Abstract
End-stage heart failure is the final common pathway of an irreversible process associated with loss of myocardial cells. In this process, the capacity for renewal and repair of myocardial tissue is inadequate and ultimately leads to ventricular remodeling. Novel therapeutic strategies have been developed to prevent it, one being cell therapy, which has emerged as a potential approach to directly repopulate and repair the damaged heart. Here, we review the use of regenerative cell therapy for different cardiac diseases and discuss the positive effect of cell therapy mediated by paracrine factors instead of turning directly into cardiomyocytes.
Keywords: heart failure, cardiac stem cell therapy, bone marrow-derived cell therapy, dilated cardiomyopathy

Introduction
Cardiovascular disease is a major public health problem that imposes a huge economic burden on health systems around the world, and patients with end-stage heart failure (HF) represent a large share of the healthcare spending.1-3
End-stage HF is the final common pathway of a process of myocardial cell death triggered by varied etiologies and characterized by myocardial dysfunction and inadequate remodeling. It is a complex and heterogeneous entity with multiple etiologies, from cardiomyopathy (CM) of ischemic origin that can improve with restoration of myocardial perfusion to other infectious, inflammatory or infiltrative processes that are less responsive to current medical treatments. Among these, nonischemic dilated CM represents one-third of all patients with HF and is more prevalent in younger patients, with an annual mortality ranging from 10% to 50%.4-8
Despite this, several treatment options such as standard pharmaceuticals, ventricular assist devices, cardiac resynchronization therapy, and cardiac transplantation have remained unchanged for several years.9-12 Although cardiac transplantation has been shown to improve outcomes in end-stage HF, the procedure comes with inherent risks.13-15
It is well known that the heart has no intrinsic muscular regeneration capacity, so regenerative medicine techniques to restore cardiac function are being increasingly investigated as potential options to treat cardiovascular disease. Among these techniques are bone marrow-derived cell (BMC) therapies.16-17 The following provides a brief review of information available on the safety of regenerative cell therapy for different cardiovascular diseases.
Cellular Cardiac Regenerative Therapies
There is a growing understanding of the anatomical and functional disorders that occur in the myocardial cell in dilated CM, such as endothelial dysfunction, impaired microvascular function (diffuse in the case of nonischemic etiology), inappropriate remodeling, increased intracardiac pressures, and progressive deterioration of ventricular function.18-19
Cell-based therapies have rapidly emerged as a potential novel therapeutic approach that attempts to regenerate cardiac myocyte contractility, improve diffuse microvascular dysfunction, and reverse ventricular structural changes such as dilation and fibrosis. In order to reverse or mitigate this cascade of events that adversely affects ventricular function, multiple therapies have been tested, including different cell strains and routes of administration (Figure 1).
Figure 1.
Source of cells and their delivery routes for the treatment of heart disease. (1) Intracoronary infusion; (2) Transendocardial; (3) Epicardial intramyocardial injection.
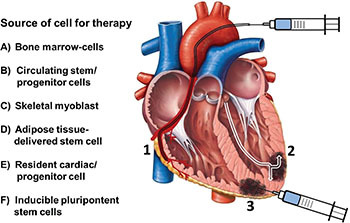
Several mechanisms have been described that may explain the effect of cell therapies, such as attenuation of cardiomyocyte and endothelial cell apoptosis, paracrine anti-inflammatory effects, promotion of angiogenesis and activation of progenitor cells in situ, increased vascularity, improved endothelial dysfunction, and decreased myocardial fibrosis.20-22 However it has also been observed that some factors may adversely affect these effects: the quantity of infused cells, cell viability, the zone of infusion, the delivery route, and, especially, the nesting rate in the myocardial tissue. In this regard, intracoronary infusion has proved to be the most practical, safe, and effective technique to elicit an adequate rate of cell nesting.23-24 Even so, when used for ischemic heart disease, this procedure has shown conflicting results regarding efficacy and safety. Moreover, stem and progenitor cell-based therapies have been applied at different stages of disease, as in the acute phase of myocardial infarction (MI) or after remote MI with chronic ischemic CM and, more sparsely, for patients with nonischemic dilated CM.25
Acute Myocardial Infarction
Acute MI has been the most studied clinical context in which to assess the safety and efficacy of cell therapies; this is based on the principle that the window of time during an acute ischemic insult is the most appropriate opportunity to prevent the death of cardiomyocytes and, therefore, subsequent remodeling (Table 1). Bone marrow cells (BMCs) are the most common cells used for therapy. They are injected into the infarcted vessel after it has been reopened by balloon dilation and stent placement, making this therapy only available to revascularized areas. In this context, it has been demonstrated that after intracoronary infusion, cardiac homing of BMCs increased in patients with an acute MI compared with chronic MI. This effect is probably due to the increased amount of chemoattractant factors secreted from the ischemic tissue and to the potential of BMCs to promote cardiac neovascularization and attenuate ischemic injury.
