, for example, non-hypothalamic CRH provokes synthesis, processing or release of POMC in the brain, but the different locations of the expression of these components may suggest it does not (Fig. 1). Similarly, there has really been no evidence that brain CRH or POMC products have any interaction with the adrenal cortex and the secretion of glucocorticoids, other than via the hypothalamus. On the contrary, it has sometimes been assumed that they do not (e.g. (53)). However, neural glucocorticoid receptor (GR) disruption, including in the PVN, ameliorates the effects of anxiety and also results in heightened HPA activity in male mice (76), consistent with the loss of glucocorticoid inhibition of CRH (20, 77). In contrast, forebrain-specific GR knockout, which does not involve the PVN, increased anxiety behaviour but has the same effect of diminishing glucocorticoid inhibition of CRH in male mice (77). It is clear from this study that the HPA is regulated partly by forebrain GR-mediated inhibition. Accordingly, what needs to be unravelled is the significance of the local brain CRH/POMC components in distinction to that of the systemic HPA, and how independent these systems really are in addiction.
Interaction between brain CRH and α-MSH
Although the main recognized function of α-MSH in the brain, regulation of food intake and nutrition seems not to be closely related to that of CRH, in fact there is ample evidence of crosstalk between them. Certainly, like the systemic HPA, POMC-processing neurones are activated by stress and play a role in the consequent behavioural response in male rats (78, 79). Furthermore, neuronal POMC-derived peptides regulate hypothalamic CRH and thus ACTH secretion in male and female mice (80). Additionally, α-MSH stimulates Crh transcription in the PVN of male rats (81, 82), although, like γ-MSH, it also inhibits interleukin-1β-induced HPA activity, apparently through central MCRs (83). That the circuit connecting brain and systemic HPA is complete is suggested by the finding that glucocorticoids enhance MC4R signalling in a hypothalamic neuronal cell line (84). We can therefore predict the existence of an extended HPA axis in which the same components, CRH, POMC products and corticosteroids as in the classical system, also interact in the brain (Fig. 1) with specific effects on mood and behaviour. The two systems, brain and somatic, interact to the extent that whatever physiological stimuli activate the systemic system, broadly ‘stress’ and the clock, must also have consequences on mood and behaviour.
Steroids in the brain
The spectrum of structures and functions of neurosteroids is so wide as to form a branch of endocrinology (or at least paracrinology) in its own right. Many are locally synthesized, although usually requiring substrates from non-neural sources. Oestrogens are prominent among these and are produced by aromatase activity in the hippocampus, acting, it is thought, on locally produced C19 steroid substrates (85). They have roles in neural plasticity (86) and neuroprotection (85, 87, 88) and regulate the function of other neurally active agents, including neuroprogesterone, which is also synthesized locally (89). There are sex-related differences in the neural responses to oestrogen (90, 91, 92). Oestrogen action in the brain is mediated through classical oestrogen receptors α and β and also through membrane metabotropic glutamate receptors (93, 94). Neuroactive steroids that primarily act through N-methyl-d-aspartate or gamma-aminobutyric acid (GABA) receptors include the adrenal androgen DHEA, which as DHEAS conjugate is the most abundant steroid in human plasma (95, 96, 97, 98). DHEA is not secreted by the rat adrenal cortex: its presence and activity in the brain reflect its local synthesis (99). DHEA and pregnenolone, both Δ5,3β-hydrosteroids, are also opioid sigma receptor agonists, whereas progesterone, which has the Δ4,3-one configuration, is an antagonist (100). Through their sigma-1 agonist actions, pretreatment with DHEA or pregnenolone potentiates cocaine-induced conditioned place preference (CPP) behaviour in mice (100) but attenuates cocaine-seeking behaviour (101). In patients, DHEA and DHEAS are associated with beneficial actions in cocaine withdrawal (102, 103), and the use of DHEA administration to assist opioid withdrawal has been studied, with variable outcomes (104, 105).
Other known neurosteroids include 3α-hydroxy-5α-pregnan-20-one (tertrahydroprogesterone, allopregnanolone, THP) and 3α,21-dihydroxy-5α-pregnan-20-one (tetrahydrodeoxycorticosterone, THDOC), and they are formed in the brain from progesterone and deoxycorticosterone (106, 107). They have anxiolytic, anti-convulsant and sedative activities and are known to be elevated in both plasma and brain in response to ethanol in rats (106, 108). In addition, the HPA axis is under tonic GABA inhibition at the hypothalamic level (75). Importantly, production in the brain of both THP and THDOC depends on precursor steroids of adrenal origin (106).
The corticosteroids themselves have neurological effects, and brain concentrations of corticosterone certainly have relevance to addictive behaviour in male rats (109), and see below. However, the relevance of local brain synthesis of corticosteroids is unclear. Certainly, all the required enzymes of the corticosteroid biosynthetic pathway from cholesterol are present, notably in the hippocampus, together with the StAR protein (110, 111, 112), but their level of production is likely to be low in comparison with concentrations crossing the blood–brain barrier, and they are not thought to be produced in the brain to any great extent (113, 114). Remarkably then, of the known neurosteroids, the corticosteroids may fall into a group of their own being predominantly dependent on an extraneural source: the adrenal cortex.
The role of the adrenal cortex
Corticosteroids and mood
Clearly, the role of corticosteroids in addiction cannot be understood without reference to the nature of the psychological and behavioural aspects of the actions of corticosteroids themselves. Almost as the corticosteroids were first characterized, their paradoxical capacity to generate both euphoria and depression in humans has been well known, although poorly understood (115, 116). Changes in mood are a feature of chronic corticosteroid therapy, with mild euphoria in the short term and increases in severity of symptoms associated with depression, or even psychosis in the long-term, and these occur most frequently in women (116, 117, 118, 119, 120), although with large variations in incidence in different studies. Moreover, both cortisol levels and the response to ACTH are higher in depression or depressive episodes (121), and animal experiments show that both of these may be linked to high CRH secretion (29). It has been suggested that corticosteroids may have a role in dopamine-related psychiatric disorders (122), and it has also been speculated that some behavioural features in animals and humans may result from structural or other changes in the brain that corticosteroids may invoke, or at least facilitate (114, 123, 124). Reduction of circulating corticosteroid levels, in combination with other indices, can also be used as a marker for response to anxiolytic therapy (125, 126). It has been postulated that depression in fact reflects GR desensitization, giving rise to impaired glucocorticoid feedback at the hypothalamus, hence increased HPA activity. In this model, one action of antidepressants is thus to resensitize GR transcriptional activity (125), independent of their action on monoamine reuptake, but perhaps involving regulation of steroid elimination from the cell through the multi-drug resistance P-glycoprotein membrane transporter system (127, 128). Together, these studies suggest that corticosteroid-evoked mood changes could be related to behavioural responses to addiction.
Corticosteroids and addiction
Although the earlier association between the adrenal cortex and addiction is derived largely from circumstantial evidence, there are now data showing a direct causal link. From their experiences with patients receiving chronic steroid treatment, some authors have been willing to label the corticosteroids as drugs of addiction themselves (129, 130, 131, 132, 133, 134), although much of the earlier evidence is based on individual case reports. These findings tend to suggest a close link between corticosteroids and addiction, a concept amply borne out by more recent studies. Alcohol administration induces ACTH secretion and thus adrenocortical stimulation in male rats (106). In habituated men smoking high- but not low-nicotine cigarettes, increased plasma ACTH and cortisol occurs within minutes of smoking (135). Further evidence for the crucial actions of elevated cortisol is given by its association with impaired learning and memory in abstinent cocaine-dependent men and women (136), although higher basal cortisol levels are associated with improved memory performance in healthy controls. These effects on memory apparently reflect the inverted U-shaped cortisol response curve; at low levels, increased cortisol is beneficial to hippocampal cognitive responses, but at higher levels, it is not (137). The degree of stress-induced cortisolaemia and mood negativity is correlated with increased positivity after amphetamine in men and women (138).
Furthermore, much experimental evidence supports the general concept (see Table 1). Male rats too self-administer corticosterone in a manner that suggests some degree of dependence (139, 140). Thus, de Jong et al. (141) found that cocaine-induced locomotor sensitization in adrenalectomized male mice was restored by replacement of both adrenaline and corticosterone, and cocaine- or alcohol-induced behaviours in female mice are inhibited in the presence of a GR inhibitor (142). Additionally, if corticosteroid synthesis is blocked, cocaine self-administration also relapses according to some authors (143). Others find the reverse that corticosterone facilitates relapse, although dexamethasone did not, suggesting mineralocorticoid receptor (NR3C2, MR) involvement (144). Such effects, like those of antipsychotic drugs, may be mediated through the mesolimbic dopaminergic system (145, 146). It is striking that dopamine-dependent responses to morphine require glucocorticoid receptors (147).
Table 1.
