Abstract
Ulcerative colitis (UC) is characterized by chronic inflammation of the colon. During inflammation, NF-κB is increased in colonic epithelial cells and in immune cells, leading to increases in proinflammatory cytokines. These events then increase DNA methyltransferases (DNMTs), which silence a subset of tumor suppressor genes by promoter methylation. Negative regulators of the Wnt pathway are frequently methylated in UC, leading to dysregulation of the pathway and, potentially, to colorectal cancer. We determined if black raspberries (BRBs) influence promoter methylation of suppressors in the Wnt pathway in dextran sodium sulfate (DSS)-induced UC. C57BL/6J mice received 1% DSS and were fed either control or 5% BRB diets. Mice were euthanized on days 7, 14 and 28, and their colons, spleen and bone marrow were collected. Berries reduced ulceration at day 28. This was accompanied by decreased staining of macrophages and neutrophils and decreased NF-κB p65 nuclear localization in the colon at all time points. At day 7, BRBs demethylated the promoter of dkk3, leading to its increased messenger RNA (mRNA) expression in colon, spleen and bone marrow. β-Catenin nuclear localization, c-Myc staining as well as protein expression of DNMT3B, histone deacetylases 1 and 2 (HDAC1 and HDAC2) and methyl-binding domain 2 (MBD2) were all decreased in colon; mRNA expression of these four proteins was decreased in bone marrow cells by BRBs. These results suggest that BRBs suppress colonic ulceration by correcting promoter hypermethylation of suppressor genes in the colon, as well as in the spleen and bone marrow that systematically regulate inflammation.
Summary: Our results suggest that dietary BRBs suppress colonic ulceration by correcting promoter hypermethylation of suppressor genes in the colon, as well as in the spleen and bone marrow that systematically regulate inflammation in DSS-induced UC.
Introduction
Ulcerative colitis (UC) is an inflammatory disease of the colon that affects as many as 750 000 Americans (1). Patients with UC are often plagued with abdominal pain, diarrhea and gastrointestinal bleeding. UC patients have a colorectal cancer incidence as high as 10% after 20 years of disease, and patients with long-standing colitis must be monitored for development of colorectal cancer. Therefore, preventing the progression of UC to colon cancer remains an important goal and chemoprevention is a promising approach to achieve this goal.
DNA methylation often occurs in the promoter regions of genes that regulate important cellular functions. A critical step in DNA methylation that can lead to gene inactivation is the overexpression of enzymes called DNA methyltransferases (DNMTs). These enzymes transfer methyl groups from S-adenosylmethionine to the 5 position of the cytosine ring in DNA (2). The extent of methylation within and/or around the promoter region of genes is negatively associated with their expression level (3,4). In addition to DNMTs, histone deacetylases (HDACs) (5) and the methyl-binding domain (MBD) (6) are linked with gene silencing. The roles of DNMTs, HDACs and MBD in regulating DNA methylation have been summarized in reviews (5,7). These enzymes work together to regulate DNA methylation and gene expression. The MBD proteins ‘read’ and ‘interpret’ the methylation moieties on DNA, and thus are critical mediators of many epigenetic processes, e.g. MBD binds to methylated CpGs and mediates repression through interactions with HDAC and DNMT proteins (8). All three of these enzymes/proteins are critical players in the regulation of gene expression.
Black raspberries (BRBs) are a natural food rich in protective antioxidant and anti-inflammatory compounds such as ellagic acid, the anthocyanins, vitamins A, C and E and folic acid, β-carotene, α-carotene, lutein, gallic acid, ferulic acid, p-coumaric acid and quercetin (9). Cancer prevention with freeze-dried BRBs or other berry types has been summarized in our reviews (10,11). Of relevance to the present study, BRB diets have been evaluated for chemopreventive activity in the colon. The initial study tested the ability of BRBs to prevent colon cancer in F344 rats induced by the carcinogen, azoxymethane (AOM) (12). AOM was administered at 15mg/kg body weight intraperitoneally once per week for 2 weeks. At 24h after the final AOM injection, rats were switched to treatment diets of 0, 2.5, 5 or 10% BRBs. Tumor multiplicity after 33 weeks was reduced significantly by 42, 45 and 71% in the 2.5, 5 and 10% BRBs + AOM groups, respectively, when compared with rats treated with AOM only. Recently, the anti-inflammatory effects of BRBs in UC were evaluated in mice (13). C57BL/6J mice were treated with water containing 3% dextran sodium sulfate (DSS) for 7 days and fed either a control diet or a diet containing 5 or 10% BRBs. Colons from one-half of the mice were collected after 7 days, and colons from the remaining mice were harvested after an additional 7 day treatment, without DSS, to determine if BRBs cause regression of UC. An acute loss in body mass, shorter colon length and colonic ulceration were observed in DSS-treated mice; BRB diets rescued DSS-induced colonic injury. In addition, BRB treatment suppressed messenger RNA (mRNA) expression of the proinflammatory cytokines, tumor necrosis factor-α and interleukin-1β (IL-1β) and protein expression of p-IκBα, cyclooxygenase-2 and plasma prostaglandin E2 (13).
Recent studies have established linkages between inflammation and DNA methylation. For example, during colonic inflammation in inflammatory bowel diseases such as UC, the level of NF-κB is increased in colonic epithelial cells and in immune cells, which leads to increases in levels of proinflammatory cytokines, e.g. IL-6 (14). Increased IL-6 can then increase levels of DNMTs, which then silence a subset of tumor suppressor genes by promoter methylation (14). Aberrant methylation of genes in the Wnt pathway has been linked to UC-induced neoplasia (15) and colorectal cancer (7). Recently, we reported that an average 4 weeks of dietary BRB administration (60 g/day) to colorectal cancer patients decreased promoter methylation of Wnt pathway negative regulators, e.g. SFRP2 and WIF1, in colorectal tumors from these patients (16).