Table 1.
Prospective randomized trials of stem cell therapy in acute myocardial infarction.
| Study | No. of Patients | LVEF (%) Baseline | Follow-up (Months) | Cell Type | Delivery Route | LVEF Increase (%) |
| ASTAMI (2006) | 100 | 46 | 6 | BMC | IC | No effect |
| FINCELL (2008) | 80 | 58.8 | 6 | BMC | IC | 7.1 |
| REGENT (2009) | 200 | 36 | 6 | BM-MNC / CD 34+ / CXCR4+ |
IC | No effect |
| BOOST (2009) | 60 | 51 | 6 | BMC | IC | 6.7 |
| BONAMI (2010) | 101 | 36.3 | 3 | BMC | IC | No Effect |
| REPAIR-AMI (2010) | 204 | 45.4 | 24 | BMC | IC | 4.7 |
| HEBE (2011) | 200 | 38.6 | 4 | BMC | IC | No Effect |
| TOPCARE (2011) | 59 | 46 | 60 | CPC / BMC | IC | 11 |
| Late Time (2011) | 87 | 48.7 | 6 | BM-MNC | IC | No Effect |
LVEF: left ventricular ejection fraction; BMC: bone marrow-derived cells; BM-MNC: bone marrow-derived unselected mononuclear cells; CPC: circulating progenitor cells; IC: intracoronary.
Other cell lineages have been tested recently, such as the autologous subtypes of tissue-resident cardiac stem and progenitor cells called cardiosphere-derived cells.26 A phase 1 study reported a reduction in myocardial scar mass and increased viability mass but with no effect on left ventricular ejection fraction (LVEF) at 6 months.27-29 A recent meta-analysis by Delewi et al.30 revealed that intracoronary BMC treatment leads to a moderate improvement in LVEF and a reduction of left ventricular end-systolic volume (LVESV) at 6 months that sustained at 12 months follow-up, without a clear significant effect on left ventricular end-diastolic volume (LVEDV) or infarct size. The authors also found that intracoronary cell therapy was significantly associated with reductions in recurrent acute MI and readmission for HF, unstable angina, or chest pain.
Chronic Ischemic Heart Disease with Myocardial Dysfunction
Patients with chronic ischemic left ventricular dysfunction may have a substantial amount of viable hibernating myocardium, which is detected by multiple methods such as cardiac magnetic resonance; therefore, coronary revascularization in these patients may result in an improvement of left ventricular function (Table 2). Moreover, the effect of the addition of BMCs by intracoronary or intramyocardial injection on these results has been tested in a few studies.31-33 Zhang et al.34 performed a meta-analysis of 11 clinical trials that evaluated the efficacy of autologous BMC transfer in 490 total patients with chronic ischemic heart disease. Compared with controls, BMC-treated patients significantly improved LVEF by 4.63% and showed a significant reduction in LVEDV and LVESV. In addition, BMC treatment was associated with a significant positive effect on survival. The authors suggest that in this subgroup of patients, BMC transfer seems to have a positive impact on myocardial remodeling, unlike patients treated in the acute phase, or within 1 week, of MI.
Table 2.