Glucocorticoids and addiction. All the direct experimental evidence for the essential role of glucocorticoids has been obtained in experimental animals, as illustrated here. Evidence from the human species is indirect and circumstantial but appears to support the general conclusion that glucocorticoids, regulated by an expanded HPA axis, underlie the important features of addiction.
| Species | Effects | Reference | |||
|---|---|---|---|---|---|
| Ratsa | Corticosterone | Administration | Up to 100 μg/ml in drinking water | Induced corticosterone self administration | 139 |
| Up to 50 μg/animal per day | Induced corticosterone self administration | 140 | |||
| Up to 0.8 mg/kg implant | Induced corticosterone self administration | 194 | |||
| Stress induced | Novel environment | Induced amphetamine self administration | 195 | ||
| Immobilisation | Impaired HPA feedback in cocaine habituated animals | 28, 29 | |||
| Synthesis blocked | By metyrapone 50 mg/kg | Reduced psychomotor effects of cocaine, and reduced reinstatement | 196 | ||
| By metyrapone 100 mg/kg | Reduced psychomotor effects of cocaine, and reduced reinstatement | 143 | |||
| By metyrapone synergistic with benzodiazepine agonist oxazepam; up to 45 mg/kg: 20 mg/kg i.p.c | Reduced psychomotor effects of cocaine, and reduced reinstatement | 197 | |||
| By adrenalectomyd | Cocaine reinstatement reduced, restored by corticosterone replacement | 198 | |||
| Reduced Fos response to dopamine agonist, enhanced dopamine response to cocaine | 199 | ||||
| Decreased cocaine-induced locomotor sensitisation | 200 | ||||
| With corticosterone hemi-succinate replacement; up to 3 mg/kg implant | Restoration of cocaine-induced sensitisation | 200 | |||
| Levels in blood | Unrelated to high or low responder to cocaine classification | 201 | |||
| Levels in brain | Related to high or low responder to cocaine classification | 109, 202 | |||
| Miceb | GR | Antagonist, mifepristone 30 mg/kg i.p. (or, less effective, MR antagonist spironolacetone 20 mg/kg i.p.) | Reduced cocaine induced reinforcement | 203 | |
| Selective GR depletion | In brain | Decreased sensitisation to cocaine self administration | 148 | ||
| In brain | Selective reduced glutamate receptor subunit, and enkephalin response to cocaine, no effect on neuropeptide or dopamine receptor response | 204 | |||
| In dopaminoceptive neurones | Decreased cocaine self administration | 149 | |||
| In dopaminoceptive or dopamine neurones | Decreased cocaine induced CPP | 150 | |||
| Selective GR overexpression | In forebrain | Increased cocaine sensitisation | 152 | ||
| Adrenalectomy | With corticosterone (20 mg in pellets) and adrenaline (5 μg/kg s.c.) replacemente | Synergistic actions on restoration of cocaine induced locomotor sensitisation | 141 |
Sprague Dawley strain except where stated
Original strain usually C57B/6
Wistar rats
Long Evans rats
DBA/2 Rj strain
In experimental animals, the definitive evidence for the pivotal role of the corticosteroids in addiction stems from recent studies in the effects of GR over- and under-expression. Brain-specific GR depletion in mice decreased cocaine self-administration, while corticosterone replacement restored it (148). Specific GR disruption in dopaminoceptive but not dopamine neurones decreased cocaine self-administration (149), whereas GR disruption in either type attenuates cocaine-induced CPP, with no effect on morphine-induced behaviour (150). Morphine-induced CPP depends on hippocampal and nucleus accumbens GR (151). In male mice, overexpression of forebrain GR results in heightened sensitization to cocaine as well as anxiety (152).
There is also evidence of the pivotal role of GR in studies of GR polymorphisms in humans, which have revealed association of particular alleles with the initiation of alcohol abuse in female adolescents (153). These and further experimental data that now link addictive behaviour and symptoms with corticosteroids, particularly in response to cocaine, are summarized in Table 1.
Sex differences in addiction
The possibility of sex differences in responses to drugs of addiction of brain CRH, POMC, neurosteroids and the HPA axis has not been addressed anywhere in the literature reviewed here. Sometimes, the sex of experimental animals used is not actually given, although this is rare. The impression is that studies are often performed on animals of the same sex – male rats are frequently used – to minimize variance. Yet sex differences in addiction are clear and the extensive evidence has been reviewed in human subjects and in experimental animals. Thus, women are more susceptible to addiction and are at greater risk of relapse than men (154, 155), and female rats are more susceptible than male rats. Substantial evidence links this to gonadal hormones (156).
There is nevertheless good reason to speculate that adrenocortical hormones are involved here as well. Both humans and rats have sex differences in adrenocortical function, and although different in nature, both may contribute to sex differences in addiction.
In humans, differences in circulating cortisol in males and females are marginal at most, though there may be differences in responsiveness to ACTH (96, 157, 158). However, the major product of the gland is in fact DHEA, which is secreted not only as the free steroid, but also, and predominantly, as the sulphate, DHEAS. Plasma concentrations of DHEA and DHEAS in young adult men are about 12 nM and 10 μM respectively, compared with about 8 nM and <7 μM in women, levels decrease with age but the sex differences are maintained (96, 159, 160, 161).
The point is that DHEA has been shown to be protective against drugs of addiction, as previously noted. Evidence from cerebrospinal fluid suggests that adrenal DHEA, and even DHEAS, may reach the brain in significant amounts (162), although how this relates to amounts synthesized within the brain cannot be assessed. Although no sex differences in cerebrospinal fluid were reported, it remains plausible that men receive more DHEA protection to addictive drugs than women (154, 162).
In rats, the situation is different, and there is no significant adrenal secretion of DHEA. However, there is a profound difference in secretion and circulating concentrations of corticosterone (the main glucocorticoid in the rat); adult female adrenals are nearly twice the size of males; and output of corticosterone is proportionately greater (163, 164, 165, 166). Although as noted earlier, DHEA is synthesized in the rat brain, there is no sex difference, and brain concentrations are similar in males and females (167). Accordingly, in the rat, it is plausible that heightened sensitivity to addictive drugs in females is associated with the higher circulating levels of corticosterone.
The adrenal, addiction and the clock
If it is the adrenal gland itself that is critical for HPA-modulated addictive processes, then other factors that are instrumental in generating adrenocortical responses may be expected to interact with addiction. Of the physiological stimuli that stimulate the adrenal cortex, stress is the most prominent and relevant. However, an equally potent regulator of the adrenal cortex is the clock.
That stress, however defined, facilitates addiction in both patients and animal models is well understood (168, 169, 170, 171, 172). It is deeply interesting to note that clock time too has its effect on addictive craving and behaviours, although this literature generally has little reference to the HPA, but has been focused on the pineal and melatonin in the brain of male mice (173), or, primarily, on clock genes. Periodicity in PER1 and cocaine sensitivity are associated in male rats and mice of various strains (174), drug reinstatement can be suppressed by photoperiod in male rats (175), and clock gene variants are associated with cocaine sensitization in Drosophila (176) as with addiction in mice (sex not given) (177) and in humans, according to some authors (178, 179, 180, 181) but not all (182). In men, alcohol consumption over a 26-hour period affected neither melatonin nor the cortisol secretory diurnal variation (183, 184).
Autonomy of the adrenal
One feature of adrenocortical function that is hardly considered, in relation to addiction or anything else, is that mechanisms exist whereby the secretion of glucocorticoid appears to be regulated in part by local stimuli. CRH is notable among these. The relationship between the functions of hypothalamic CRH and CRH formed locally in the adrenal is currently obscure. That the adrenal gland of various species may secrete CRH from the medulla in response to splanchnic nerve stimulation has been shown, as has the direct stimulatory effect of CRH on corticosteroid secretion (185, 186, 187, 188). How does adrenal CRH vary with addiction? This is a topic for the future.
Conclusion
There is a clear pattern in the relationship of HPA activation to the development of addictive behaviours in response to quite different drugs. What is it they all have in common? Is there a unifying pathway that in so many cases leads to what may sometimes appear to be an addiction to the adrenal cortex and the secretion of glucocorticoids?
One point is becoming clear: CRH and POMC at different brain sites have clear functional links with the classical HPA (Fig. 1), and together, they may play similar roles in the adaptation that underlies addictive behaviour. They may be considered in the context of addiction as an expanded HPA, of which the terminal, and crucial, component is the adrenal cortex itself.
The evidence for the key importance of the adrenal cortex and glucocorticoids in behaviour and symptoms in drug withdrawal and reinstatement seems conclusive. Therapeutic control of glucocorticoid secretion or inhibition of glucocorticoid action at its receptor may be important future developments (148, 189) in what otherwise is a bleak therapeutic landscape (48, 189, 190, 191).
