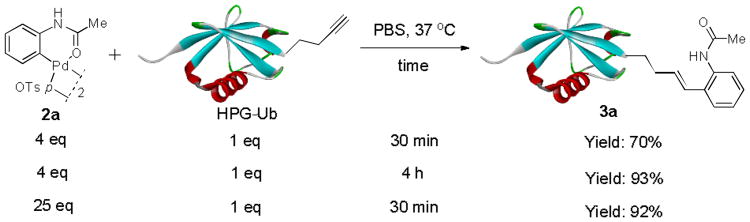
Abstract
We report the facile preparation of palladacycles as the storable arylpalladium(II) reagents from the acetanilides via cyclopalladation. The palladacycles exhibit good stability in PBS buffer and are capable of functionalizing a metabolically encoded HPG-containing protein, thus providing a new type of biocompatible organometallic reagents for selectively functionalizing the alkyne-encoded proteins.
Bioorthogonal reactions have provided chemical tools to study biomolecular dynamics and function in living systems.1-4 These reactions typically proceed in two steps: first, a biomolecule substrate is tagged with a bioorthogonal functional group (chemical reporter); second, a biophysical probe containing a complementary reactive group is used to selectively react with the pre-tagged substrate. Similar to azides, terminal alkynes have become attractive bioorthogonal chemical reporters due to their small sizes, excellent biocompatibility, and ease of incorporation into proteins. Importantly, the alkynes readily react with the azide probes via Cu-catalyzed azide-alkyne cycloaddition5-8 or strain-promoted cycloaddition reaction.9
Recently, we developed a new protein bioconjugation strategy through a palladium-mediated cross-coupling reaction to label an alkyne-encoded protein in vitro and in E. coli.10 In this strategy, we employed a two-step procedure to generate the ‘preactivated’ aryl-Pd(II)complex first followed by the cross-coupling reaction with the alkyne-encoded protein. The reaction was clean and efficient; however, the in situ generated aryl-Pd(II) complexes gradually lose its reactivity in phosphate buffered saline (PBS) over the time. Hence, excess amounts of reagent are typically required in order to achieve high conversion. At about the same time, Myers and co-workers reported the preparation of storable arylpalladium(II) reagents11 through the decarboxylative palladation and showed that these reagents were capable of labeling the olefinic substrates via stoichiometric Heck-type reaction in aqueous media. Inspired by this report, we envisioned that by introducing a weak-coordination directing group12, 13 in our palladium complex, we may achieve an optimum balance between reactivity and stability. Herein, we describe the preparation of the storable palladacycles with an ortho-directing group, the characterization of their stability in PBS buffer, and their use in selective functionalization of an alkyne-encoded protein in PBS under the mild condition.
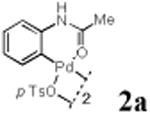
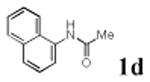
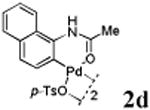
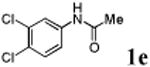
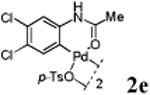
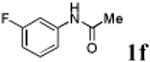
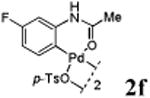
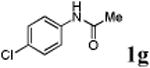
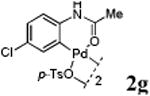
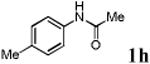
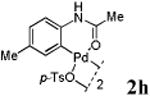
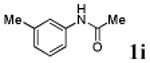
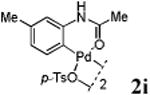
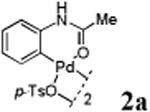
We first prepared a series of palladacycles with varying substituents on the aromatic ring from the acetanilide derivatives by following Yu's cyclopalladation protocol.14 We found that substrates with methoxy substituent (except at the ortho position which is probably due to ortho-effect14) provided the desired palladacycles in excellent yields (Table 1, entries 2 and 12) whereas substrates with methyl substituent afforded modest yields15(entries 8 and 9) similar to 2a without any substituents.14, 16, 17 Interestingly, fluorine substitution gave rise to high yields (entries 6 and 10) compared to chlorine which gave much lower yields (entries 5 and 7). BODIPY substrate 1k served as a poor substrate for the cyclopalladation reaction, giving only 35% yield (entry 11). Notably, high regioselectivity was observed in the formation of palladacycles 2d from 1-acetamidonaphthalene 1d (see supplemental information). Using Pd(O2CCF3) as palladium source,18 we can also generate readily palladacycles containing a fluorescein or PEG (MW ≈ 5 kDa) group (entries 14, 15).