The spleen is a site for storage and rapid deployment of monocytes; splenic monocytes have been identified as a resource that the body exploits to regulate inflammation (17). Similarly, bone marrow transplantation appears to be the only curative measure for UC in some patients, suggesting the importance of bone marrow in regulating inflammation (18). In view of these observations, the present study investigated whether the anti-inflammatory activities of BRBs are associated with their ability to modulate DNA methylation in the colon, spleen and bone marrow. Our results provide insight as to the mechanisms by which BRBs exert their anti-inflammatory activities during DSS-induced UC in mice.
Materials and methods
Preparation of BRB powder
The BRB powder used in this study was the same lot as the powder used in a previous DSS study conducted in the laboratory of D.R. (13). Conditions used to grow and harvest the berries and to prepare the powder, as well as to analyze 26 of its nutrient and non-nutrient components, have been detailed by Stoner (9) and Montrose et al. (13). Briefly, BRBs of the Jewel variety were grown on an Ohio farm in 2006, picked mechanically when ripe and washed and frozen at −20°C within 2–3h of the time of picking. They were freeze dried and ground into a powder at Van Drunen Farms (Momence, IL) and the powder was shipped frozen to the Ohio State University, where it was kept frozen until mixed into American Institute of Nutrition-76A synthetic diet at a concentration of 5%. Diets containing 5% BRB powder have been shown to inhibit carcinogen-induced tumors in the rat esophagus, colon and mammary gland and in the hamster cheek pouch (9). The starch in the diet was reduced by 5% to maintain an isocaloric diet. No residual pesticides, herbicides or fungicides were detected in the berry powder. BRBs contain four major polyphenolic anthocyanins that are responsible for the color of the berries. Their content in the powder was: cyanidin-3-glucoside [278.5mg/100g dry weight (DW)], cyanidin-3-sambubioside (56.0mg/100g DW), cyanidin-3-rutinoside (1790mg/100g DW) and cyanidin-3-xylosylrutinoside (853.5mg/100g DW), resulting in a total anthocyanin content of 2978mg/100g DW. We have shown previously that the anthocyanins in BRBs are chemopreventive in a rat model of esophageal cancer (19).
Animals
C57BL/6J male mice, aged 4–5 weeks, were purchased from The Jackson Laboratory (Bar Harbor, ME). They were acclimated to the animal facility for 1 week before use in the bioassay. Male mice were chosen for study because they are hardier than the females and have been used in previous studies (9,13). One group of 4- to 5-week-old C57BL/6J male mice (n = 9) were given drinking water and fed American Institute of Nutrition-76A diet ad libitum for 28 days (GP1, Figure 1A). Three mice in GP1 were euthanized on each of days 7, 14 and 28. Two groups of the same mice (n = 18 per group) were administered 1% DSS (MP Biomedical, Irvine, CA) in drinking water for 7 days and then switched to drinking water without DSS for 21 days. These mice were fed either American Institute of Nutrition-76A diet (GP2, Figure 1A) or a diet containing 5% BRBs (GP3, Figure 1A) for the entire 28 days. Six mice in GPs 2 and 3 were euthanized on each of days 7, 14 and 28 and their colons, spleen and bone marrow were collected. Colons were Swiss rolled, formalin fixed and paraffin embedded; cells from spleen and bone marrow were isolated as described below and stored at −80°C. All animal procedures were approved by the Ohio State University Animal Care and Use Committee, and mice were treated in accordance with institutional guidelines for animal care.
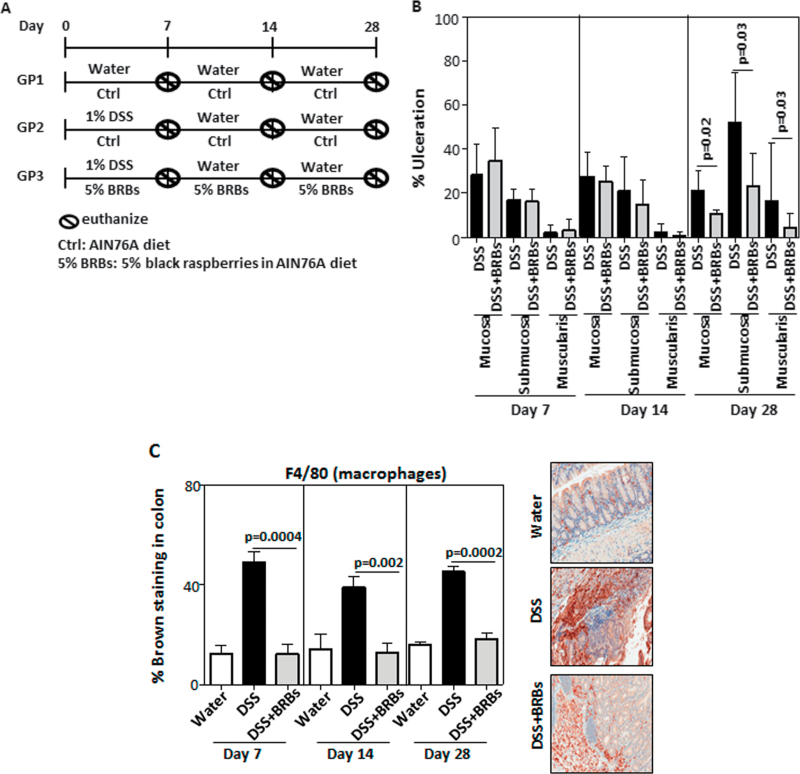
Fig. 1.