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the review.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- 1.Lewis JT. Sensibility to intoxication in albino rats after double adrenalectomy. American Journal of Physiology. 1923;64:506–511. [Google Scholar]
- 2.Mackay EM, Mackay LL. Susceptibility of adrenalectomized rats to intoxication. Journal of Pharmacology and Experimental Therapeutics. 1929;35:67–74. [Google Scholar]
- 3.Mackay EM. The relation of acquired morphine tolerance to the adrenal cortex. Journal of Pharmacology and Experimental Therapeutics. 1931;43:51–60. [Google Scholar]
- 4.Sung CY, Way EL, Scott KG. Studies on the relationship of metabolic fate and hormonal effects of d,l-methadone to the development of drug tolerance. Journal of Pharmacology and Experimental Therapeutics. 1953;107:12–23. [PubMed] [Google Scholar]
- 5.Boswell WH. Narcotic addiction. Management of withdrawal symptoms with cortisone. United States Armed Forces Medical Journal. 1951;2:1347–1351. [PubMed] [Google Scholar]
- 6.Lovell HW, Tintera JW. Hypoadrenocorticism in alcoholism and drug addiction. Geriatrics. 1951;6:1–11. [PubMed] [Google Scholar]
- 7.Fraser HF, Isbell H. Failure of cortisone and ACTH in treatment of the morphine abstinence syndrome. Annals of Internal Medicine. 1953;38:234–238. doi: 10.7326/0003-4819-38-2-234. [DOI] [PubMed] [Google Scholar]
- 8.Altman LC, Hill JS, Hairfield WM, Mullarkey MF. Effects of corticosteroids on eosinophil chemotaxis and adherence. Journal of Clinical Investigation. 1981;67:28–36. doi: 10.1172/JCI110024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraser HF. Tolerance to and physical dependence on opiates, barbiturates, and alcohol. Annual Review of Medicine. 1957;8:427–440. doi: 10.1146/annurev.me.08.020157.002235. [DOI] [PubMed] [Google Scholar]
- 10.Eisenman AJ, Fraser HF, Sloan J, Isbell H. Urinary 17-ketosteroid excretion during a cycle of addiction to morphine. Journal of Pharmacology and Experimental Therapeutics. 1958;124:305–311. [PubMed] [Google Scholar]
- 11.Eisenman AJ, Fraser HF, Brooks JW. Urinary excretion and plasma levels of 17-hydroxycorticosteroids during a cycle of addiction to morphine. Journal of Pharmacology and Experimental Therapeutics. 1961;132:226–231. [PubMed] [Google Scholar]
- 12.Hochberg Z, Pacak K, Chrousos GP. Endocrine withdrawal syndromes. Endocrine Reviews. 2003;24:523–538. doi: 10.1210/er.2001-0014. [DOI] [PubMed] [Google Scholar]
- 13.Lloyd RB, Nemeroff CB. The role of corticotropin-releasing hormone in the pathophysiology of depression: therapeutic implications. Current Topics in Medicinal Chemistry. 2011;11:609–617. doi: 10.2174/1568026611109060609. [DOI] [PubMed] [Google Scholar]
- 14.Orth DN. Corticotropin-releasing hormone in humans. Endocrine Reviews. 1992;13:164–191. doi: 10.1210/edrv-13-2-164. [DOI] [PubMed] [Google Scholar]
- 15.Chen R, Lewis KA, Perrin MH, Vale WW. Expression cloning of a human corticotropin-releasing-factor receptor. PNAS. 1993;90:8967–8971. doi: 10.1073/pnas.90.19.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vita N, Laurent P, Lefort S, Chalon P, Lelias JM, Kaghad M, Le Fur G, Caput D, Ferrara P. Primary structure and functional expression of mouse pituitary and human brain corticotrophin releasing factor receptors. FEBS Letters. 1993;335:1–5. doi: 10.1016/0014-5793(93)80427-V. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell AJ. The role of corticotropin releasing factor in depressive illness: a critical review. Neuroscience and Biobehavioral Reviews. 1998;22:635–651. doi: 10.1016/S0149-7634(97)00059-6. [DOI] [PubMed] [Google Scholar]
- 18.Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacological Reviews. 2001;53:209–243. [PubMed] [Google Scholar]
- 19.Koob GF. Brain stress systems in the amygdala and addiction. Brain Research. 2009;1293:61–75. doi: 10.1016/j.brainres.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aguilera G, Liu Y. The molecular physiology of CRH neurons. Frontiers in Neuroendocrinology. 2012;33:67–84. doi: 10.1016/j.yfrne.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spina M, Merlo-Pich E, Chan RK, Basso AM, Rivier J, Vale W, Koob GF. Appetite-suppressing effects of urocortin, a CRF-related neuropeptide. Science. 1996;273:1561–1564. doi: 10.1126/science.273.5281.1561. [DOI] [PubMed] [Google Scholar]
- 22.Pelleymounter MA, Joppa M, Carmouche M, Cullen MJ, Brown B, Murphy B, Grigoriadis DE, Ling N, Foster AC. Role of corticotropin-releasing factor (CRF) receptors in the anorexic syndrome induced by CRF. Journal of Pharmacology and Experimental Therapeutics. 2000;293:799–806. [PubMed] [Google Scholar]
- 23.Ho SP, Takahashi LK, Livanov V, Spencer K, Lesher T, Maciag C, Smith MA, Rohrbach KW, Hartig PR, Arneric SP. Attenuation of fear conditioning by antisense inhibition of brain corticotropin releasing factor-2 receptor. Brain Research. Molecular Brain Research. 2001;89:29–40. doi: 10.1016/S0169-328X(01)00050-X. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi LK, Ho SP, Livanov V, Graciani N, Arneric SP. Antagonism of CRF(2) receptors produces anxiolytic behavior in animal models of anxiety. Brain Research. 2001;902:135–142. doi: 10.1016/S0006-8993(01)02405-2. [DOI] [PubMed] [Google Scholar]
- 25.Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Research. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goeders NE. A neuroendocrine role in cocaine reinforcement. Psychoneuroendocrinology. 1997;22:237–259. doi: 10.1016/S0306-4530(97)00027-9. [DOI] [PubMed] [Google Scholar]
- 27.Goeders NE. The HPA axis and cocaine reinforcement. Psychoneuroendocrinology. 2002;27:13–33. doi: 10.1016/S0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- 28.Mantsch JR, Cullinan WE, Tang LC, Baker DA, Katz ES, Hoks MA, Ziegler DR. Daily cocaine self-administration under long-access conditions augments restraint-induced increases in plasma corticosterone and impairs glucocorticoid receptor-mediated negative feedback in rats. Brain Research. 2007;1167:101–111. doi: 10.1016/j.brainres.2007.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mantsch JR, Taves S, Khan T, Katz ES, Sajan T, Tang LC, Cullinan WE, Ziegler DR. Restraint-induced corticosterone secretion and hypothalamic CRH mRNA expression are augmented during acute withdrawal from chronic cocaine administration. Neuroscience Letters. 2007;415:269–273. doi: 10.1016/j.neulet.2007.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. Journal of Neuroscience. 1997;17:2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology. 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- 32.O'Callaghan MJ, Croft AP, Jacquot C, Little HJ. The hypothalamopituitary–adrenal axis and alcohol preference. Brain Research Bulletin. 2005;68:171–178. doi: 10.1016/j.brainresbull.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Graf EN, Hoks MA, Baumgardner J, Sierra J, Vranjkovic O, Bohr C, Baker DA, Mantsch JR. Adrenal activity during repeated long-access cocaine self-administration is required for later CRF-Induced and CRF-dependent stressor-induced reinstatement in rats. Neuropsychopharmacology. 2011;36:1444–1454. doi: 10.1038/npp.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Enoch MA, Shen PH, Ducci F, Yuan Q, Liu J, White KV, Albaugh B, Hodgkinson CA, Goldman D. Common genetic origins for EEG, alcoholism and anxiety: the role of CRH-BP. PLoS ONE. 2008;3:e3620. doi: 10.1371/journal.pone.0003620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ray LA. Stress-induced and cue-induced craving for alcohol in heavy drinkers: preliminary evidence of genetic moderation by the OPRM1 and CRH-BP genes. Alcoholism, Clinical and Experimental Research. 2011;35:166–174. doi: 10.1111/j.1530-0277.2010.01333.x. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Y, Franck J, Spangler R, Maggos CE, Ho A, Kreek MJ. Reduced hypothalamic POMC and anterior pituitary CRF1 receptor mRNA levels after acute, but not chronic, daily "binge" intragastric alcohol administration. Alcoholism, Clinical and Experimental Research. 2000;24:1575–1582. [PubMed] [Google Scholar]
- 37.Richardson HN, Lee SY, O'Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic–pituitary–adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. European Journal of Neuroscience. 2008;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee B, Tiefenbacher S, Platt DM, Spealman RD. Role of the hypothalamic–pituitary–adrenal axis in reinstatement of cocaine-seeking behavior in squirrel monkeys. Psychopharmacology. 2003;168:177–183. doi: 10.1007/s00213-003-1391-4. [DOI] [PubMed] [Google Scholar]
- 39.Buckingham JC, Cooper TA. Pharmacological characterization of opioid receptors influencing the secretion of corticotrophin releasing factor in the rat. Neuroendocrinology. 1986;44:36–40. doi: 10.1159/000124618. [DOI] [PubMed] [Google Scholar]
- 40.Tsagarakis S, Rees LH, Besser M, Grossman A. Opiate receptor subtype regulation of CRF-41 release from rat hypothalamus in vitro. Neuroendocrinology. 1990;51:599–605. doi: 10.1159/000125397. [DOI] [PubMed] [Google Scholar]
- 41.Kreek MJ, Borg L, Zhou Y & Schluger J. Relationships between endocrine functions and substance abuse syndromes: heroin and related short-acting opiates in addiction contrasted with methadone and other long acting agonists used in pharmacotherapy of addiction. In Hormones, Brain, and Behaviour, 2nd edn, pp 781–829. Eds DW Pfaff, AP Arnold, AM Etgen, RT Rubin & SE Fahrbach. San Diego, CA: Elsevier, 2002.