Prospective randomized trials of stem cell therapy in ischemic heart failure.
| Study | No. of Patients | LVEF (%) Baseline | Follow-up (Months) | Cell Type | Delivery Route | LVEF Increase (%) |
| REPAIR-AMI (2006) | 204 | 48 | 12 | BMC | IC | 2.5 |
| TOPCARE-CHD (2006) | 121 | 40 | 12 | BMC | IC | 1.8 |
| STAR-heart (2010) | 391 | 33 | 24 | BMC | IC | 6.2 |
| FocusHF (2011) | 30 | 37 | 6 | BM-MNC | IM | No Effect |
| SCIPO (2011) | 14 | 30 | 4 | CDC | IC | 8.2 |
| CADUCEUS (2012) | 25 | 39 | 6 | CDC | IC | No Effect |
| Sürder et all.28 (2013) | 200 | 37.4 | 4 | BM-MNC | IC | No Effect |
LVEF: left ventricular ejection fraction; BMC: bone marrow-derived cells; BM-MNC: bone marrow-derived unselected mononuclear cells; CDC: cardiosphere-derived cells; IC: intracoronary; IM: intramyocardial.
Strauer et al.35-36 have recently reported long-term follow-up data on the intracoronary application of BMC in patients with chronic HF due to ischemic CM (LVEF <35%) from the nonrandomized STAR study. Throughout a 5-year follow-up, the authors reported improved LVEF, quality of life, and survival in patients with HF who received BMC (191 patients with mean NYHA class 3.22) compared to the control group (200 patients) with a similar LVEF.
Nonischemic Dilated Cardiomyopathy
There is little evidence of the potential benefit of cell therapies in nonischemic etiologies, as some patients exhibit nonhomogeneous tissue perfusion on nuclear imaging, which is the basis of target-area selection for stem cell administration. The studies performed have shown that BMC administration attenuates the effects of circulating autoantibodies, which are thought to be involved in the pathogenesis of nonischemic dilated CM (Table 3). In the study by Vrtovec et al.,37 55 patients were randomized to intracoronary infusion transplant of CD34 + progenitor cells or placebo. At 1 year, cell therapy resulted in significant improvement in LVEF (25.5%±7.5% to 30.1%±6.7%, P=.03), an increase in the 6-minutes walk distance ( 359±104 m to 485±127 m, P=0.001 ), and a decrease of NT-proBNP levels (2069±1996 pg/mL to 1037±950 pg/mL, P=0.01); cell therapy was the only independent prognostic factor to remain free of death or cardiac transplantation (2/28, 7% to 8/27, 30%, P=.03). The 5-year follow-up, in addition to demonstrating the middle-term safety of the procedure, also showed a persistent improvement in LVEF and exercise capacity, maintaining the benefit of reduced mortality from HF.38
Table 3.
Prospective randomized trials of stem cell therapy in nonischemic heart failure.
| Study | No. of Patients | LVEF (%) Baseline | Follow-up (Months) | Cell Type | Delivery Route | LVEF Increase (%) |
| Bocchi et al.49 (2008) | 22 | 21 | 15 | BMC | IC | 8.8 |
| Fischer-Rasokat et al.40 (2009) | 33 | 30 | 3 | BMC | IC | 3.4 |
| Seth et al.39 (2010) | 85 | 23 | 36 | BMC | IC | 5.9 |
| Vrtovec et al.37 (2011) | 55 | 26 | 12 | Autologous CD 34+ |
IC | 4.6 |
| Vrtovec et al.38 (2013) | 55 | 24 | 60 | Autologous CD 34+ |
IC | 5.6 |
LVEF: left ventricular ejection fraction; BMC: bone marrow cells; IC: intracoronary.