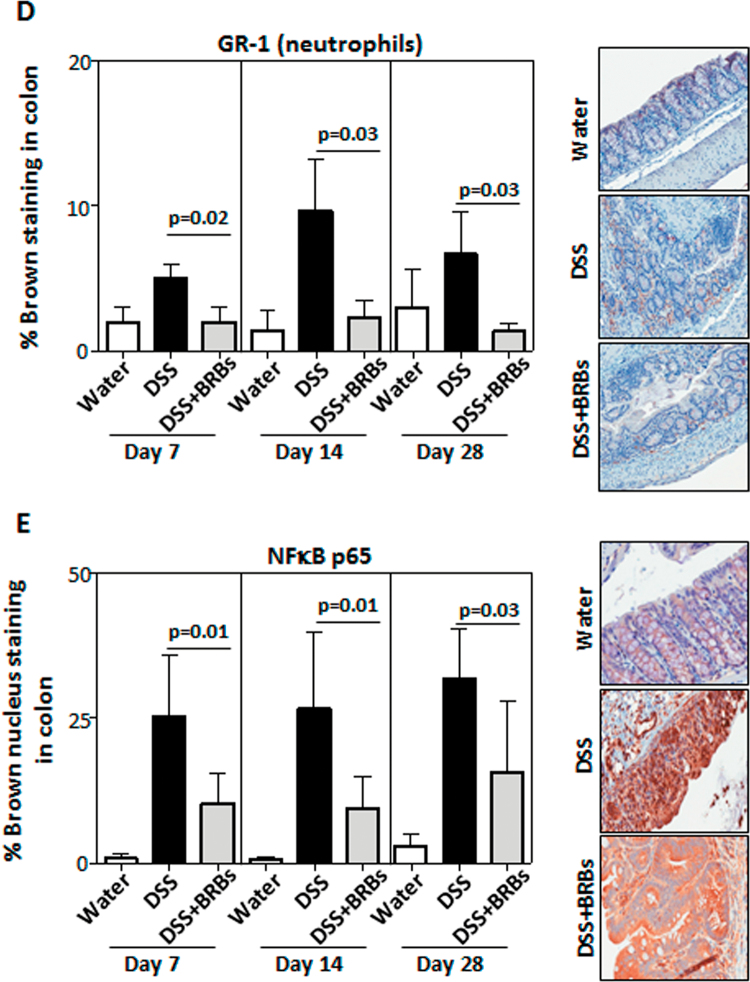
BRBs decrease DSS-induced colonic ulceration. (A) Study protocol. (B) Five percentage BRB dietary administration significantly decreased ulceration in mucosa, submucosa and muscularis in DSS-treated mice at day 28. There was no ulceration in GP1 (data not shown). Interestingly, berries significantly decreased staining of macrophages (C), neutrophils (D) and nuclear localization of NF-κB p65 (E), a proinflammatory biomarker, in UC tissue at days 7, 14 and 28. AIN 76A, American Institute of Nutrition-76A; ctrl, control.
Evaluation of colonic ulceration
Formalin-fixed and paraffin-embedded colons were stained by hematoxylin and eosin for colonic ulceration analysis. The entire colon was viewed under ×200 magnification (high power view). The percentage of ulceration was calculated as involved tissue in high power areas over total high power areas of the entire length of the colon. Areas of ulceration in the mucosa, submucosa and muscularis were counted separately. Colonic ulceration analysis was performed in a blinded manner by our pathologist, M.Y. At each time point, three mice from the water control group (GP1), six DSS-treated mice fed control diet (GP2) and six DSS-treated mice fed BRB diet (GP3) were used for analysis of colonic ulceration.
Isolations of splenocytes and bone marrow cells
Spleens were collected and kept in 5ml modified Eagle’s medium media (Cellgro, Corning, Manassas, VA) supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY) and 1% penicillin–streptomycin (Gibco). They were then minced using dissection scissors and forceps into a homogeneous paste that was passed through 40 µm cell strainers (BD Falcon, San Jose, CA) to obtain single-cell suspensions. Bone marrow from femur and tibia was flushed with 5ml of the above media using 27G needles. Then the 27G needles were changed to 18G, and the bone marrow-media mixture was passed through the 18G needles several times in order to make single-cell suspensions. The single-cell suspensions of spleens and bone marrow were then centrifuged at 1200rpm, and supernatant was discarded. Cell pellets were then suspended with 5ml cold 1× red blood cell lysis buffer and placed on ice for 5 min. Ten milliliters of 1× phosphate-buffered saline supplemented with 5% fetal bovine serum was then added to neutralize the red blood cell lysis buffer. The solution was then centrifuged at 1200rpm, and supernatant was discarded. Cell pellets were resuspended with 5ml 1× phosphate-buffered saline supplemented with 5% fetal bovine serum and centrifuged at 1200rpm. The supernatant was discarded, and splenocytes and bone marrow cells in pellets were subjected to DNA and RNA extraction.
Pyrosequencing
To determine methylation levels of apc, sfrp1, dkk2, dkk3 and sox17 genes in colon, spleen and bone marrow, a Pyrosequencing system (Qiagen, Valencia, CA) was used to detect methylated CpG sites in the promoter regions of the genes using the sequencing reactions described previously (20). Genomic DNA from colon, spleen and bone marrow was extracted using a DNA extraction kit (Qiagen) and 500ng of genomic DNA was treated with sodium bisulfite using the EZ DNA Methylation kit (Zymo Research, Orange, CA). Bisulfite-treated DNA was amplified with specific primers. Primer sequences within the promoter regions of the aforementioned five genes are given in Supplementary Table 1, available at Carcinogenesis Online. Average methylation levels of individual CpG sites for each DNA sample were calculated and results are presented as percent methylation. Three mice from GP1, six mice from GP2 and six mice from GP3 at day 7 were used for Pyrosequencing.
Real-time PCR analysis
Colons, spleen and bone marrow from three mice from GP1, six mice from GP2 and six mice from GP3 were collected on day 7. Paraffin-embedded mouse colon tissues were used for mRNA extraction using RecoverAll Total Nucleic Acid Isolation Kit for formalin fixed and paraffin embedded (Ambion, Grand Island, NY). Total RNA from cells collected from spleen or bone marrow was extracted using Trizol. Two micrograms of total RNA per sample was reverse transcribed using Superscript III RT (Invitrogen, Grand Island, NY). Quantitative PCR procedures and primer information are provided in Supplementary Table 2, available at Carcinogenesis Online.