- 42.Buckingham JC, Cooper TA. Differences in hypothalamo–pituitary–adrenocortical activity in the rat after acute and prolonged treatment with morphine. Neuroendocrinology. 1984;38:411–417. doi: 10.1159/000123927. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Y, Spangler R, Maggos CE, Wang XM, Han JS, Ho A, Kreek MJ. Hypothalamic–pituitary–adrenal activity and pro-opiomelanocortin mRNA levels in the hypothalamus and pituitary of the rat are differentially modulated by acute intermittent morphine with or without water restriction stress. Journal of Endocrinology. 1999;163:261–267. doi: 10.1677/joe.0.1630261. [DOI] [PubMed] [Google Scholar]
- 44.Kupferschmidt DA, Newman AE, Boonstra R, Erb S. Antagonism of cannabinoid 1 receptors reverses the anxiety-like behavior induced by central injections of corticotropin-releasing factor and cocaine withdrawal. Neuroscience. 2012;204:125–133. doi: 10.1016/j.neuroscience.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 45.Buckingham JC, Cooper TA. Effects of naloxone on hypothalamo–pituitary–adrenocortical activity in the rat. Neuroendocrinology. 1986;42:421–426. doi: 10.1159/000124481. [DOI] [PubMed] [Google Scholar]
- 46.Tsagarakis S, Navarra P, Rees LH, Besser M, Grossman A, Navara P. Morphine directly modulates the release of stimulated corticotrophin-releasing factor-41 from rat hypothalamus in vitro. Endocrinology. 1989;124:2330–2335. doi: 10.1210/endo-124-5-2330. [DOI] [PubMed] [Google Scholar]
- 47.Oswald LM, Wand GS. Opioids and alcoholism. Physiology & Behavior. 2004;81:339–358. doi: 10.1016/j.physbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Heilig M, Goldman D, Berrettini W, O'Brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nature Reviews. Neuroscience. 2011;12:670–684. doi: 10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Armario A. Activation of the hypothalamic–pituitary–adrenal axis by addictive drugs: different pathways, common outcome. Trends in Pharmacological Sciences. 2010;31:318–325. doi: 10.1016/j.tips.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Sliwowska JH, Weinberg J. Prenatal alcohol exposure and fetal programming: effects on neuroendocrine and immune function. Experimental Biology and Medicine. 2005;230:376–388. doi: 10.1177/15353702-0323006-05. [DOI] [PubMed] [Google Scholar]
- 51.Allen CD, Rivier CL, Lee SY. Adolescent alcohol exposure alters the central brain circuits known to regulate the stress response. Neuroscience. 2011;182:162–168. doi: 10.1016/j.neuroscience.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cippitelli A, Damadzic R, Singley E, Thorsell A, Ciccocioppo R, Eskay RL, Heilig M. Pharmacological blockade of corticotropin-releasing hormone receptor 1 (CRH1R) reduces voluntary consumption of high alcohol concentrations in non-dependent Wistar rats. Pharmacology, Biochemistry and Behavior. 2012;100:522–529. doi: 10.1016/j.pbb.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pastor R, McKinnon CS, Scibelli AC, Burkhart-Kasch S, Reed C, Ryabinin AE, Coste SC, Stenzel-Poore MP, Phillips TJ. Corticotropin-releasing factor-1 receptor involvement in behavioral neuroadaptation to ethanol: a urocortin1-independent mechanism. PNAS. 2008;105:9070–9075. doi: 10.1073/pnas.0710181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giardino WJ, Pastor R, Anacker AM, Spangler E, Cote DM, Li J, Stenzel-Poore MP, Phillips TJ, Ryabinin AE. Dissection of corticotropin-releasing factor system involvement in locomotor sensitivity to methamphetamine. Genes, Brain and Behavior. 2012;10:78–89. doi: 10.1111/j.1601-183X.2010.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raffin-Sanson ML, de Keyzer Y, Bertagna X. Proopiomelanocortin, a polypeptide precursor with multiple functions: from physiology to pathological conditions. European Journal of Endocrinology. 2003;149:79–90. doi: 10.1530/eje.0.1490079. [DOI] [PubMed] [Google Scholar]
- 56.Cone RD. Anatomy and regulation of the central melanocortin system. Nature Neuroscience. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 57.King CM, Hentges ST. Relative number and distribution of murine hypothalamic proopiomelanocortin neurons innervating distinct target sites. PLoS ONE. 2011;6:e25864. doi: 10.1371/journal.pone.0025864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Starowicz K, Bilecki W, Sieja A, Przewlocka B, Przewlocki R. Melanocortin 4 receptor is expressed in the dorsal root ganglions and down-regulated in neuropathic rats. Neuroscience Letters. 2004;358:79–82. doi: 10.1016/j.neulet.2003.12.096. [DOI] [PubMed] [Google Scholar]
- 59.Bergland R, Blume H, Hamilton A, Monica P, Paterson R. Adrenocorticotropic hormone may be transported directly from the pituitary to the brain. Science. 1980;210:541–543. doi: 10.1126/science.6252607. [DOI] [PubMed] [Google Scholar]
- 60.Kapcala LP, Lechan R, Reichlin S. Origin of immunoreactive ACTH in brain sites outside the ventral hypothalamus. Neuroendocrinology. 1983;37:440–445. doi: 10.1159/000123590. [DOI] [PubMed] [Google Scholar]
- 61.Carr DB, Jones KJ, Bergland RM, Hamilton A, Kasting NW, Fisher JE, Martin JB. Causal links between plasma and CSF endorphin levels in stress: vector-ARMA analysis. Peptides. 1985;6(Suppl 1):5–10. doi: 10.1016/0196-9781(85)90004-X. [DOI] [PubMed] [Google Scholar]
- 62.Twyman RM. Hormonal signalling to the brain for the control of feeding/energy balance. In Encyclopedia of Neuroscience, pp 1201–1206. Ed LR Squire. Oxford: Academic Press, 2009.
- 63.Marcinkiewicz M, Day R, Seidah NG, Chretien M. Ontogeny of the prohormone convertases PC1 and PC2 in the mouse hypophysis and their colocalization with corticotropin and α-melanotropin. PNAS. 1993;90:4922–4926. doi: 10.1073/pnas.90.11.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Allen RG, Peng B, Pellegrino MJ, Miller ED, Grandy DK, Lundblad JR, Washburn CL, Pintar JE. Altered processing of pro-orphanin FQ/nociceptin and pro-opiomelanocortin-derived peptides in the brains of mice expressing defective prohormone convertase 2. Journal of Neuroscience. 2001;21:5864–5870. doi: 10.1523/JNEUROSCI.21-16-05864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grill HJ. Distributed neural control of energy balance: contributions from hindbrain and hypothalamus. Obesity. 2006;14(Suppl 5):216S–221S. doi: 10.1038/oby.2006.312. [DOI] [PubMed] [Google Scholar]
- 66.Millington GW. The role of proopiomelanocortin (POMC) neurones in feeding behaviour. Nutrition and Metabolism. 2007;4:18. doi: 10.1186/1743-7075-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berglund ED, Vianna CR, Donato J, Jr, Kim MH, Chuang JC, Lee CE, Lauzon DA, Lin P, Brule LJ, Scott MM, et al. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. Journal of Clinical Investigation. 2012;122:1000–1009. doi: 10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roubos EW, Dahmen M, Kozicz T, Xu L. Leptin and the hypothalamo–pituitary–adrenal stress axis. General and Comparative Endocrinology. 2012;177:28–36. doi: 10.1016/j.ygcen.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 69.Alvaro JD, Tatro JB, Duman RS. Melanocortins and opiate addiction. Life Sciences. 1997;61:1–9. doi: 10.1016/S0024-3205(97)00029-5. [DOI] [PubMed] [Google Scholar]