Seth et al.39 analyzed a cohort of 44 patients with nonischemic HF, comparing 20 controls to 24 who were randomized to cell therapy using intracoronary infusion of bone marrow-derived mononuclear cells. There was a significant improvement in NYHA functional class in the treatment group, with 16 patients (62%) who improved by at least one degree of functional class. In addition, ejection fraction improved by 5.4% (20±7.4% to 25±12%, P <0.05) with no change in left ventricular end-diastolic volume. The 3-year follow-up showed persistent improvement in LVEF, mainly by decreases in left ventricular end-systolic volume without changes in end-diastolic volume. It also showed an improvement in functional class (although less pronounced in NYHA class IV) and improvement in quality of life, although it did not demonstrate improved survival.39
Fischer et al.40 performed intracoronary infusion of bone marrow-derived cells on 33 patients with dilated nonischemic cardiomyopathy and analyzed hemodynamics and cardiac function by Doppler at 3 months. There was an improvement in global and segmental contractility, with a significant increase in LVEF (30.2%±10.9% to 33.4%±11.5%, P=.001). Dynamics showed a lower coronary vascular resistance index unchanged in the reference vessel diameter, which could result in improved micro- and macrovascular endothelial function; they also showed a significant decrease at 1 year in NT-proBNP levels (1610±993 to 1473±1147 pg/mL, P=0.038), a known neuroimmunomodulator with well-established prognostic implications in patients with HF.40
Inflammatory Paracrine Response to Stem Cell Therapy
Several studies have focused on the ability of stem cells to improve or regenerate myocardium by injecting cell suspensions containing either mixed or purified cellular population into the heart. Despite the apparent benefit of this experimental procedure, the mechanisms remain controversial and unclear, leaving large gaps in the understanding of the actual outcome of stem cell therapies and its future implications in the field of medicine. Few reports have focused on the immunologic aspects of the inflammatory paracrine response to stem cell therapy that might lead to improved cardiac function, cell proliferation, angiogenesis, or vasculogenesis by secreted chemical mediators via inflammatory cell infiltration and immunologic reactions.
Preclinical models have confirmed the main role of paracrine effects as part of stem cell therapy benefits, demonstrating attenuated apoptosis of endothelial cells and cardiomyocytes41 as well as cardiac function improvement42 and tissue perfusion related to angiogenesis and arteriogenesis.43 These effects are apparently significantly related to lymphohistiocytic infiltration at stem cell injection sites.44 The importance of monocytes and macrophages in myocardial tissue healing and prevention of ventricular remodeling has been tested in several models45-46 and has shown that macrophages act as producing factors that protect hypoxic cardiac cells from apoptosis.47
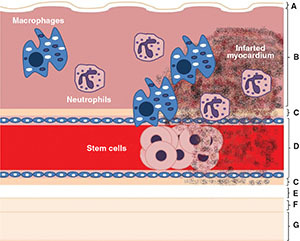
Some authors suggest that the beneficial action of stem cells depends on their ability to recruit lymphohistiocytic compounds more than on cell differentiation to new cardiomyocytes, and that the most important positive effects are related to the death of implanted cells in the site of transplantation rather than the intact stem cells by themselves (Figure 2).48 This hypothesis could be well established by clinical trials that have shown how intracoronary stem cell transplantation can lead to improved ventricular remodeling and function, exercise tolerance, and long-term survival in patients with initial higher intramyocardial homing, despite low cell retention at the end of the study.37-38 One of the main factors affecting the efficacy of stem cell therapies seems to be the number of viable cells that achieve nesting on the affected myocardium. All cell subtypes may have different regenerative properties insofar as they tolerate adverse ischemic environments and interact with chemoreceptor expression; therefore, any measure to improve homing could have a significant impact on the effectiveness of cell therapy. Several techniques are currently being studied to better support cells, including multicellular therapy, modification of cell properties prior to infusion, increasing myocardial chemokine expression by electroshock, transport polymers, and tissue engineering gel.49-52
Figure 2.
Inflammatory paracrine response to stem cell therapy. The presence of neutrophils and macrophages on myocardial tissue (lymphohistiocytic infiltration) heals and prevents ventricular remodeling at stem cell injection sites. (A) Endocardium; (B) Myocardium; (C) Epicardium; (D) Coronary blood vessel; (E) Pericardial cavity; (F) Parietal pericardium; (G) Fibrous pericardium.
Conclusion
Stem cell regenerative cardiac therapy appears to be a safe treatment modality for patients with ischemic and nonischemic cardiac disease, mainly promoting neovascularization and improving endothelial dysfunction. The results of meta-analysis addressing the clinical applicability suggest middle- and long-term improvement in cardiac function, specifically LVEF, exercise tolerance, functional class, quality of life, and scar size; however, the effect on adverse remodeling processes is less clear. Several important aspects need to be addressed, namely discriminating cell populations, dosing, timing, homing modulation, and delivery routes. Clarification of these issues may translate into better outcomes for patients. Further studies are needed to define the underlying mechanisms of stem cell therapy response and develop methods to further improve stem cell homing and survival.