Table 1.
Synthesis of Palladacycles.a

| |||
|---|---|---|---|
| Entry | Aryl acetanilide | Palladacycles | Yield (%)b |
| 1 |
|
|
75 |
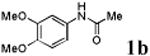
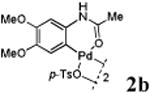
| 2 |
|
|
93 |
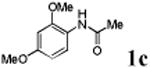
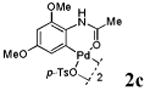
| 3 |
|
|
68 |
| 4 |
|
|
87 |
| 5 |
|
|
54 |
| 6 |
|
|
95 |
| 7 |
|
|
67 |
| 8 |
|
|
70 |
| 9 |
|
|
71 |
| 10 |
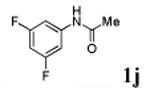
|
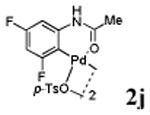
|
73 |
| 11 |
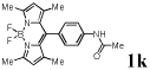
|
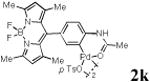
|
35 |
| 12 |
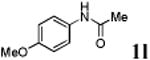
|
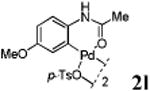
|
92 |
| 13 |
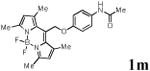
|
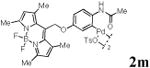
|
72 |
| 14 |
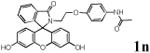
|
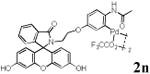
|
60 |
| 15 |
|
|
85c |
Reactions were carried out with 0.1 mmol acetanilide, 1 equiv Pd(OAc)2 and 1 equiv p-TsOH·H2O in 1mL dioxane at room temperature for 1-10 h.
Isolated yields.
Estimated based 1H NMR.
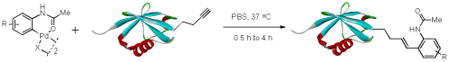
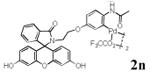
With the palladacycles in hand, we examined their stability in DMSO as well as in PBS buffer. Gratifyingly, all palladacycles were stable in DMSO at room temperature, with no detectable change in 1H-NMR spectra after storage for 10 days. Similarly, most palladacycles exhibited high tolerance to PBS buffer/DMSO (19:1) mixture as monitored by 1H NMR within 24 h (see Table S1 in supplemental information). Next, we assessed whether palladacycle 2a can be employed to modify a metabolically encoded homopropargylglycine (HPG)-containing ubiquitin (HPG-Ub)10 via a cross-coupling reaction process (Scheme 1). Treating 2.5 μM of HPG-Ub with 4 equiv of palladacycle 2a in PBS buffer at 37 °C for 30 min afforded the ligation product 3a in 70% yield, based on LC-MS analysis. Prolonging reaction time to 4 h increased the yield to 93%. Likewise, addition of large excess of palladacycle 2a (25 equiv) increased the yield to 92% (Scheme 1). This encouraging result led us to investigate the rest of the palladacycles (2b-l) in the series using 4 equiv at three time points, namely, 30 min, 2 h, and 4 h, and the results are summarized in Table 2. Among palladacycle series, 2d with the naphthyl ring showed the highest ligation efficiency, reaching essentially quantitative yield within 30 min. Palladacycles with methyl, methoxy, or fluorine substituents on the aromatic ring (Table 2, entries 2, 3, 8, 9, 12, 6, and 10) also gave high yields. However, the reaction with chloro-substituted palladacycles 2e and 2g proceeded sluggishly, giving lower yields (Table 2, entries 5 and 7). In addition, palladacycles 2k and 2m carrying a BODIPY group gave only 30% and 14% yield, respectively, when 25 equiv of palladacycles were employed (Table 2, entries 11, 13). The lower reactivity can be attributed to their poor solubility in PBS buffer. Palladacycle 2n with a fluorescein group gave a relatively higher yield (60%) under the same condition (Table 2, entry 14).
Scheme 1.
Optimization of reaction conditions.
Table 2.