- 70.Starowicz K, Sieja A, Bilecki W, Obara I, Przewlocka B. The effect of morphine on MC4 and CRF receptor mRNAs in the rat amygdala and attenuation of tolerance after their blockade. Brain Research. 2003;990:113–119. doi: 10.1016/S0006-8993(03)03444-9. [DOI] [PubMed] [Google Scholar]
- 71.Starowicz K, Obara I, Przewlocki R, Przewlocka B. Inhibition of morphine tolerance by spinal melanocortin receptor blockade. Pain. 2005;117:401–411. doi: 10.1016/j.pain.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 72.Ercil NE, Galici R, Kesterson RA. HS014, a selective melanocortin-4 (MC4) receptor antagonist, modulates the behavioral effects of morphine in micemodulates the behavioral effects of morphine in mice. Psychopharmacology. 2005;180:279–285. doi: 10.1007/s00213-005-2166-x. [DOI] [PubMed] [Google Scholar]
- 73.Hsu R, Taylor JR, Newton SS, Alvaro JD, Haile C, Han G, Hruby VJ, Nestler EJ, Duman RS. Blockade of melanocortin transmission inhibits cocaine reward. European Journal of Neuroscience. 2005;21:2233–2242. doi: 10.1111/j.1460-9568.2005.04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kokare DM, Singru PS, Dandekar MP, Chopde CT, Subhedar NK. Involvement of α-melanocyte stimulating hormone (α-MSH) in differential ethanol exposure and withdrawal related depression in rat: neuroanatomical–behavioral correlates. Brain Research. 2008;1216:53–67. doi: 10.1016/j.brainres.2008.03.064. [DOI] [PubMed] [Google Scholar]
- 75.Jessop DS. Review: central non-glucocorticoid inhibitors of the hypothalamo–pituitary–adrenal axis. Journal of Endocrinology. 1999;160:169–180. doi: 10.1677/joe.0.1600169. [DOI] [PubMed] [Google Scholar]
- 76.Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nature Genetics. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- 77.Arnett MG, Kolber BJ, Boyle MP, Muglia LJ. Behavioral insights from mouse models of forebrain- and amygdala-specific glucocorticoid receptor genetic disruption. Molecular and Cellular Endocrinology. 2011;336:2–5. doi: 10.1016/j.mce.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu J, Garza JC, Truong HV, Henschel J, Zhang W, Lu XY. The melanocortinergic pathway is rapidly recruited by emotional stress and contributes to stress-induced anorexia and anxiety-like behavior. Endocrinology. 2007;148:5531–5540. doi: 10.1210/en.2007-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kawashima S, Sakihara S, Kageyama K, Nigawara T, Suda T. Corticotropin-releasing factor (CRF) is involved in the acute anorexic effect of α-melanocyte-stimulating hormone: a study using CRF-deficient mice. Peptides. 2008;29:2169–2174. doi: 10.1016/j.peptides.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 80.Smart JL, Tolle V, Otero-Corchon V, Low MJ. Central dysregulation of the hypothalamic–pituitary–adrenal axis in neuron-specific proopiomelanocortin-deficient mice. Endocrinology. 2007;148:647–659. doi: 10.1210/en.2006-0990. [DOI] [PubMed] [Google Scholar]
- 81.Fekete C, Legradi G, Mihaly E, Huang QH, Tatro JB, Rand WM, Emerson CH, Lechan RM. α-Melanocyte-stimulating hormone is contained in nerve terminals innervating thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and prevents fasting-induced suppression of prothyrotropin-releasing hormone gene expression. Journal of Neuroscience. 2000;20:1550–1558. doi: 10.1523/JNEUROSCI.20-04-01550.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lu XY, Barsh GS, Akil H, Watson SJ. Interaction between α-melanocyte-stimulating hormone and corticotropin-releasing hormone in the regulation of feeding and hypothalamo–pituitary–adrenal responses. Journal of Neuroscience. 2003;23:7863–7872. doi: 10.1523/JNEUROSCI.23-21-07863.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cragnolini AB, Perello M, Schioth HB, Scimonelli TN. α-MSH and γ-MSH inhibit IL-1β induced activation of the hypothalamic–pituitary–adrenal axis through central melanocortin receptors. Regulatory Peptides. 2004;122:185–190. doi: 10.1016/j.regpep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 84.Sebag JA, Hinkle PM. Regulation of endogenous melanocortin-4 receptor expression and signaling by glucocorticoids. Endocrinology. 2006;147:5948–5955. doi: 10.1210/en.2006-0984. [DOI] [PubMed] [Google Scholar]
- 85.Fester L, Prange-Kiel J, Jarry H, Rune GM. Estrogen synthesis in the hippocampus. Cell and Tissue Research. 2011;345:285–294. doi: 10.1007/s00441-011-1221-7. [DOI] [PubMed] [Google Scholar]
- 86.Prange-Kiel J, Rune GM. Direct and indirect effects of estrogen on rat hippocampus. Neuroscience. 2006;138:765–772. doi: 10.1016/j.neuroscience.2005.05.061. [DOI] [PubMed] [Google Scholar]
- 87.Peri A, Danza G, Benvenuti S, Luciani P, Deledda C, Rosati F, Cellai I, Serio M. New insights on the neuroprotective role of sterols and sex steroids: the seladin-1/DHCR24 paradigm. Frontiers in Neuroendocrinology. 2009;30:119–129. doi: 10.1016/j.yfrne.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 88.Saldanha CJ, Duncan KA, Walters BJ. Neuroprotective actions of brain aromatase. Frontiers in Neuroendocrinology. 2009;30:106–118. doi: 10.1016/j.yfrne.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Micevych P, Soma KK, Sinchak K. Neuroprogesterone: key to estrogen positive feedback? Brain Research Reviews. 2008;57:470–480. doi: 10.1016/j.brainresrev.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacological Reviews. 2010;62:155–198. doi: 10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gillies GE, McArthur S. Independent influences of sex steroids of systemic and central origin in a rat model of Parkinson's disease: a contribution to sex-specific neuroprotection by estrogens. Hormones and Behavior. 2010;57:23–34. doi: 10.1016/j.yhbeh.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 92.Fester L, Prange-Kiel J, Zhou L, Blittersdorf BV, Bohm J, Jarry H, Schumacher M, Rune GM. Estrogen-regulated synaptogenesis in the hippocampus: sexual dimorphism in vivo but not in vitro. Journal of Steroid Biochemistry and Molecular Biology. 2012;131:24–29. doi: 10.1016/j.jsbmb.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 93.Mermelstein PG, Micevych PE. Nervous system physiology regulated by membrane estrogen receptors. Reviews in the Neurosciences. 2008;19:413–424. doi: 10.1515/REVNEURO.2008.19.6.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Micevych PE, Mermelstein PG. Membrane estrogen receptors acting through metabotropic glutamate receptors: an emerging mechanism of estrogen action in brain. Molecular Neurobiology. 2008;38:66–77. doi: 10.1007/s12035-008-8034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baulieu EE, Corpechot C, Dray F, Emiliozzi R, Lebeau MC, Mauvais Jarvis P, Robel P. An adrenal-secreted "androgen": dehydroisoandrosterone sulfate. Its metabolism and a tentative generalization on the metabolism of other steroid conjugates in man. Recent Progress in Hormone Research. 1965;21:411–500. [PubMed] [Google Scholar]
- 96.Vinson GP, Whitehouse BJ & Hinson JP. In The Adrenal Cortex, ch 3, pp 65–139. Englewood Heights, NJ, USA: Prentice-Hall, 1992.