Immunohistochemical staining and computer-assisted image analysis
Paraffin-embedded colons from three mice in GP1, six mice in GP2 and six mice in GP3 collected on days 7, 14 and 28 were cut into 4 µm sections and placed on slides. The slides were placed in a 60°C oven for 1 h, cooled, deparaffinized and rehydrated through xylene and graded ethanol solutions to water. All slides were treated for 5min with a 3% H2O2 solution in water to block endogenous peroxidase. Antigens were retrieved by placing the slides in a vegetable steamer in Dako Target Retrieval Solution for 25min, after which they were cooled for 15min. The slides were then placed on a Dako Autostainer for automated staining with primary antibodies to DNMT3B (dilution 1:200; Santa Cruz Biotechnology, Santa Cruz, CA), HDAC1 (dilution 1:250; Novus Biologicals, Littleton, CO), HDAC2 (dilution 1:100; Santa Cruz Biotechnology), MBD2 (dilution 1:1000; Novus Biologicals), NF-κB p65 (dilution 1:250; Cell Signaling Technology, Danvers, MA), F4/80 (dilution 1:150; Abcam, Cambridge, MA) to stain macrophages, GR-1 (dilution 1:50; Abcam) to stain neutrophils or c-Myc (dilution 1:50; Abcam) for 1 h at room temperature.
Stained tissues were viewed and photographed at ×200 magnification with a bright-field microscope mounted with a high-resolution spot camera. The camera was interfaced with a computer containing a matrix frame grabber board and image analysis software (Simple PCI Imaging Systems, Compix) as described before (19). We were assisted by M.Y. in the selection of stained areas within UC tissue for quantification of specific antigens. For all antigens, up to 30 high power (×200) regions of UC were analyzed and the staining intensities quantified.
Statistical analysis
Data from evaluation of colonic ulceration, pyrosequencing, real-time PCR, and immunohistochemistry was compared by Students t-test. All analyses were two sided, and a P value <0.05 was considered to be significant.
Results
The current study was designed to determine whether BRBs can prevent ulceration when given simultaneously with DSS and continuously administered in the recovery stage. The study protocol is depicted in Figure 1A. Colons were collected on days 7, 14 and 28 from mice given either drinking water and control diet (GP1), 1% DSS in the drinking water and control diet (GP2) or 1% DSS in drinking water and a 5% BRB diet (GP3). Analysis of colonic ulceration from these animals is shown in Figure 1B. There was no ulceration in colons from GP1 mice at all time points (data not shown). The 5% BRB diet significantly decreased DSS-induced ulceration in the mucosa, submucosa and muscularis on day 28 but not on days 7 or 14 (Figure 1B). Both macrophages and neutrophils have been shown to contribute to inflammation in DSS-induced colitis (21,22). DSS exposure increased staining of macrophages (Figure 1C) and neutrophils (Figure 1D) in the colon and BRBs decreased the staining of macrophages and neutrophils at days 7, 14 and 28. Interestingly, early at day 7, the berry diet significantly inhibited the nuclear localization of NF-κB p65, a potent proinflammatory transcription factor, and the suppression continued through days 14 and 28 (Figure 1E).