Funding Statement
Funding/Support: This work was partially supported by the Endowed Chair in Cardiology – Tec de Monterrey 0020CAT131 as well as CONACYT-México grant 151136 (G G-R). Dr. Guerrero-Beltrán was supported by a CONACYT Postdoctoral Fellowship.
Footnotes
Conflict of Interest Disclosure: The authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
References
- 1.Teuteberg JJ, Lewis EF, Nohria A, Tsang SW, Fang JC, Givertz MM, et al. Characteristics of patients who die with heart failure and a low ejection fraction in the new millennium. J Card Fail. 2006 Feb;12(1):47–53.. doi: 10.1016/j.cardfail.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, et al. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006 Mar 21;113(11): 1424–33.. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 3.Watson RD, Gibbs CR, Lip GY. ABC of heart failure. Clinical features and complications. BMJ. 2000 Jan 22;320(7229): 236–9.. doi: 10.1136/bmj.320.7229.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parakh K, Kittleson MM, Heidecker B, Wittstein IS, Judge DP, Champion HC, et al. The variable natural history of idiopathic dilated cardiomyopathy. Isr Med Assoc J. 2012 Nov;14(11): 666–71.. [PubMed] [Google Scholar]
- 5.Arad M, Freimark D. Predicting prognosis in dilated cardiomyopathy. Isr Med Assoc J. 2012 Nov;14(11):687–9.. [PubMed] [Google Scholar]
- 6.Eckardt L, Haverkamp W, Johna R, Böcker D, Deng MC, Breithardt G, et al. Arrhythmias in heart failure: current concepts of mechanisms and therapy. J Cardiovasc Electrophysiol. 2000 Jan;11(1):106–17.. doi: 10.1111/j.1540-8167.2000.tb00746.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee DS, Gona P, Albano I, Larson MG, Benjamin EJ, Levy D, et al. A systematic assessment of causes of death after heart failure onset in the community: impact of age at death, time period, and left ventricular systolic dysfunction. Circ Heart Fail. 2011 Jan;4(1):36–43.. doi: 10.1161/CIRCHEARTFAILURE.110.957480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grigorian-Shamagian L, Otero Raviña F, Abu Assi E, Vidal Perez R, Teijeira-Fernandez E, Varela Roman A, et al. Why and when do patients with heart failure and normal left ventricular ejection fraction die? Analysis of >600 deaths in a community long-term study. Am Heart J. 2008 Dec;156(6):1184–90.. doi: 10.1016/j.ahj.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Garg N, Senthilkumar A, Nusair MB, Goyal N, Garg RK, Alpert MA. Heart Failure With a Normal Left Ventricular Ejection Fraction: Epidemiology Pathophysiology, Diagnosis and Management. Am J Med Sci. doi: 10.1097/MAJ.0b013e31828c586e. 2013 Mar 14. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Kamdar F, John R, Eckman P, Colvin-Adams M, Shumway SJ, Liao K. Postcardiac transplant survival in the current era in patients receiving continuous-flow left ventricular assist devices. J Thorac Cardiovasc Surg. 2013 Feb;145(2):575–81.. doi: 10.1016/j.jtcvs.2012.09.095. [DOI] [PubMed] [Google Scholar]
- 11.Attisani M, Centofanti P, La Torre M, Boffini M, Ricci D, Ribezzo M, et al. Advanced heart failure in critical patients (INTERMACS 1 and 2 levels): ventricular assist devices or emergency transplantation?. Interact Cardiovasc Thorac Surg. 2012 Oct;15(4):678–84.. doi: 10.1093/icvts/ivs256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verbrugge FH, De Vusser P, Rivero-Ayerza M, Van Herendael H, Rondelez K, Dupont M, et al. Cardiac resynchronization therapy with or without defibrillator: experience from a high-volume Belgian implantation centre. Acta Cardiol. 2013 Feb;68(1):37–45.. doi: 10.1080/ac.68.1.2959630. [DOI] [PubMed] [Google Scholar]