- 97.Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology. 1998;23:963–987. doi: 10.1016/S0306-4530(98)00071-7. [DOI] [PubMed] [Google Scholar]
- 98.Mellon SH. Neurosteroid regulation of central nervous system development. Pharmacology & Therapeutics. 2007;116:107–124. doi: 10.1016/j.pharmthera.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baulieu EE, Robel P. Dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) as neuroactive neurosteroids. PNAS. 1998;95:4089–4091. doi: 10.1073/pnas.95.8.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Romieu P, Martin-Fardon R, Bowen WD, Maurice T. Sigma 1 receptor-related neuroactive steroids modulate cocaine-induced reward. Journal of Neuroscience. 2003;23:3572–3576. doi: 10.1523/JNEUROSCI.23-09-03572.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maayan R, Lotan S, Doron R, Shabat-Simon M, Gispan-Herman I, Weizman A, Yadid G. Dehydroepiandrosterone (DHEA) attenuates cocaine-seeking behavior in the self-administration model in rats. European Neuropsychopharmacology. 2006;16:329–339. doi: 10.1016/j.euroneuro.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 102.Wilkins JN, Majewska MD, Van Gorp W, Li SH, Hinken C, Plotkin D, Setoda D. DHEAS and POMS measures identify cocaine dependence treatment outcome. Psychoneuroendocrinology. 2005;30:18–28. doi: 10.1016/j.psyneuen.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 103.Doron R, Fridman L, Gispan-Herman I, Maayan R, Weizman A, Yadid G. DHEA, a neurosteroid, decreases cocaine self-administration and reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology. 2006;31:2231–2236. doi: 10.1038/sj.npp.1301013. [DOI] [PubMed] [Google Scholar]
- 104.Maayan R, Touati-Werner D, Shamir D, Yadid G, Friedman A, Eisner D, Weizman A, Herman I. The effect of DHEA complementary treatment on heroin addicts participating in a rehabilitation program: a preliminary study. European Neuropsychopharmacology. 2008;18:406–413. doi: 10.1016/j.euroneuro.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 105.Yadid G, Sudai E, Maayan R, Gispan I, Weizman A. The role of dehydroepiandrosterone (DHEA) in drug-seeking behavior. Neuroscience and Biobehavioral Reviews. 2010;35:303–314. doi: 10.1016/j.neubiorev.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 106.Boyd KN, Kumar S, O'Buckley TK, Porcu P, Morrow AL. Ethanol induction of steroidogenesis in rat adrenal and brain is dependent upon pituitary ACTH release and de novo adrenal StAR synthesis. Journal of Neurochemistry. 2010;112:784–796. doi: 10.1111/j.1471-4159.2009.06509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vinson GP. The mislabelling of deoxycorticosterone: making sense of corticosteroid structure and function. Journal of Endocrinology. 2011;211:3–16. doi: 10.1530/JOE-11-0178. [DOI] [PubMed] [Google Scholar]
- 108.Barbaccia ML, Affricano D, Trabucchi M, Purdy RH, Colombo G, Agabio R, Gessa GL. Ethanol markedly increases "GABAergic" neurosteroids in alcohol-preferring rats. European Journal of Pharmacology. 1999;384:R1–R2. doi: 10.1016/S0014-2999(99)00678-0. [DOI] [PubMed] [Google Scholar]
- 109.Palamarchouk V, Smagin G, Goeders NE. Self-administered and passive cocaine infusions produce different effects on corticosterone concentrations in the medial prefrontal cortex (MPC) of rats. Pharmacology, Biochemistry and Behavior. 2009;94:163–168. doi: 10.1016/j.pbb.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gomez-Sanchez CE, Zhou MY, Cozza EN, Morita H, Foecking MF, Gomez-Sanchez EP. Aldosterone biosynthesis in the rat brain. Endocrinology. 1997;138:3369–3373. doi: 10.1210/en.138.8.3369. [DOI] [PubMed] [Google Scholar]
- 111.Higo S, Hojo Y, Ishii H, Komatsuzaki Y, Ooishi Y, Murakami G, Mukai H, Yamazaki T, Nakahara D, Barron A, et al. Endogenous synthesis of corticosteroids in the hippocampus. PLoS ONE. 2011;6:e21631. doi: 10.1371/journal.pone.0021631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Taves MD, Gomez-Sanchez CE, Soma KK. Extra-adrenal glucocorticoids and mineralocorticoids: evidence for local synthesis, regulation, and function. American Journal of Physiology. Endocrinology and Metabolism. 2011;301:E11–E24. doi: 10.1152/ajpendo.00100.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Davies E, MacKenzie SM. Extra-adrenal production of corticosteroids. Clinical and Experimental Pharmacology & Physiology. 2003;30:437–445. doi: 10.1046/j.1440-1681.2003.03867.x. [DOI] [PubMed] [Google Scholar]
- 114.Herbert J, Goodyer IM, Grossman AB, Hastings MH, de Kloet ER, Lightman SL, Lupien SJ, Roozendaal B, Seckl JR. Do corticosteroids damage the brain? Journal of Neuroendocrinology. 2006;18:393–411. doi: 10.1111/j.1365-2826.2006.01429.x. [DOI] [PubMed] [Google Scholar]
- 115.Ingle D. The biologic properties of cortisone: a review. Journal of Clinical Endocrinology. 1950;10:1312–1354. doi: 10.1210/jcem-10-10-1312. [DOI] [PubMed] [Google Scholar]
- 116.Bolanos SH, Khan DA, Hanczyc M, Bauer MS, Dhanani N, Brown ES. Assessment of mood states in patients receiving long-term corticosteroid therapy and in controls with patient-rated and clinician-rated scales. Annals of Allergy, Asthma & Immunology. 2004;92:500–505. doi: 10.1016/S1081-1206(10)61756-5. [DOI] [PubMed] [Google Scholar]
- 117.Klein JF. Adverse psychiatric effects of systemic glucocorticoid therapy. American Family Physician. 1992;46:1469–1474. [PubMed] [Google Scholar]
- 118.Brown ES, Suppes T. Mood symptoms during corticosteroid therapy: a review. Harvard Review of Psychiatry. 1998;5:239–246. doi: 10.3109/10673229809000307. [DOI] [PubMed] [Google Scholar]
- 119.Sirois F. Steroid psychosis: a review. General Hospital Psychiatry. 2003;25:27–33. doi: 10.1016/S0163-8343(02)00241-4. [DOI] [PubMed] [Google Scholar]
- 120.Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clinic Proceedings. 2006;81:1361–1367. doi: 10.4065/81.10.1361. [DOI] [PubMed] [Google Scholar]
- 121.Michael RP, Gibbons JL. Interrelationships between the endocrine system neuropsychiatry. International Review of Neurobiology. 1963;5:243–302. doi: 10.1016/s0074-7742(08)60597-8. [DOI] [PubMed] [Google Scholar]
- 122.Van Craenenbroeck K, De Bosscher K, Vanden Berghe W, Vanhoenacker P, Haegeman G. Role of glucocorticoids in dopamine-related neuropsychiatric disorders. Molecular and Cellular Endocrinology. 2005;245:10–22. doi: 10.1016/j.mce.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 123.Montaron MF, Piazza PV, Aurousseau C, Urani A, Le Moal M, Abrous DN. Implication of corticosteroid receptors in the regulation of hippocampal structural plasticity. European Journal of Neuroscience. 2003;18:3105–3111. doi: 10.1111/j.1460-9568.2003.03048.x. [DOI] [PubMed] [Google Scholar]
- 124.Zunszain PA, Anacker C, Cattaneo A, Carvalho LA, Pariante CM. Glucocorticoids, cytokines and brain abnormalities in depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2011;35:722–729. doi: 10.1016/j.pnpbp.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Anacker C, Zunszain PA, Carvalho LA, Pariante CM. The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology. 2011;36:415–425. doi: 10.1016/j.psyneuen.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Horstmann S, Binder EB. Glucocorticoids as predictors of treatment response in depression. Harvard Review of Psychiatry. 2011;19:125–143. doi: 10.3109/10673229.2011.586550. [DOI] [PubMed] [Google Scholar]
- 127.Medh RD, Lay RH, Schmidt TJ. Agonist-specific modulation of glucocorticoid receptor-mediated transcription by immunosuppressants. Molecular and Cellular Endocrinology. 1998;138:11–23. doi: 10.1016/S0303-7207(98)00055-0. [DOI] [PubMed] [Google Scholar]
- 128.Pariante CM, Thomas SA, Lovestone S, Makoff A, Kerwin RW. Do antidepressants regulate how cortisol affects the brain? Psychoneuroendocrinology. 2004;29:423–447. doi: 10.1016/j.psyneuen.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 129.Kelly M. Steroids: drugs of addiction to patient and doctor. Journal of Chronic Diseases. 1964;17:461–464. doi: 10.1016/0021-9681(64)90106-7. [DOI] [PubMed] [Google Scholar]
- 130.Kelly M. Steroids are drugs of addiction. Rheumatism. 1965;21:50–54. [PubMed] [Google Scholar]
- 131.Morgan HG, Boulnois J, Burns-Cox C. Addiction to prednisone. BMJ. 1973;2:93–94. doi: 10.1136/bmj.2.5858.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kligman AM, Frosch PJ. Steroid addiction. International Journal of Dermatology. 1979;18:23–31. doi: 10.1111/j.1365-4362.1979.tb01905.x. [DOI] [PubMed] [Google Scholar]
- 133.Brown ES. Chemical dependence involving glucocorticoids. Annals of Clinical Psychiatry. 1997;9:185–187. doi: 10.3109/10401239709147796. [DOI] [PubMed] [Google Scholar]
- 134.Anfinson TJ, Channappa C, Vo HT. Drug dependence involving prednisone: two cases and a review of the literature. Psychopharmacology Bulletin. 2008;41:154–163. [PubMed] [Google Scholar]