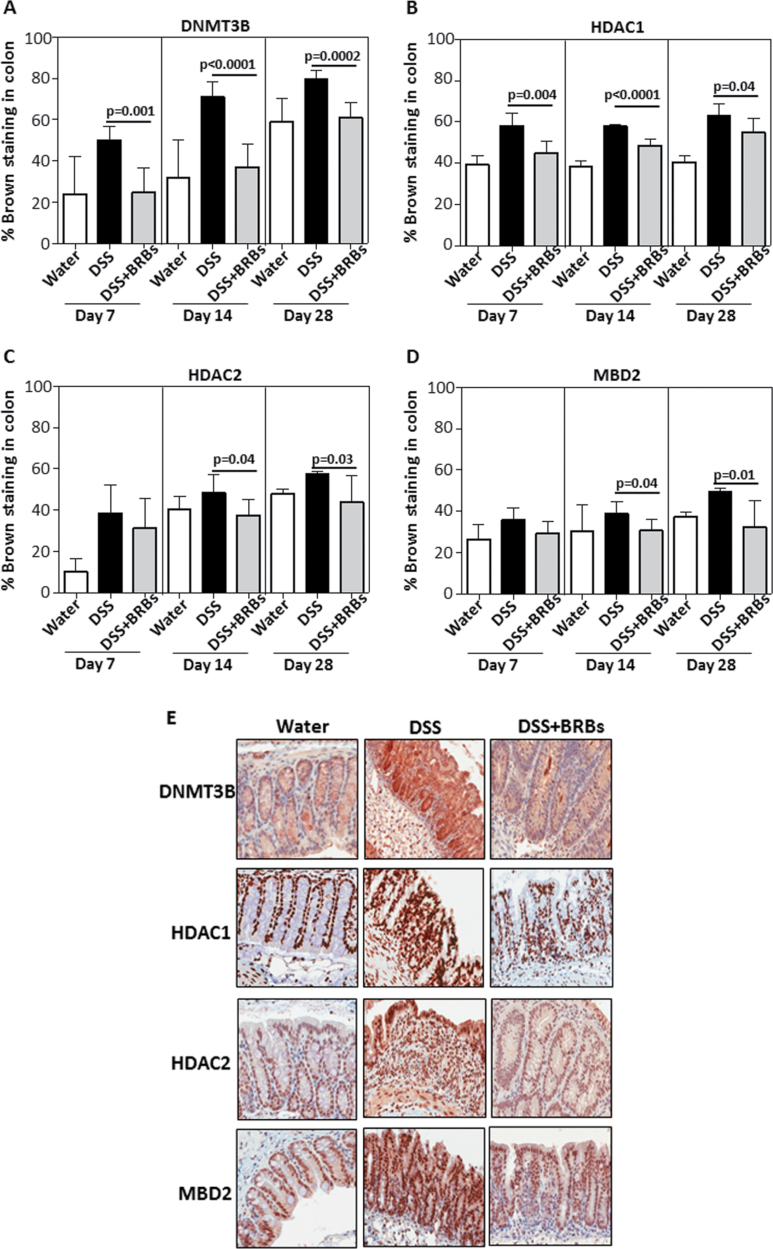
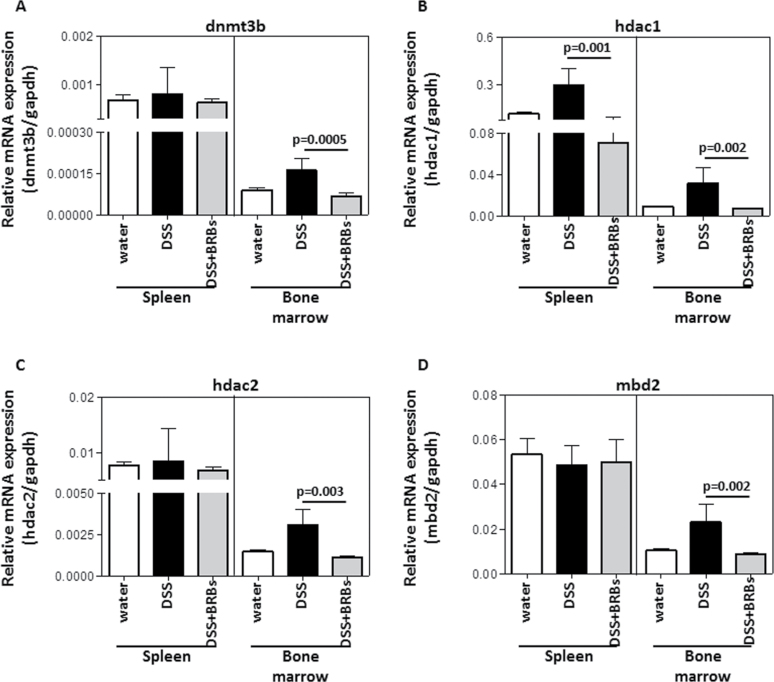
Protein expression of DNMT3B, HDAC1, HDAC2 and MBD2 in UC tissues of the colon from DSS-treated mice was evaluated by quantitative immunohistochemistry. As early as day 7, the berry diet exerted its protective effects on these enzymes. For example, the protein expressions of DNMT3B (Figure 2A) and HDAC1 (Figure 2B) were decreased by BRBs on days 7, 14 and 28, and those of HDAC2 (Figure 2C) and MBD2 (Figure 2D) were decreased on days 14 and 28. In addition, only hdac1 mRNA expression was significantly decreased by BRBs in spleen; mRNA expression of dnmt3B, hdac1, hdac2 and mbd2 was significantly decreased by BRBs in bone marrow (Figure 3). These results suggest that BRBs regulate methylation in spleen mainly through hdac1.
Fig. 2.
BRBs suppress enzymes/proteins regulating DNA methylation. At days 7, 14 and 28, BRBs significantly decreased protein expression of DNMT3B (A) and HDAC1 (B) in DSS-induced ulcerative colon. HDAC2 (C) and MBD2 (D) were significantly decreased by BRBs at days 14 and 28. (E) Representative staining of DNMT3B, HDAC1, HDAC2 and MBD2 in normal colon from water control mice fed control diet and ulcerative colon from mice treated with DSS and fed control or BRB diets.
Fig. 3.
Effects of BRBs on mRNA expression of dnmt3b, hdac1, hdac2 and mbd2 in cells from spleen and bone marrow. Interestingly, BRBs decreased mRNA expression of dnmt3b (A), hdac1 (B), hdac2 (C) and mbd2 (D) in bone marrow from DSS-treated mice. BRBs only decreased hdac1 mRNA in spleen.
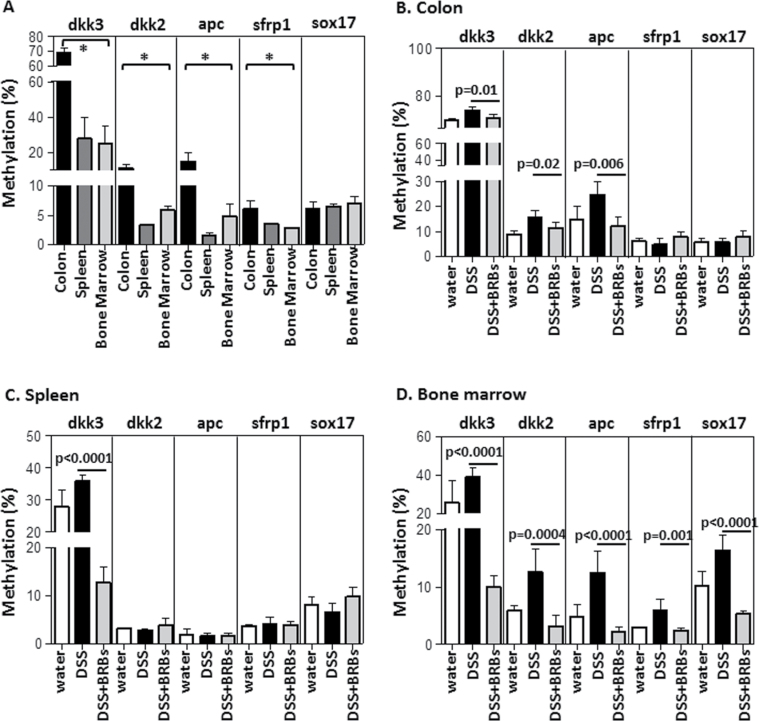
Promoter DNA methylation of the Wnt pathway tumor suppressor genes was measured by Pyrosequencing at day 7 to coincide with the observed reductions in NF-κB p65 (Figure 1E) and DNMT3B (Figure 2A) protein expression at this time point. In mice given plain drinking water and fed control diet (GP1 in Figure 1A), promoter methylation of dkk3, dkk2, apc and sfrp1 was significantly higher in the colon than in spleen or bone marrow (Figure 4A). Moreover, the methylation level of these genes varied from one gene to another. For example, dkk3 was methylated to a greater extent than the other four genes in colon, spleen and bone marrow (Figure 4A). DSS (1%) treatment significantly increased methylation of (i) dkk3, dkk2 and apc in mouse colon (Figure 4B); (ii) dkk3 in the spleen (Figure 4C) and (iii) dkk3, dkk2, apc, sfrp1 and sox17 in the bone marrow (Figure 4D). BRBs decreased the methylation level of all genes in the colon, spleen and bone marrow whose methylation was increased by DSS (Figure 4B–D).
