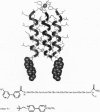
Abstract
Flash photolysis and pulse radiolysis measurements demonstrate a conformational dependence of electron transfer rates across a 16-mer helical bundle (three-helix metalloprotein) modified with a capping CoIII(bipyridine)3 electron acceptor at the N terminus and a 1-ethyl-1'-ethyl-4,4'- bipyridinium donor at the C terminus. For the CoIII(peptide)3-1-ethyl-1'-ethyl-4,4'-bipyridinium maquettes, the observed transfer is a first order, intramolecular process, independent of peptide concentration or laser pulse energy. In the presence of 6 M urea, the random coil bundle (approximately 0% helicity) has an observed electron transfer rate constant of kobs = 900 +/- 100 s-1. In the presence of 25% trifluoroethanol (TFE), the helicity of the peptide is 80% and the kobs increases to 2000 +/- 200 s-1. Moreover, the increase in the rate constant in TFE is consistent with the observed decrease in donor-acceptor distance in this solvent. Such bifunctional systems provide a class of molecules for testing the effects of conformation on electron transfer in proteins and peptides.
Full text
PDF
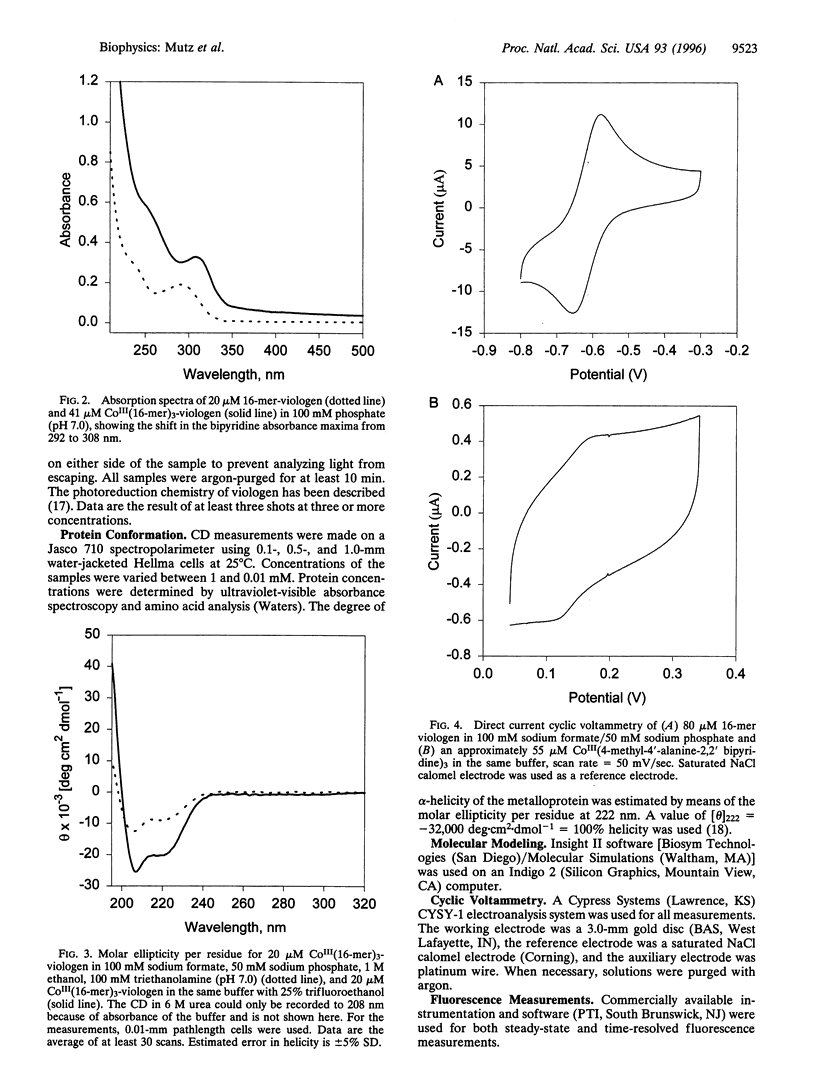
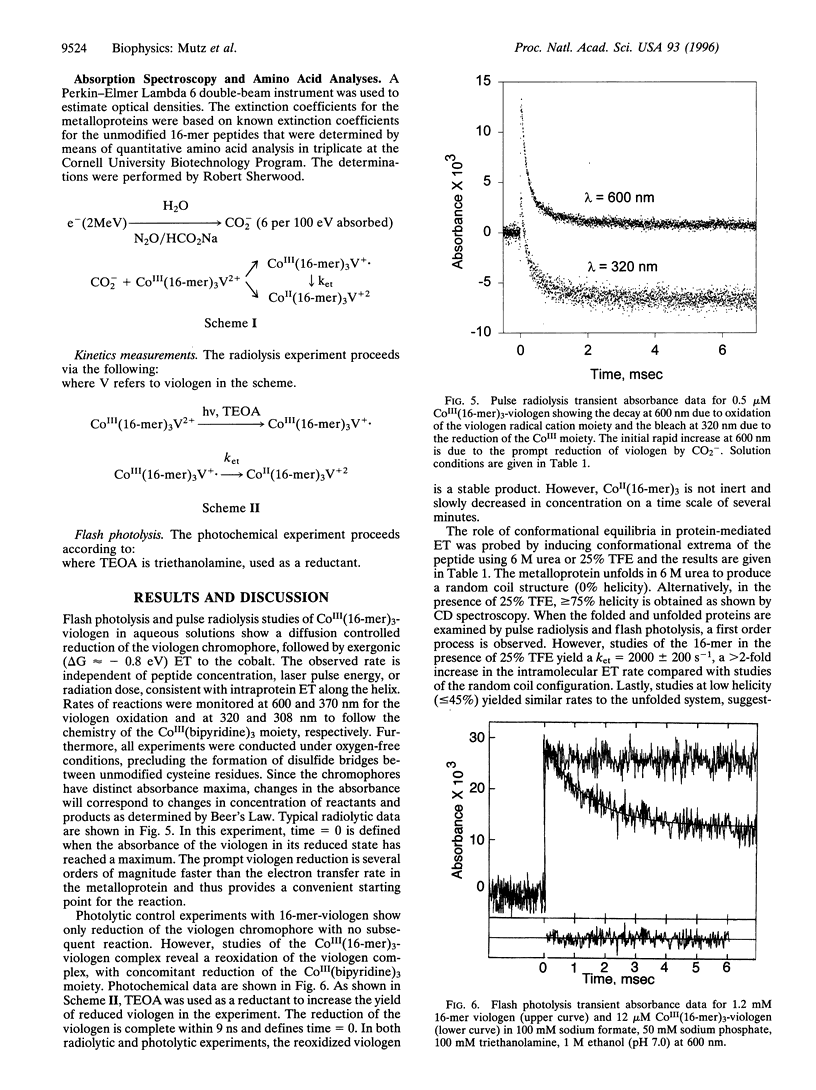