- 135.Mendelson JH, Sholar MB, Goletiani N, Siegel AJ, Mello NK. Effects of low- and high-nicotine cigarette smoking on mood states and the HPA axis in men. Neuropsychopharmacology. 2005;30:1751–1763. doi: 10.1038/sj.npp.1300753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fox HC, Jackson ED, Sinha R. Elevated cortisol and learning and memory deficits in cocaine dependent individuals: relationship to relapse outcomes. Psychoneuroendocrinology. 2009;34:1198–1207. doi: 10.1016/j.psyneuen.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.de Kloet ER, Oitzl MS, Joels M. Stress and cognition: are corticosteroids good or bad guys? Trends in Neurosciences. 1999;22:422–426. doi: 10.1016/S0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- 138.Hamidovic A, Childs E, Conrad M, King A, de Wit H. Stress-induced changes in mood and cortisol release predict mood effects of amphetamine. Drug and Alcohol Dependence. 2010;109:175–180. doi: 10.1016/j.drugalcdep.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Deroche V, Piazza PV, Deminiere JM, Le Moal M, Simon H. Rats orally self-administer corticosterone. Brain Research. 1993;622:315–320. doi: 10.1016/0006-8993(93)90837-D. [DOI] [PubMed] [Google Scholar]
- 140.Piazza PV, Deroche V, Deminiere JM, Maccari S, Le Moal M, Simon H. Corticosterone in the range of stress-induced levels possesses reinforcing properties: implications for sensation-seeking behaviors. PNAS. 1993;90:11738–11742. doi: 10.1073/pnas.90.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.de Jong IE, Steenbergen PJ, de Kloet ER. Behavioral sensitization to cocaine: cooperation between glucocorticoids and epinephrine. Psychopharmacology. 2009;204:693–703. doi: 10.1007/s00213-009-1498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Roberts AJ, Lessov CN, Phillips TJ. Critical role for glucocorticoid receptors in stress- and ethanol-induced locomotor sensitization. Journal of Pharmacology and Experimental Therapeutics. 1995;275:790–797. [PubMed] [Google Scholar]
- 143.Piazza PV, Marinelli M, Jodogne C, Deroche V, Rouge-Pont F, Maccari S, Le Moal M, Simon H. Inhibition of corticosterone synthesis by metyrapone decreases cocaine-induced locomotion and relapse of cocaine self-administration. Brain Research. 1994;658:259–264. doi: 10.1016/S0006-8993(09)90034-8. [DOI] [PubMed] [Google Scholar]
- 144.Mantsch JR, Saphier D, Goeders NE. Corticosterone facilitates the acquisition of cocaine self-administration in rats: opposite effects of the type II glucocorticoid receptor agonist dexamethasone. Journal of Pharmacology and Experimental Therapeutics. 1998;287:72–80. [PubMed] [Google Scholar]
- 145.Piazza PV, Barrot M, Rouge-Pont F, Marinelli M, Maccari S, Abrous DN, Simon H, Le Moal M. Suppression of glucocorticoid secretion and antipsychotic drugs have similar effects on the mesolimbic dopaminergic transmission. PNAS. 1996;93:15445–15450. doi: 10.1073/pnas.93.26.15445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Danilczuk Z, Ossowska G, Wrobel A, Lupina T. Glucocorticoids modulate behavioral effects induced by dopaminergic agonists in rats. Polish Journal of Pharmacology. 2001;53:467–473. [PubMed] [Google Scholar]
- 147.Marinelli M, Aouizerate B, Barrot M, Le Moal M, Piazza PV. Dopamine-dependent responses to morphine depend on glucocorticoid receptors. PNAS. 1998;95:7742–7747. doi: 10.1073/pnas.95.13.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Deroche-Gamonet V, Sillaber I, Aouizerate B, Izawa R, Jaber M, Ghozland S, Kellendonk C, Le Moal M, Spanagel R, Schutz G, et al. The glucocorticoid receptor as a potential target to reduce cocaine abuse. Journal of Neuroscience. 2003;23:4785–4790. doi: 10.1523/JNEUROSCI.23-11-04785.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ambroggi F, Turiault M, Milet A, Deroche-Gamonet V, Parnaudeau S, Balado E, Barik J, van der Veen R, Maroteaux G, Lemberger T, et al. Stress and addiction: glucocorticoid receptor in dopaminoceptive neurons facilitates cocaine seeking. Nature Neuroscience. 2009;12:247–249. doi: 10.1038/nn.2282. [DOI] [PubMed] [Google Scholar]
- 150.Barik J, Parnaudeau S, Saint Amaux AL, Guiard BP, Golib Dzib JF, Bocquet O, Bailly A, Benecke A, Tronche F. Glucocorticoid receptors in dopaminoceptive neurons, key for cocaine, are dispensable for molecular and behavioral morphine responses. Biological Psychiatry. 2010;68:231–239. doi: 10.1016/j.biopsych.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 151.Dong Z, Han H, Wang M, Xu L, Hao W, Cao J. Morphine conditioned place preference depends on glucocorticoid receptors in both hippocampus and nucleus accumbens. Hippocampus. 2006;16:809–813. doi: 10.1002/hipo.20216. [DOI] [PubMed] [Google Scholar]
- 152.Wei Q, Fentress HM, Hoversten MT, Zhang L, Hebda-Bauer EK, Watson SJ, Seasholtz AF, Akil H. Early-life forebrain glucocorticoid receptor overexpression increases anxiety behavior and cocaine sensitization. Biological Psychiatry. 2012;71:224–231. doi: 10.1016/j.biopsych.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Desrivieres S, Lourdusamy A, Muller C, Ducci F, Wong CP, Kaakinen M, Pouta A, Hartikainen AL, Isohanni M, Charoen P, et al. Glucocorticoid receptor (NR3C1) gene polymorphisms and onset of alcohol abuse in adolescents. Addiction Biology. 2011;16:510–513. doi: 10.1111/j.1369-1600.2010.00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Devaud LL, Alele P, Ritu C. Sex differences in the central nervous system actions of ethanol. Critical Reviews in Neurobiology. 2003;15:41–59. doi: 10.1615/CritRevNeurobiol.v15.i1.20. [DOI] [PubMed] [Google Scholar]
- 155.Becker JB, Hu M. Sex differences in drug abuse. Frontiers in Neuroendocrinology. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Festa ED, Russo SJ, Gazi FM, Niyomchai T, Kemen LM, Lin SN, Foltz R, Jenab S, Quinones-Jenab V. Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacology. 2004;46:672–687. doi: 10.1016/j.neuropharm.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 157.Weekes N, Lewis R, Patel F, Garrison-Jakel J, Berger DE, Lupien SJ. Examination stress as an ecological inducer of cortisol and psychological responses to stress in undergraduate students. Stress. 2006;9:199–206. doi: 10.1080/10253890601029751. [DOI] [PubMed] [Google Scholar]
- 158.Keenan DM, Roelfsema F, Carroll BJ, Iranmanesh A, Veldhuis JD. Sex defines the age dependence of endogenous ACTH-cortisol dose responsiveness. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2009;297:R515–R523. doi: 10.1152/ajpregu.00200.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Parker CR, Jr, Porter JC. Developmental changes in molecular forms of immunoreactive adrenocorticotropin in the anterior pituitary gland of humans. Endocrine Research. 1999;25:397–410. doi: 10.1080/07435809909066156. [DOI] [PubMed] [Google Scholar]
- 160.Rainey WE, Carr BR, Sasano H, Suzuki T, Mason JI. Dissecting human adrenal androgen production. Trends in Endocrinology and Metabolism. 2002;13:234–239. doi: 10.1016/S1043-2760(02)00609-4. [DOI] [PubMed] [Google Scholar]
- 161.Muniyappa R, Wong KA, Baldwin HL, Sorkin JD, Johnson ML, Bhasin S, Harman SM, Blackman MR. Dehydroepiandrosterone secretion in healthy older men and women: effects of testosterone and growth hormone administration in older men. Journal of Clinical Endocrinology and Metabolism. 2006;91:4445–4452. doi: 10.1210/jc.2006-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Guazzo EP, Kirkpatrick PJ, Goodyer IM, Shiers HM, Herbert J. Cortisol, dehydroepiandrosterone (DHEA), and DHEA sulfate in the cerebrospinal fluid of man: relation to blood levels and the effects of age. Journal of Clinical Endocrinology and Metabolism. 1996;81:3951–3960. doi: 10.1210/jc.81.11.3951. [DOI] [PubMed] [Google Scholar]
- 163.Jones IC. Role of the adrenal cortex in reproduction. British Medical Bulletin. 1955;11:156–160. doi: 10.1093/oxfordjournals.bmb.a069470. [DOI] [PubMed] [Google Scholar]
- 164.Cortes JM, Peron FG, Dorfman RI. Secretion of 18-hydroxydeoxycorticosterone by the rat adrenal gland. Endocrinology. 1963;73:713–720. doi: 10.1210/endo-73-6-713. [DOI] [PubMed] [Google Scholar]
- 165.Critchlow V, Liebelt RA, Bar-Sela M, Mountcastle W, Lipscomb HS. Sex difference in resting pituitary–adrenal function in the rat. American Journal of Physiology. 1963;205:807–815. doi: 10.1152/ajplegacy.1963.205.5.807. [DOI] [PubMed] [Google Scholar]
- 166.Malendowicz LK, Robba C, Nussdorfer GG. Sex differences in adrenocortical structure and function. XXII. Light- and electron-microscopic morphometric studies on the effects of gonadectomy and gonadal hormone replacement on the rat adrenal cortex. Cell and Tissue Research. 1986;244:141–145. doi: 10.1007/BF00218391. [DOI] [PubMed] [Google Scholar]
- 167.Torres JM, Ortega E. DHEA, PREG and their sulphate derivatives on plasma and brain after CRH and ACTH administration. Neurochemical Research. 2003;28:1187–1191. doi: 10.1023/A:1024276328127. [DOI] [PubMed] [Google Scholar]