Reactions of Palladacycles with HPG-Ub.a
| ||||
|---|---|---|---|---|
| Entry | Palladacycles | Yield(%)b | ||
| 0.5 h | 2 h | 4 h | ||
| 1 |
|
70 | 96 | 93 |
| 2 |
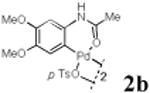
|
65 | 84 | 93 |
| 3 |
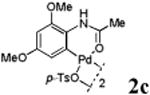
|
49 | 70 | 80 |
| 4 |
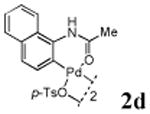
|
96 | 97 | 99 |
| 5 |
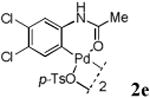
|
20 | 33 | 42 |
| 6 |
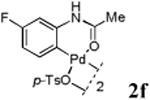
|
68 | 85 | 89 |
| 7 |
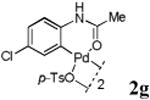
|
45 | 56 | 61 |
| 8 |
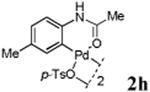
|
82 | 90 | 98 |
| 9 |
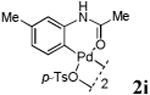
|
83 | 88 | 92 |
| 10 |
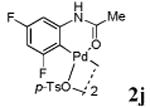
|
59 | 70 | 80 |
| 11 |
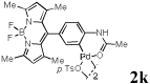
|
30c | ND | ND |
| 12 |
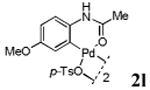
|
60 | 76 | 85 |
| 13 |
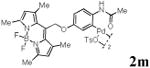
|
14c | ND | ND |
| 14 |
|
60c | ND | ND |
Reactions were carried out using 2.5 μM of HPG-Ub and 4 equiv of palladacycle at 37 °C.
Yields were determined based on LC-MS analyses of the reaction mixtures: Yield % = Iproduct/(IHPG-Ub + Iproduct), where Iproduct and IHPG-Ub, represent the ion counts of the ligated product and HPG-Ub, respectively.
25 equiv of the palladacycle was used.
To elucidate the structure of the ligation product, the adduct of HPG-Ub with palladacycle 2d was digested with trypsin, and the tryptic peptides were analysed LC-MS. The mass of the naphthyl acetanilide-modified C-terminal pentapeptide is consistent with a structure in which the linkage between naphthalene and the peptide fragment is a double bond (Figure S1, Tables S2 and S3).
To verify the selectivity of this type of ligation, we analysed the reaction mixture of HPG-Ub with the BODIPY functionalized palladacycle 2k by SDS-PAGE. In-gel fluorescence analysis reealed that only HPG-Ub was fluorescently labelled, while no fluorescent band was detected with recombinant ubiquitin under identical conditions (Figure S2). Similarly, the reaction of HPG-Ub with PEG-containing palladacycle 2o showed the concentration-dependent formation of a distinct higher molecular weight band on SDS-PAGE, indicating the selective formation of the PEGylated HPG-Ub adduct (Figure S3).
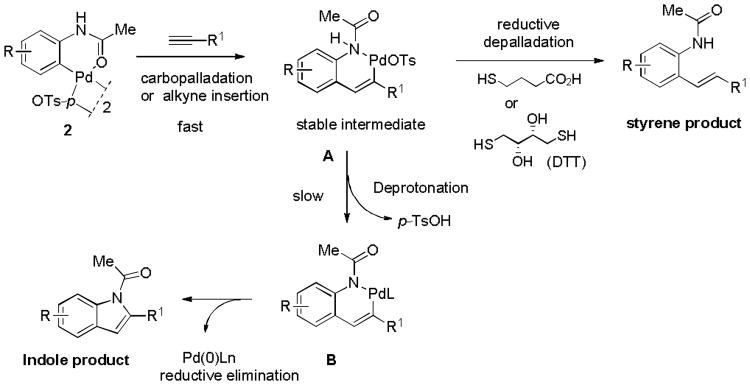
To provide insights into the reaction mechanism, we tested the reaction of 2d with N-acylated HPG-dipeptide 310 at diluted concentration (2.5 μM) in PBS buffer for 30 min (Figure S4). LC-MS analysis showed the formation of a complex mixture containing styrene, alkyne (or indole), and some uncharacterized side products. Compared with HPG-dipeptide substrate, HPG-Ub reacts much cleaner with the palladacycles. To explain this discrepancy, we propose that palladacycle 2 would undergo carbopalladation with alkyne (alkyne insertion) to afford a vinyl-palladium(II) intermediate A (scheme 2), which could be stabilized by coordination with nearby residues such as Arg in the protein substrate, thus preventing a path to indole formation via intermediate B or other side reactions such as multi-alkyne-insertion. Upon treatment with a reducing reagent such as 3-mercaptopropanoic acid (added before LC-MS analysis) or dithiothreitol (added before SDS-PAGE analysis), intermediate A would undergo reductive depalladation to generate the styrenyl product.