- 13.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012 Aug;14(8):803–69.. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 14.Herrera-Garza EH, Molina-Gamboa JD, Ortega-Durán OA, Chavarria-Martánez U, Martínez-Chapa HD, Elizondo-Sifuentes LA, et al. [Heart transplant in Monterrey, Nuevo Leon]. Rev Invest Clin. 2011 Sep;63 Suppl 1:91–5.. [PubMed] [Google Scholar]
- 15.Herrera-Garza E, Molina-Gamboa J, Decanini-Arcaute H, Ibarra-Flores M, Torres-García M, Macías-Hidalgo C, et al. [Heart transplant in “Nuevo Leon”: the first 33 cases]. Arch Cardiol Mex. 2006 Apr-Jun;76(2):151–62.. [PubMed] [Google Scholar]
- 16.Gulati A, Ismail TF, Jabbour A, Ismail NA, Morarji K, Ali A, et al. Clinical utility and prognostic value of left atrial volume assessment by cardiovascular magnetic resonance in non-ischaemic dilated cardiomyopathy. Eur J Heart Fail. 2013 Jun;15(6):660–70.. doi: 10.1093/eurjhf/hft019. [DOI] [PubMed] [Google Scholar]
- 17.Sanbe A. Dilated cardiomyopathy: a disease of the myocardium. Biol Pharm Bull. 2013;36(1):18–22.. doi: 10.1248/bpb.b212023. [DOI] [PubMed] [Google Scholar]
- 18.Kubanek M, Sramko M, Maluskova J, Kautznerova D, Weichet J, Lupinek P, et al. Novel predictors of left ventricular reverse remodeling in individuals with recent-onset dilated cardiomyopathy. J Am Coll Cardiol. 2013 Jan 8;61(1):54–63.. doi: 10.1016/j.jacc.2012.07.072. [DOI] [PubMed] [Google Scholar]
- 19.McNally EM, Golbus JR, Puckelwartz MJ. Genetic mutations and mechanisms in dilated cardiomyopathy. J Clin Invest. 2013 Jan 2;123(1):19–26.. doi: 10.1172/JCI62862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jakob P, Landmesser U. Current Status of Cell-Based Therapy for Heart Failure. Curr Heart Fail Rep. 2013 Jun;10(2):165–76.. doi: 10.1007/s11897-013-0134-z. [DOI] [PubMed] [Google Scholar]
- 21.Tongers J, Losordo DW, Landmesser U. Stem and progenitor cell-based therapy in ischaemic heart disease: promise uncertainties, and challenges. Eur Heart J. 2011 May;32(10): 1197–206.. doi: 10.1093/eurheartj/ehr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maltais S, Perrault LP, Ly HQ. The bone marrow-cardiac axis: role of endothelial progenitor cells in heart failure. Eur J Cardiothorac Surg. 2011 Mar;39(3):368–74.. doi: 10.1016/j.ejcts.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 23.De Rosa S, Seeger FH, Honold J, Fischer-Rasokat U, Lehmann R, Fichtlscherer S, et al. Procedural safety and predictors of acute outcome of intracoronary administration of progenitor cells in 775 consecutive procedures performed for acute myocardial infarction or chronic heart failure. Circ Cardiovasc Interv. 2013 Feb;6(1):44–51.. doi: 10.1161/CIRCINTERVENTIONS.112.971705. [DOI] [PubMed] [Google Scholar]
- 24.Messori A, Fadda V, Maratea D, Trippoli S. Intracoronary infusion of bone-marrow derived mononuclear cells in acute myocardial infarction: Are outcomes influenced by the number of infused cells?. Heart Lung Circ. doi: 10.1016/j.hlc.2012.10.008. 2012 Dec 21. pii: S1443-9506(12)01335-2. [DOI] [PubMed] [Google Scholar]
- 25.Stamm C, Nasseri B, Choi YH, Hetzer R. Cell therapy for heart disease: great expectations as yet unmet. Heart Lung Circ. 2009 Aug;18(4):245–56.. doi: 10.1016/j.hlc.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Kreke M, Smith RR, Marbán L, Marbán E. Cardiospheres and cardiosphere-derived cells as therapeutic agents following myocardial infarction. Expert Rev Cardiovasc Ther. 2012 Sep;10(9):1185–94.. doi: 10.1586/erc.12.102. [DOI] [PubMed] [Google Scholar]
