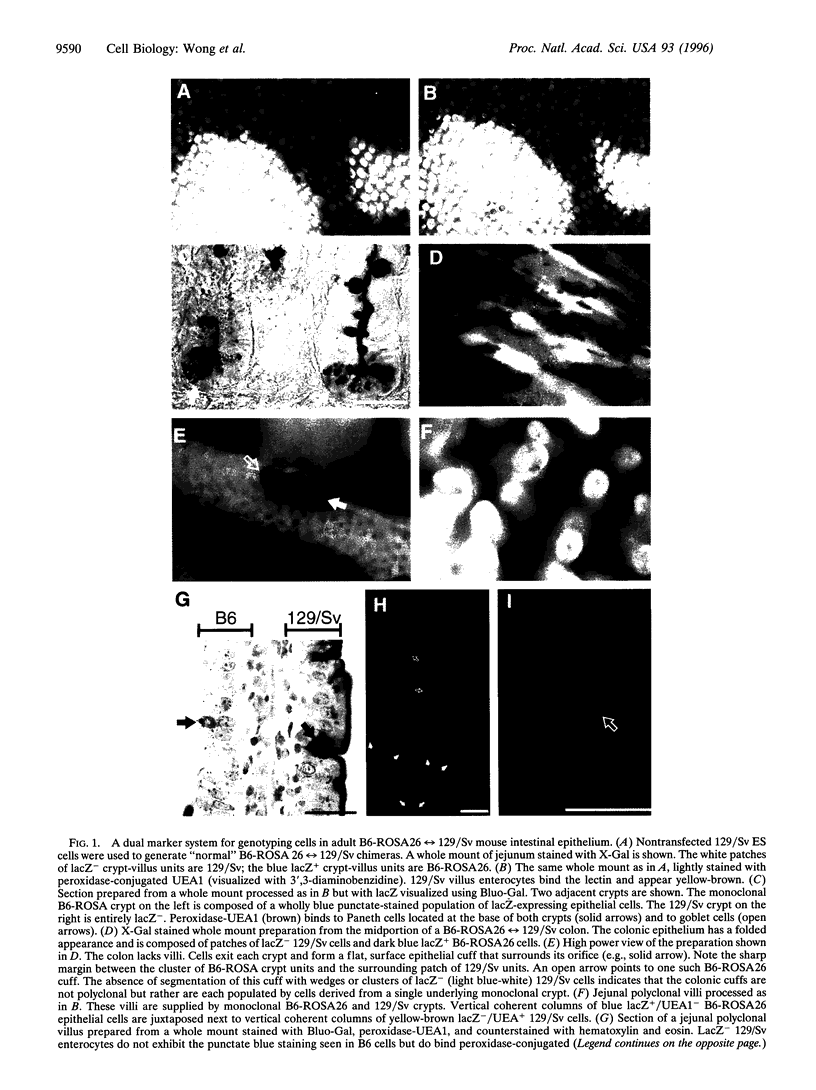
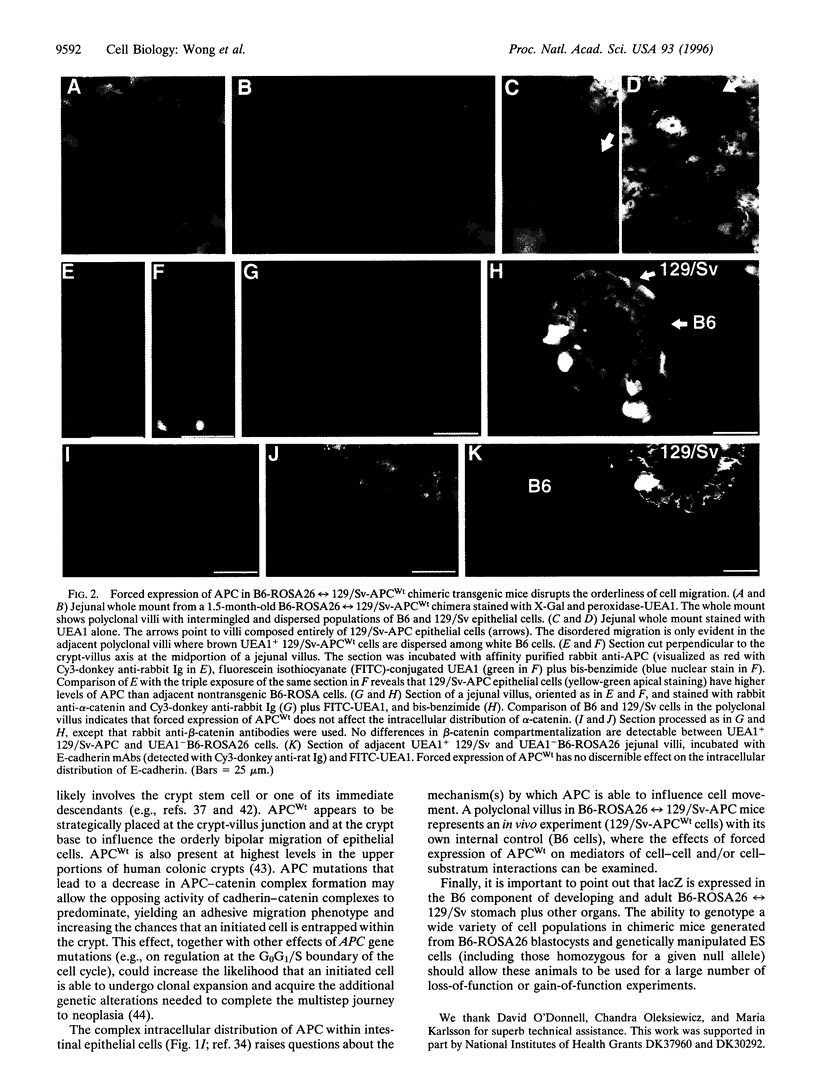
Abstract
Mutations of the human adenomatosis polyposis coli (APC) gene are associated with the development of familial as well as sporadic intestinal neoplasia. To examine the in vivo function of APC, 129/Sv embryonic stem (ES) cells were transfected with DNA encoding the wild-type human protein under the control of a promoter that is active in all four of the small intestine's principal epithelial lineages during their migration-associated differentiation. ES-APC cells were then introduced into C57BL/6-ROSA26 blastocysts. Analyses of adult B6-ROSA26<-->129/Sv-APC chimeric mice revealed that forced expression of APC results in markedly disordered cell migration. When compared with the effects of forced expression of E-cadherin, the data suggest that APC-catenin and E-cadherin-catenin complexes have opposing effects on intestinal epithelial cell movement/adhesiveness; augmentation of E-cadherin-beta-catenin complexes produces a highly ordered, "adhesive" migration, whereas augmentation of APC-beta-catenin complexes produces a disordered, nonadhesive migratory phenotype. We propose that APC mutations may promote tumorigenesis by increasing the relative activity of cadherin-catenin complexes, resulting in enhanced adhesiveness and functional anchorage of initiated cells within the intestinal crypt. Our studies also indicate that chimeric mice generated from B6-ROSA26 blastocysts and genetically manipulated ES cells should be useful for auditing gene function in the gastrointestinal tract and in other tissues.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeg G. H., Matsumine A., Kuroda T., Bhattacharjee R. N., Miyashiro I., Toyoshima K., Akiyama T. The tumour suppressor gene product APC blocks cell cycle progression from G0/G1 to S phase. EMBO J. 1995 Nov 15;14(22):5618–5625. doi: 10.1002/j.1460-2075.1995.tb00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bry L., Falk P., Huttner K., Ouellette A., Midtvedt T., Gordon J. I. Paneth cell differentiation in the developing intestine of normal and transgenic mice. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10335–10339. doi: 10.1073/pnas.91.22.10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove W. F., Luongo C., Connelly C. S., Gould K. A., Shoemaker A. R., Moser A. R., Gardner R. L. The adenomatous polyposis coli gene of the mouse in development and neoplasia. Cold Spring Harb Symp Quant Biol. 1994;59:501–508. doi: 10.1101/sqb.1994.059.01.055. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Friedrich G., Soriano P. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 1991 Sep;5(9):1513–1523. doi: 10.1101/gad.5.9.1513. [DOI] [PubMed] [Google Scholar]