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen Y. H., Yang J. T., Chau K. H. Determination of the helix and beta form of proteins in aqueous solution by circular dichroism. Biochemistry. 1974 Jul 30;13(16):3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- Fields G. B., Noble R. L. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int J Pept Protein Res. 1990 Mar;35(3):161–214. doi: 10.1111/j.1399-3011.1990.tb00939.x. [DOI] [PubMed] [Google Scholar]
- Geren L., Hahm S., Durham B., Millett F. Photoinduced electron transfer between cytochrome c peroxidase and yeast cytochrome c labeled at Cys 102 with (4-bromomethyl-4'-methylbipyridine)[bis(bipyridine)]ruthenium2+. Biochemistry. 1991 Oct 1;30(39):9450–9457. doi: 10.1021/bi00103a009. [DOI] [PubMed] [Google Scholar]
- Haas E., Katchalski-Katzir E., Steinberg I. Z. Effect of the orientation of donor and acceptor on the probability of energy transfer involving electronic transitions of mixed polarization. Biochemistry. 1978 Nov 14;17(23):5064–5070. doi: 10.1021/bi00616a032. [DOI] [PubMed] [Google Scholar]
- Handel T. M., Williams S. A., DeGrado W. F. Metal ion-dependent modulation of the dynamics of a designed protein. Science. 1993 Aug 13;261(5123):879–885. doi: 10.1126/science.8346440. [DOI] [PubMed] [Google Scholar]
- Langen R., Chang I. J., Germanas J. P., Richards J. H., Winkler J. R., Gray H. B. Electron tunneling in proteins: coupling through a beta strand. Science. 1995 Jun 23;268(5218):1733–1735. doi: 10.1126/science.7792598. [DOI] [PubMed] [Google Scholar]