Scheme 2.
Proposed mechanism for the generation of styrene product.
In conclusion, we have synthesized stable palladacycles that are suitable for protein labelling in aqueous medium. Trypsin digestion/LC-MS analysis suggested that the cross-coupling of the palladacycles with a terminal alkyne-encoded protein produces a styrene adduct, likely through a tandem stoichiometric carbopalladation followed by reductive depalladation. These palladacycles were used to modify a terminal alkyne-encoded protein in PBS buffer at 37 °C to form the styrene adducts with moderate to high yields. Because of their superior stability and reactivity, these palladacycles, 2d in particular, may be useful for functionalizing the terminal alkyne-encoded proteins in cellular systems.
Supplementary Material
Acknowledgments
We gratefully acknowledge the National Institutes of Health (GM 085092) for financial support.
Footnotes
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/b000000x/. This manuscript is dedicated to Professor Andrew Hamilton on the occasion of his 60th birthday.
References
- 1.Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Proc Natl Acad Sci USA. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaiser ET, Lawrence DS. Science. 1984;226:505–511. doi: 10.1126/science.6238407. [DOI] [PubMed] [Google Scholar]
- 3.Lim RK, Lin Q. Chem Comm. 2010;46:1589–1600. doi: 10.1039/b925931g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sletten EM, Bertozzi CR. Angew Chem Int Ed. 2009;48:6974–6998. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Besanceney-Webler C, Jiang H, Zheng T, Feng L, Soriano del Amo D, Wang W, Klivansky LM, Marlow FL, Liu Y, Wu P. Angew Chem Int Ed. 2011;50:8051–8056. doi: 10.1002/anie.201101817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong V, Presolski SI, Ma C, Finn MG. Angew Chem Int Ed. 2009;48:9879–9883. doi: 10.1002/anie.200905087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy DC, McKay CS, Legault MC, Danielson DC, Blake JA, Pegoraro AF, Stolow A, Mester Z, Pezacki JP. J Am Chem Soc. 2011;133:17993–18001. doi: 10.1021/ja2083027. [DOI] [PubMed] [Google Scholar]
- 8.Moses JE, Moorhouse AD. Chem Soc Rev. 2007;36:1249–1262. doi: 10.1039/b613014n. [DOI] [PubMed] [Google Scholar]
- 9.Sletten EM, Bertozzi CR. Acc Chem Res. 2011;44:666–676. doi: 10.1021/ar200148z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li N, Lim RK, Edwardraja S, Lin Q. J Am Chem Soc. 2011;133:15316–15319. doi: 10.1021/ja2066913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simmons RL, Yu RT, Myers AG. J Am Chem Soc. 2011;133:15870–15873. doi: 10.1021/ja206339s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engle KM, Mei TS, Wasa M, Yu JQ. Acc Chem Res. 2012;45:788–802. doi: 10.1021/ar200185g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leow D, Li G, Mei TS, Yu JQ. Nature. 2012;486:518–522. doi: 10.1038/nature11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giri R, Lam JK, Yu JQ. J Am Chem Soc. 2010;132:686–693. doi: 10.1021/ja9077705. [DOI] [PubMed] [Google Scholar]
- 15.Rauf W, Thompson AL, Brown JM. Dalton Trans. 2010;39:10414–10421. doi: 10.1039/c0dt00378f. [DOI] [PubMed] [Google Scholar]
- 16.Bedford RB, Haddow MF, Mitchell CJ, Webster RL. Angew Chem Int Ed. 2011;50:5524–5527. doi: 10.1002/anie.201101606. [DOI] [PubMed] [Google Scholar]
- 17.Rauf W, Thompson AL, Brown JM. Chem Comm. 2009;45:3874–3876. doi: 10.1039/b905717j. [DOI] [PubMed] [Google Scholar]
- 18.Yeung CS, Zhao X, Borduas N, Dong VM. Chem Sci. 2010;1:331–336. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.