- 168.Goeders NE. The impact of stress on addiction. European Neuropsychopharmacology. 2003;13:435–441. doi: 10.1016/j.euroneuro.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 169.Uhart M, Wand GS. Stress, alcohol and drug interaction: an update of human research. Addiction Biology. 2009;14:43–64. doi: 10.1111/j.1369-1600.2008.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kosten TR. Stress and addiction. American Journal of Psychiatry. 2011;168:566–568. doi: 10.1176/appi.ajp.2011.11020180. [DOI] [PubMed] [Google Scholar]
- 171.Logrip ML, Zorrilla EP, Koob GF. Stress modulation of drug self-administration: implications for addiction comorbidity with post-traumatic stress disorder. Neuropharmacology. 2011;62:552–564. doi: 10.1016/j.neuropharm.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Schwabe L, Dickinson A, Wolf OT. Stress, habits, and drug addiction: a psychoneuroendocrinological perspective. Experimental and Clinical Psychopharmacology. 2011;19:53–63. doi: 10.1037/a0022212. [DOI] [PubMed] [Google Scholar]
- 173.Uz T, Akhisaroglu M, Ahmed R, Manev H. The pineal gland is critical for circadian Period1 expression in the striatum and for circadian cocaine sensitization in mice. Neuropsychopharmacology. 2003;28:2117–2123. doi: 10.1038/sj.npp.1300254. [DOI] [PubMed] [Google Scholar]
- 174.Akhisaroglu M, Ahmed R, Kurtuncu M, Manev H, Uz T. Diurnal rhythms in cocaine sensitization and in Period1 levels are common across rodent species. Pharmacology, Biochemistry and Behavior. 2004;79:37–42. doi: 10.1016/j.pbb.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 175.Sorg BA, Stark G, Sergeeva A, Jansen HT. Photoperiodic suppression of drug reinstatement. Neuroscience. 2011;176:284–295. doi: 10.1016/j.neuroscience.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Andretic R, Chaney S, Hirsh J. Requirement of circadian genes for cocaine sensitization in Drosophila. Science. 1999;285:1066–1068. doi: 10.1126/science.285.5430.1066. [DOI] [PubMed] [Google Scholar]
- 177.Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, et al. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nature Medicine. 2005;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- 178.Perreau-Lenz S, Zghoul T, Spanagel R. Clock genes running amok. Clock genes and their role in drug addiction and depression. EMBO Reports. 2007;8:S20–S23. doi: 10.1038/sj.embor.7401016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Falcón E, McClung CA. A role for the circadian genes in drug addiction. Neuropharmacology. 2009;56(Suppl 1):91–96. doi: 10.1016/j.neuropharm.2008.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kovanen L, Saarikoski ST, Haukka J, Pirkola S, Aromaa A, Lonnqvist J, Partonen T. Circadian clock gene polymorphisms in alcohol use disorders and alcohol consumption. Alcohol and Alcoholism. 2010;45:303–311. doi: 10.1093/alcalc/agq035. [DOI] [PubMed] [Google Scholar]
- 181.Albrecht U. The circadian clock, reward, and memory. Frontiers in Molecular Neuroscience. 2011;4:41. doi: 10.3389/fnmol.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Malison RT, Kranzler HR, Yang BZ, Gelernter J. Human clock, PER1 and PER2 polymorphisms: lack of association with cocaine dependence susceptibility and cocaine-induced paranoia. Psychiatric Genetics. 2006;16:245–249. doi: 10.1097/01.ypg.0000242198.59020.ca. [DOI] [PubMed] [Google Scholar]
- 183.Danel T, Touitou Y. Alcohol consumption does not affect melatonin circadian synchronization in healthy men. Alcohol and Alcoholism. 2006;41:386–390. doi: 10.1093/alcalc/agl036. [DOI] [PubMed] [Google Scholar]
- 184.Danel T, Vantyghem MC, Touitou Y. Responses of the steroid circadian system to alcohol in humans: importance of the time and duration of intake. Chronobiology International. 2006;23:1025–1034. doi: 10.1080/07420520600920742. [DOI] [PubMed] [Google Scholar]
- 185.Edwards AV, Jones CT. Secretion of corticotrophin releasing factor from the adrenal during splanchnic nerve stimulation in conscious calves. Journal of Physiology. 1988;400:89–100. doi: 10.1113/jphysiol.1988.sp017112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ehrhart-Bornstein M, Hinson JP, Bornstein SR, Scherbaum WA, Vinson GP. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocrine Reviews. 1998;19:101–143. doi: 10.1210/er.19.2.101. [DOI] [PubMed] [Google Scholar]
- 187.Fukuda T, Takahashi K, Suzuki T, Saruta M, Watanabe M, Nakata T, Sasano H. Urocortin 1, urocortin 3/stresscopin, and corticotropin-releasing factor receptors in human adrenal and its disorders. Journal of Clinical Endocrinology and Metabolism. 2005;90:4671–4678. doi: 10.1210/jc.2005-0090. [DOI] [PubMed] [Google Scholar]
- 188.Tsatsanis C, Dermitzaki E, Venihaki M, Chatzaki E, Minas V, Gravanis A, Margioris AN. The corticotropin-releasing factor (CRF) family of peptides as local modulators of adrenal function. Cellular and Molecular Life Sciences. 2007;64:1638–1655. doi: 10.1007/s00018-007-6555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.van den Brink W, van Ree JM. Pharmacological treatments for heroin and cocaine addiction. European Neuropsychopharmacology. 2003;13:476–487. doi: 10.1016/j.euroneuro.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 190.Sabino V, Cottone P, Zhao Y, Steardo L, Koob GF, Zorrilla EP. Selective reduction of alcohol drinking in Sardinian alcohol-preferring rats by a sigma-1 receptor antagonist. Psychopharmacology. 2009;205:327–335. doi: 10.1007/s00213-009-1548-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Moreno AY, Azar MR, Warren NA, Dickerson TJ, Koob GF, Janda KD. A critical evaluation of a nicotine vaccine within a self-administration behavioral model. Molecular Pharmaceutics. 2010;7:431–441. doi: 10.1021/mp900213u. [DOI] [PubMed] [Google Scholar]
- 192.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 193.Miquel M, Toledo R, Garcia LI, Coria-Avila GA, Manzo J. Why should we keep the cerebellum in mind when thinking about addiction? Current Drug Abuse Reviews. 2009;2:26–40. doi: 10.2174/1874473710902010026. [DOI] [PubMed] [Google Scholar]
- 194.Deroche V, Marinelli M, Le Moal M, Piazza PV. Glucocorticoids and behavioral effects of psychostimulants. II: Cocaine intravenous self-administration and reinstatement depend on glucocorticoid levels. Journal of Pharmacology and Experimental Therapeutics. 1997;281:1401–1407. [PubMed] [Google Scholar]
- 195.Piazza PV, Maccari S, Deminiere JM, Le Moal M, Mormede P, Simon H. Corticosterone levels determine individual vulnerability to amphetamine self-administration. PNAS. 1991;88:2088–2092. doi: 10.1073/pnas.88.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Marinelli M, Rouge-Pont F, De Jesus-Oliveira C, Le Moal M, Piazza PV. Acute blockade of corticosterone secretion decreases the psychomotor stimulant effects of cocaine. Neuropsychopharmacology. 1997;16:156–161. doi: 10.1016/S0893-133X(96)00169-8. [DOI] [PubMed] [Google Scholar]
- 197.Goeders NE, Guerin GF. Effects of the combination of metyrapone and oxazepam on cocaine and food self-administration in rats. Pharmacology, Biochemistry and Behavior. 2008;91:181–189. doi: 10.1016/j.pbb.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Shalev U, Marinelli M, Baumann MH, Piazza PV, Shaham Y. The role of corticosterone in food deprivation-induced reinstatement of cocaine seeking in the rat. Psychopharmacology. 2003;168:170–176. doi: 10.1007/s00213-002-1200-5. [DOI] [PubMed] [Google Scholar]
- 199.Barrot M, Abrous DN, Marinelli M, Rouge-Pont F, Le Moal M, Piazza PV. Influence of glucocorticoids on dopaminergic transmission in the rat dorsolateral striatum. European Journal of Neuroscience. 2001;13:812–818. doi: 10.1046/j.1460-9568.2001.01434.x. [DOI] [PubMed] [Google Scholar]
- 200.Marinelli M, Rouge-Pont F, Deroche V, Barrot M, De Jesus-Oliveira C, Le Moal M, Piazza PV. Glucocorticoids and behavioral effects of psychostimulants. I: Locomotor response to cocaine depends on basal levels of glucocorticoids. Journal of Pharmacology and Experimental Therapeutics. 1997;281:1392–1400. [PubMed] [Google Scholar]
- 201.Nelson AM, Kleschen MJ, Zahniser NR. Individual differences in cocaine-induced locomotor activity of male Sprague–Dawley rats are not explained by plasma corticosterone levels. Neuroscience Letters. 2010;476:9–13. doi: 10.1016/j.neulet.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rose AK, Shaw SG, Prendergast MA, Little HJ. The importance of glucocorticoids in alcohol dependence and neurotoxicity. Alcoholism, Clinical and Experimental Research. 2010;34:2011–2018. doi: 10.1111/j.1530-0277.2010.01298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Fiancette JF, Balado E, Piazza PV, Deroche-Gamonet V. Mifepristone and spironolactone differently alter cocaine intravenous self-administration and cocaine-induced locomotion in C57BL/6J mice. Addiction Biology. 2010;15:81–87. doi: 10.1111/j.1369-1600.2009.00178.x. [DOI] [PubMed] [Google Scholar]
- 204.Izawa R, Jaber M, Deroche-Gamonet V, Sillaber I, Kellendonk C, Le Moal M, Tronche F, Piazza PV. Gene expression regulation following behavioral sensitization to cocaine in transgenic mice lacking the glucocorticoid receptor in the brain. Neuroscience. 2006;137:915–924. doi: 10.1016/j.neuroscience.2005.10.006. [DOI] [PubMed] [Google Scholar]