- Frisch S. M., Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994 Feb;124(4):619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. I., Hermiston M. L. Differentiation and self-renewal in the mouse gastrointestinal epithelium. Curr Opin Cell Biol. 1994 Dec;6(6):795–803. doi: 10.1016/0955-0674(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Groden J., Thliveris A., Samowitz W., Carlson M., Gelbert L., Albertsen H., Joslyn G., Stevens J., Spirio L., Robertson M. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991 Aug 9;66(3):589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- Hall P. A., Coates P. J., Ansari B., Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994 Dec;107(Pt 12):3569–3577. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- Hamilton S. R., Liu B., Parsons R. E., Papadopoulos N., Jen J., Powell S. M., Krush A. J., Berk T., Cohen Z., Tetu B. The molecular basis of Turcot's syndrome. N Engl J Med. 1995 Mar 30;332(13):839–847. doi: 10.1056/NEJM199503303321302. [DOI] [PubMed] [Google Scholar]
- Hermiston M. L., Gordon J. I. In vivo analysis of cadherin function in the mouse intestinal epithelium: essential roles in adhesion, maintenance of differentiation, and regulation of programmed cell death. J Cell Biol. 1995 Apr;129(2):489–506. doi: 10.1083/jcb.129.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermiston M. L., Gordon J. I. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995 Nov 17;270(5239):1203–1207. doi: 10.1126/science.270.5239.1203. [DOI] [PubMed] [Google Scholar]
- Hermiston M. L., Green R. P., Gordon J. I. Chimeric-transgenic mice represent a powerful tool for studying how the proliferation and differentiation programs of intestinal epithelial cell lineages are regulated. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8866–8870. doi: 10.1073/pnas.90.19.8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermiston M. L., Wong M. H., Gordon J. I. Forced expression of E-cadherin in the mouse intestinal epithelium slows cell migration and provides evidence for nonautonomous regulation of cell fate in a self-renewing system. Genes Dev. 1996 Apr 15;10(8):985–996. doi: 10.1101/gad.10.8.985. [DOI] [PubMed] [Google Scholar]
- Hinck L., Näthke I. S., Papkoff J., Nelson W. J. Dynamics of cadherin/catenin complex formation: novel protein interactions and pathways of complex assembly. J Cell Biol. 1994 Jun;125(6):1327–1340. doi: 10.1083/jcb.125.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joslyn G., Carlson M., Thliveris A., Albertsen H., Gelbert L., Samowitz W., Groden J., Stevens J., Spirio L., Robertson M. Identification of deletion mutations and three new genes at the familial polyposis locus. Cell. 1991 Aug 9;66(3):601–613. doi: 10.1016/0092-8674(81)90022-2. [DOI] [PubMed] [Google Scholar]
- Joslyn G., Richardson D. S., White R., Alber T. Dimer formation by an N-terminal coiled coil in the APC protein. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11109–11113. doi: 10.1073/pnas.90.23.11109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., Roth K. A., Moser A. R., Gordon J. I. Transgenic mouse models that explore the multistep hypothesis of intestinal neoplasia. J Cell Biol. 1993 Nov;123(4):877–893. doi: 10.1083/jcb.123.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzler K. W., Nilbert M. C., Vogelstein B., Bryan T. M., Levy D. B., Smith K. J., Preisinger A. C., Hamilton S. R., Hedge P., Markham A. Identification of a gene located at chromosome 5q21 that is mutated in colorectal cancers. Science. 1991 Mar 15;251(4999):1366–1370. doi: 10.1126/science.1848370. [DOI] [PubMed] [Google Scholar]