Fig. 4.
BRBs decreased promoter methylation of tumor suppressor genes in the Wnt pathway. (A) Promoter methylation of dkk3, dkk2, apc and sfrp1 was significantly higher in colon than in spleen and bone marrow in mice given plain drinking water and fed control diet (GP1) at day 7. BRBs differentially demethylated dkk3, dkk2, apc, sfrp1 and sox17 in colon (B), spleen (C) and bone marrow (D) from DSS-treated mice at day 7. *P < 0.05 for (A) indicates that the methylation levels of genes in the colon are significantly higher than those in spleen and bone marrow.
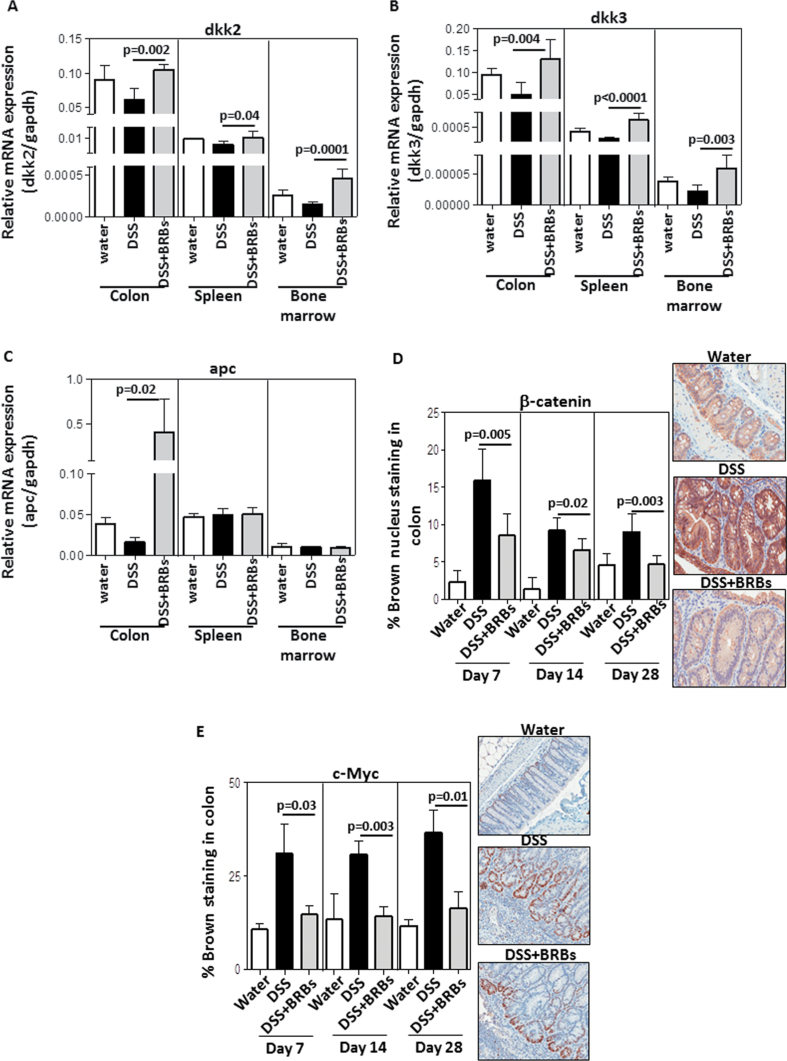
mRNA expression of dkk2 (Figure 5A) and dkk3 (Figure 5B) was increased by BRBs in the colon, spleen and bone marrow from DSS-treated mice; mRNA expression of apc (Figure 5C) was increased only in colon by BRBs. mRNA expression of sfrp1 and sox17 was not significantly altered by berry treatment (data not shown). β-Catenin nuclear localization was increased by DSS and decreased by dietary BRBs in the ulcerative region of the colon (Figure 5D). c-Myc is one of the well-known downstream genes in the Wnt pathway. c-Myc immunohistochemistry in colon was performed and the data are presented in Figure 5E. Our data show that similar to β-catenin, c-Myc protein expression was decreased by BRBs in DSS-treated mice. Therefore, demethylation of Wnt negative regulators by BRBs may alter the functional parameters such as β-catenin and c-Myc.
Fig. 5.
BRBs protectively modulate expression of Wnt pathway genes. BRBs significantly increased mRNA expression of dkk2 (A) and dkk3 (B) in colon, spleen and bone marrow at day 7; apc mRNA expression (C) was only increased in colon from DSS-treated mice at day 7. β-Catenin nuclear localization (D) and c-Myc protein expression (E) were decreased by BRBs in ulcerative colon at days 7, 14 and 28.
Discussion
The data from this study clearly demonstrate that BRBs possess strong anti-inflammatory activities in DSS-induced colonic injury in mice and confirm our previous results (13). Our previous results showed that BRB treatment suppressed mRNA expression of the proinflammatory cytokines, tumor necrosis factor-α and IL-1β and protein expression of p-IκBα, cyclooxygenase-2 and plasma prostaglandin E2 in DSS-treated mice (13). Data from the present study indicate that other mechanism(s) by which berries exert their anti-inflammatory effects is through decreasing NF-κB p65 expression leading to decrease of DNMT3B expression, which in turn reverse aberrant DNA methylation of tumor suppressor genes, e.g. dkk2, dkk3, in the Wnt pathway, resulting in their enhanced mRNA expression locally in colon and systematically in spleen and bone marrow. Based upon our published data and the results of this study, it seems likely that BRBs might prevent DSS-induced colon tumorigenesis in mice and a study is ongoing to evaluate this possibility.