- 27.Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004 Jul 1;94(1):92–5.. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 28.Sürder D, Schwitter J, Moccetti T, Astori G, Rufibach K, Plein S, et al. Cell-based therapy for myocardial repair in patients with acute myocardial infarction: rationale and study design of the SWiss multicenter Intracoronary Stem cells Study in Acute Myocardial Infarction (SWISS-AMI). Am Heart J. 2010 Jul;160(1):58–64.. doi: 10.1016/j.ahj.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 29.Wöhrle J, Merkle N, Mailänder V, Nusser T, Schauwecker P, von Scheidt F, et al. Results of intracoronary stem cell therapy after acute myocardial infarction. Am J Cardiol. 2010 Mar 15;105(6):804–12.. doi: 10.1016/j.amjcard.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 30.Delewi R, Andriessen A, Tijssen JG, Zijlstra F, Piek JJ, Hirsch A. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a meta-analysis of randomised controlled clinical trials. Heart. 2013 Feb;99(4): 225–32.. doi: 10.1136/heartjnl-2012-302230. [DOI] [PubMed] [Google Scholar]
- 31.Anversa P, Kajstura J, Rota M, Leri A. Regenerating new heart with stem cells. J Clin Invest. 2013 Jan 2;123(1):62–70.. doi: 10.1172/JCI63068. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Turan RG, Bozdag-T I, Ortak J, Kische S, Akin I, Schneider H, et al. Improved functional activity of bone marrow derived circulating progenitor cells after intra coronary freshly isolated bone marrow cells transplantation in patients with ischemic heart disease. Stem Cell Rev. 2011 Sep;7(3):646–56.. doi: 10.1007/s12015-010-9220-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beeres SL, Bax JJ, Dibbets-Schneider P, Stokkel MP, Fibbe WE, van der Wall EE, et al. Intramyocardial injection of autologous bone marrow mononuclear cells in patients with chronic myocardial infarction and severe left ventricular dysfunction. Am J Cardiol. 2007 Oct 1;100(7):1094–8.. doi: 10.1016/j.amjcard.2007.04.056. [DOI] [PubMed] [Google Scholar]
- 34.Zhang CY, Sun AJ, Ge JB, Zhang SN, Wang KQ, Zou YZ. [Efficacy of autologous bone marrow-derived cells transfer for patients with chronic ischemic heart disease: a meta-analysis]. Zhonghua Xin Xue Guan Bing Za Zhi. 2010 Jul;38(7):656–61.. [PubMed] [Google Scholar]
- 35.Strauer BE, Steinhoff G. 10 years of intracoronary and intramyocardial bone marrow stem cell therapy of the heart: from the methodological origin to clinical practice. J Am Coll Cardiol. 2011 Sep 6;58(11):1095–104.. doi: 10.1016/j.jacc.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 36.Strauer BE, Yousef M, Schannwell CM. Schannwell The acute and long-term effects of intracoronary Stem cell Transplantation in 191 patients with chronic heARt failure: the STAR-heart study. Eur J Heart Fail. 2010 Jul;12(7):721–9.. doi: 10.1093/eurjhf/hfq095. [DOI] [PubMed] [Google Scholar]
- 37.Vrtovec B, Poglajen G, Sever M, Lezaic L, Domanovic D, Cernelc P, et al. Effects of intracoronary stem cell transplantation in patients with dilated cardiomyopathy. J Card Fail. 2011 Apr;17(4):272–81.. doi: 10.1016/j.cardfail.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Vrtovec B, Poglajen G, Lezaic L, Sever M, Domanovic D, Cernelc P, et al. Effects of intracoronary CD34+ stem cell transplantation in nonischemic dilated cardiomyopathy patients: 5-year follow-up. Circ Res. 2013 Jan 4;112(1):165–73.. doi: 10.1161/CIRCRESAHA.112.276519. [DOI] [PubMed] [Google Scholar]
- 39.Seth S, Bhargava B, Narang R, Ray R, Mohanty S, Gulati G, et al. The ABCD (Autologous Bone Marrow Cells in Dilated Cardiomyopathy) trial a long-term follow-up study. J Am Coll Cardiol. 2010 Apr 13;55(15):1643–4.. doi: 10.1016/j.jacc.2009.11.070. [DOI] [PubMed] [Google Scholar]