In addition, we have shown that a 5% BRB diet reduces DSS-induced damage in the colonic mucosa of mice, resulting in a lesser inflammatory response in the submucosa and muscularis mucosa at day 28 but not at days 7 and 14 (Figure 1B). However, the berries inhibited nuclear localization of the transcription activator, NF-κB p65, at all three time points (Figure 1E). As one might expect, these results suggest that molecular changes occur antecedent to pathological changes. The suppressing effects of BRBs on proinflammatory biomarker(s) at early time points likely contributed to the decreased colonic ulceration at later time points.
The damaging effects of DSS have been shown to occur in part as a result of the increased production of free radicals causing oxidative damage, which then activates NF-κB within the colonic mucosa (23,24). Although we previously reported that BRBs do not affect reactive nitrogen species production in DSS-treated mice (13), results from our current study suggest that BRBs are able to inhibit NF-κB, leading to aforementioned correction of aberrant DNA methylation.
Oxidative damage in Bacteroides fragilis-induced colitis can induce recruitment of members of a gene silencing complex, Sirtuin-1 (SIRT1), polycomb members and DNMTs, which inactivate suppressor genes through promoter methylation (25). The silencing protein complex is enriched in tumor suppressor genes including sfrp5 and sox17, Wnt pathway regulators. In particular, sox17 has been found to be methylated and is a polycomb-targeted gene in glutathione peroxidase, Gpx1 and Gpx2, double knockout mice, a model of inflammatory bowel diseases (26).
Is there cancer-specific and/or inflammation-induced aberrant DNA methylation and transcriptional silencing? O’Hagan et al. (25) reported that when human colon cancer SW480 cells were treated with H2O2 to induce oxidative damage, aforementioned complexes containing DNMTs, SIRT1 and polycomb members are recruited to damaged chromatin. Further, H2O2 treatment causes relocalization of the complexes from non-GC-rich to GC-rich areas. In the same study, the authors showed that promoter CpG islands in high-expression oncogenes, e.g. MYC, do not become hypermethylated nor are they methylated in cancer (25). However, promoter methylation of SFRP4 and SFRP5, Wnt pathway negative regulators, is increased and they have low basal expression (25). The authors thereby suggest that cancer-specific promoter DNA methylation mostly targets genes that have low basal expression (25).
Reversal of DNA methylation can occur through either a passive or active mechanism. Passive, or replicative-dependent, demethylation is characterized by an inhibition of known DNA methylating enzymes, e.g. DNMT1 or DNMT3b. Because these enzymes are inactivated, over the course of replication cycles, new DNA from each daughter cell remains unmethylated (27). Conversely, active, or replicative-independent, demethylation is characterized by the demethylation of DNA that is already methylated (27). This can occur by upregulation of demethylating enzymes, however this mechanism is less understood (28,29). We previously reported that dietary intervention of BRBs to colorectal cancer patients resulted in decreased DNA methylation in colorectal tumor tissue, specifically in Wnt inhibitor genes (16). The mechanism for this was through passive demethylation via inhibition of the DNA methylating enzymes, DNMT1 and DNMT3b (16,30). In the current study in DSS-treated mice, BRBs are shown again to prevent Wnt inhibitor methylation through passive demethylation by inhibiting expression of DNMT3b.
The importance of the spleen and bone marrow in the development of colonic inflammation is now appreciated (17,31). The bone marrow produces and contains numerous (pro)monocytes (31), and the spleen is a reservoir of extramedullary monocytes that contribute to the rapid onset of inflammation (17). Several lines of evidence suggest that bone marrow cells are critical in maintaining homeostasis of the colonic microenvironment. Bone marrow cell transplantation can attenuate mucosal inflammation through an immune reset with the generation of new self-tolerant lymphocytes (32). In addition, bone marrow cell transplantation inhibits T-cell activation, reduces levels of proinflammatory cytokines and upregulates IL-10 expression (33). All these events dampen immune responses. Further, transplanted bone marrow cells can be incorporated into the epithelial compartment and induce chronic injury in DSS-induced colitis (32). We showed that 5% BRBs in the diet reduced the number of CD4+ and CD8+ T cells in the spleen of IL-10 knockout mice, another mouse model of UC (34), which may have also happened in this study. In the current study, berries demethylated promoter CpGs of tumor suppressor genes and increased their expression in the spleen and bone marrow, which might contribute to their ability to dampen proinflammatory activities in these tissues through the mechanisms described above.
In summary, the present study provides one of anti-inflammatory mechanisms that BRBs exerts in DSS-induced UC in mice. BRBs decrease NF-κB p65 protein expression, leading to decreased DNMT3B, thereby decreased promoter methylation of tumor suppressor genes in the Wnt pathway and decreased translocation of β-catenin to the nucleus prohibiting the activation of the pathway. However, we do not know if berries selectively demethylate certain tumor suppressor genes in the Wnt pathway and if berries also demethylate tumor suppressor genes involved in controlling other cellular functions and/or pathways, e.g. DNA repair. Future studies in an attempt to investigate and answer these questions will allow us to better define the anti-inflammatory and anti-colitis-associated carcinogenic mechanisms of berries. Ultimately, these findings may in turn support the inclusion of berries in the diet for the prevention of UC in patients.
Supplementary material
Supplementary Tables 1 and 2 can be found at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health, National Cancer Institute (R01 CA148818).
Conflict of Interest Statement: None declared.
Supplementary Material
Glossary
Abbreviations:
- AOM
azoxymethane
- BRBs
black raspberries
- DNMT
DNA methyltransferase
- DSS
dextran sodium sulfate
- DW
dry weight
- HDAC
histone deacetylase
- IL
interleukin
- MBD
methyl-binding domain
- mRNA
messenger RNA
- UC
ulcerative colitis.
References
- 1. Loftus E.V., Jr, et al. (2002). Epidemiology of inflammatory bowel disease. Gastroenterol. Clin. North Am., 31, 1–20 [DOI] [PubMed] [Google Scholar]
- 2. Gal-Yam E.N., et al. (2008). Cancer epigenetics: modifications, screening, and therapy. Annu. Rev. Med., 59, 267–280 [DOI] [PubMed] [Google Scholar]
- 3. Ushijima T. (2005). Detection and interpretation of altered methylation patterns in cancer cells. Nat. Rev. Cancer, 5, 223–231 [DOI] [PubMed] [Google Scholar]
- 4. Herman J.G., et al. (2003). Gene silencing in cancer in association with promoter hypermethylation. N. Engl. J. Med., 349, 2042–2054 [DOI] [PubMed] [Google Scholar]
- 5. Baylin S.B. (2002). Mechanisms underlying epigenetically mediated gene silencing in cancer. Semin. Cancer Biol., 12, 331–337 [DOI] [PubMed] [Google Scholar]
- 6. Lopez-Serra L., et al. (2006). A profile of methyl-CpG binding domain protein occupancy of hypermethylated promoter CpG islands of tumor suppressor genes in human cancer. Cancer Res., 66, 8342–8346 [DOI] [PubMed] [Google Scholar]
- 7. Baylin S.B., et al. (2006). Epigenetic gene silencing in cancer - a mechanism for early oncogenic pathway addiction? Nat. Rev. Cancer, 6, 107–116 [DOI] [PubMed] [Google Scholar]
- 8. Sansom O.J., et al. (2007). Mechanisms of disease: methyl-binding domain proteins as potential therapeutic targets in cancer. Nat. Clin. Pract. Oncol., 4, 305–315 [DOI] [PubMed] [Google Scholar]
- 9. Stoner G.D. (2009). Foodstuffs for preventing cancer: the preclinical and clinical development of berries. Cancer Prev. Res. (Phila)., 2, 187–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang L.S., et al. (2008). Anthocyanins and their role in cancer prevention. Cancer Lett., 269, 281–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stoner G.D., et al. (2007). Cancer prevention with freeze-dried berries and berry components. Semin. Cancer Biol., 17, 403–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harris G.K., et al. (2001). Effects of lyophilized black raspberries on azoxymethane-induced colon cancer and 8-hydroxy-2′-deoxyguanosine levels in the Fischer 344 rat. Nutr. Cancer, 40, 125–133 [DOI] [PubMed] [Google Scholar]
- 13. Montrose D.C., et al. (2011). Anti-inflammatory effects of freeze-dried black raspberry powder in ulcerative colitis. Carcinogenesis, 32, 343–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hartnett L., et al. (2012). Inflammation, DNA methylation and colitis-associated cancer. Carcinogenesis, 33, 723–731 [DOI] [PubMed] [Google Scholar]
- 15. Dhir M., et al. (2008). Epigenetic regulation of WNT signaling pathway genes in inflammatory bowel disease (IBD) associated neoplasia. J. Gastrointest. Surg., 12, 1745–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang L.S., et al. (2011). Modulation of genetic and epigenetic biomarkers of colorectal cancer in humans by black raspberries: a phase I pilot study. Clin. Cancer Res., 17, 598–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Swirski F.K., et al. (2009). Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science, 325, 612–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Actis G.C., et al. (2011). Inflammatory bowel disease: beyond the boundaries of the bowel. Expert Rev. Gastroenterol. Hepatol., 5, 401–410 [DOI] [PubMed] [Google Scholar]
- 19. Wang L.S., et al. (2009). Anthocyanins in black raspberries prevent esophageal tumors in rats. Cancer Prev. Res. (Phila)., 2, 84–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zuo T., et al. (2011). Epigenetic silencing mediated through activated PI3K/AKT signaling in breast cancer. Cancer Res., 71, 1752–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Waddell A., et al. (2013). Intestinal CCL11 and eosinophilic inflammation is regulated by myeloid cell-specific RelA/p65 in mice. J. Immunol., 190, 4773–4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koelink P.J., et al. (2013). Collagen degradation and neutrophilic infiltration: a vicious circle in inflammatory bowel disease. Gut. PMID: 23525573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oz H.S., et al. (2005). Antioxidants as novel therapy in a murine model of colitis. J. Nutr. Biochem., 16, 297–304 [DOI] [PubMed] [Google Scholar]
- 24. Bhattacharyya S., et al. (2009). ROS, Hsp27, and IKKβ mediate dextran sodium sulfate (DSS) activation of IκBα, NFκB, and IL-8. Inflamm. Bowel Dis., 15, 673–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O’Hagan H.M., et al. (2011). Oxidative damage targets complexes containing DNA methyltransferases, SIRT1, and polycomb members to promoter CpG Islands. Cancer Cell, 20, 606–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hahn M.A., et al. (2008). Methylation of polycomb target genes in intestinal cancer is mediated by inflammation. Cancer Res., 68, 10280–10289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen Z.X., et al. (2011). DNA methylation and demethylation in mammals. J. Biol. Chem., 286, 18347–18353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mayer W., et al. (2000). Demethylation of the zygotic paternal genome. Nature, 403, 501–502 [DOI] [PubMed] [Google Scholar]
- 29. Oswald J., et al. (2000). Active demethylation of the paternal genome in the mouse zygote. Curr. Biol., 10, 475–478 [DOI] [PubMed] [Google Scholar]
- 30. Wang L.S., et al. (2013). Black raspberry-derived anthocyanins demethylate tumor suppressor genes through the inhibition of DNMT1 and DNMT3B in colon cancer cells. Nutr. Cancer, 65, 118–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Furth R., et al. (1968). The origin and kinetics of mononuclear phagocytes. J. Exp. Med., 128, 415–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alison M.R., et al. (2012). The ailing gut: a therapeutic role for bone marrow cells? Transplantation, 93, 565–571 [DOI] [PubMed] [Google Scholar]
- 33. Tanaka F., et al. (2008). Exogenous administration of mesenchymal stem cells ameliorates dextran sulfate sodium-induced colitis via anti-inflammatory action in damaged tissue in rats. Life Sci., 83, 771–779 [DOI] [PubMed] [Google Scholar]
- 34. Kuo C.T., et al. (2010). Effects of dietary black raspberries on DNA methylation in ulcerative colitis-induced carcinogenesis in IL-10 knock-out mice. Inflamm. Bowel Dis., 17, S15 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.