- 40.Fischer-Rasokat U, Assmus B, Seeger FH, Honold J, Leistner D, Fichtlscherer S, et al. A pilot trial to assess potential effects of selective intracoronary bone marrow-derived progenitor cell infusion in patients with nonischemic dilated cardiomyopathy: final 1-year results of the transplantation of progenitor cells and functional regeneration enhancement pilot trial in patients with nonischemic dilated cardiomyopathy. Circ Heart Fail. 2009 Sep;2(5):417–23.. doi: 10.1161/CIRCHEARTFAILURE.109.855023. [DOI] [PubMed] [Google Scholar]
- 41.Fuchs S, Baffour R, Zhou YF, Shou M, Pierre A, Tio FO, et al. Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional function in pigs with chronic experimental myocardial ischemia. J Am Coll Cardiol. 2001 May;37(6):1726–32.. doi: 10.1016/s0735-1097(01)01200-1. [DOI] [PubMed] [Google Scholar]
- 42.Laflamme MA, Zbinden S, Epstein SE, Murry CE. Cell-based therapy for myocardial ischemia and infarction: pathophysiological mechanisms. Annu Rev Pathol. 2007;2: 307–39.. doi: 10.1146/annurev.pathol.2.010506.092038. [DOI] [PubMed] [Google Scholar]
- 43.Tse HF, Lau CP. Therapeutic angiogenesis with bone marrow-derived stem cells. J Cardiovasc Pharmacol Ther. 2007 Jun;12(2):89–97.. doi: 10.1177/1074248407303139. [DOI] [PubMed] [Google Scholar]
- 44.Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A. 2005 Aug 9;102(32):11474–9.. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lambert JM, Lopez EF, Lindsey ML. Macrophage roles following myocardial infarction. Int J Cardiol. 2008 Nov 12;130(2):147–58.. doi: 10.1016/j.ijcard.2008.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res. 2008 Aug;58(2):88–111.. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trial J, Rossen RD, Rubio J, Knowlton AA. Inflammation and ischemia: macrophages activated by fibronectin fragments enhance the survival of injured cardiac myocytes. Exp Biol Med (Maywood). 2004 Jun;229(6):538–45.. doi: 10.1177/153537020422900612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeghiazarians Y, Zhang Y, Prasad M, Shih H, Saini SA, Takagawa J, et al. Injection of bone marrow cell extract into infarcted hearts results in functional improvement comparable to intact cell therapy. Mol Ther. 2009 Jul;17(7):1250–6.. doi: 10.1038/mt.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bocchi EA, Bacal F, Guimarães G, Mendroni A, Mocelin A, Filho AE, et al. Granulocyte-colony stimulating factor or granulocyte-colony stimulating factor associated to stem cell intracoronary infusion effects in non ischemic refractory heart failure. Int J Cardiol. 2010 Jan 7;138(1):94–7.. doi: 10.1016/j.ijcard.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 50.Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, et al. REPAIR-AMI Investigators.Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur Heart J. 2006 Dec;27(23):2775–83.. doi: 10.1093/eurheartj/ehl388. [DOI] [PubMed] [Google Scholar]
- 51.Assmus B, Fischer-Rasokat U, Honold J, Seeger FH, Fichtlscherer S, Tonn T, et al. Transcoronary transplantation of functionally competent BMCs is associated with a decrease in natriuretic peptide serum levels and improved survival of patients with chronic postinfarction heart failure: results of the TOPCARE-CHD Registry. Circ Res. 2007 Apr 27;100(8): 1234–41.. doi: 10.1161/01.RES.0000264508.47717.6b. [DOI] [PubMed] [Google Scholar]
- 52.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006 Sep 21;355(12):1199–209.. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]