- Luongo C., Moser A. R., Gledhill S., Dove W. F. Loss of Apc+ in intestinal adenomas from Min mice. Cancer Res. 1994 Nov 15;54(22):5947–5952. [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Miyashiro I., Senda T., Matsumine A., Baeg G. H., Kuroda T., Shimano T., Miura S., Noda T., Kobayashi S., Monden M. Subcellular localization of the APC protein: immunoelectron microscopic study of the association of the APC protein with catenin. Oncogene. 1995 Jul 6;11(1):89–96. [PubMed] [Google Scholar]
- Moser A. R., Dove W. F., Roth K. A., Gordon J. I. The Min (multiple intestinal neoplasia) mutation: its effect on gut epithelial cell differentiation and interaction with a modifier system. J Cell Biol. 1992 Mar;116(6):1517–1526. doi: 10.1083/jcb.116.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser A. R., Shoemaker A. R., Connelly C. S., Clipson L., Gould K. A., Luongo C., Dove W. F., Siggers P. H., Gardner R. L. Homozygosity for the Min allele of Apc results in disruption of mouse development prior to gastrulation. Dev Dyn. 1995 Aug;203(4):422–433. doi: 10.1002/aja.1002030405. [DOI] [PubMed] [Google Scholar]
- Munemitsu S., Albert I., Souza B., Rubinfeld B., Polakis P. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):3046–3050. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemitsu S., Souza B., Müller O., Albert I., Rubinfeld B., Polakis P. The APC gene product associates with microtubules in vivo and promotes their assembly in vitro. Cancer Res. 1994 Jul 15;54(14):3676–3681. [PubMed] [Google Scholar]
- Nagafuchi A., Takeichi M. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO J. 1988 Dec 1;7(12):3679–3684. doi: 10.1002/j.1460-2075.1988.tb03249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishisho I., Nakamura Y., Miyoshi Y., Miki Y., Ando H., Horii A., Koyama K., Utsunomiya J., Baba S., Hedge P. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991 Aug 9;253(5020):665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- Näthke I. S., Hinck L., Swedlow J. R., Papkoff J., Nelson W. J. Defining interactions and distributions of cadherin and catenin complexes in polarized epithelial cells. J Cell Biol. 1994 Jun;125(6):1341–1352. doi: 10.1083/jcb.125.6.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M., Baribault H., Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989 Jun;8(6):1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkoff J., Rubinfeld B., Schryver B., Polakis P. Wnt-1 regulates free pools of catenins and stabilizes APC-catenin complexes. Mol Cell Biol. 1996 May;16(5):2128–2134. doi: 10.1128/mcb.16.5.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peifer M. Regulating cell proliferation: as easy as APC. Science. 1996 May 17;272(5264):974–975. doi: 10.1126/science.272.5264.974. [DOI] [PubMed] [Google Scholar]
- Polakis P. Mutations in the APC gene and their implications for protein structure and function. Curr Opin Genet Dev. 1995 Feb;5(1):66–71. doi: 10.1016/s0959-437x(95)90055-1. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B., Albert I., Porfiri E., Fiol C., Munemitsu S., Polakis P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996 May 17;272(5264):1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B., Souza B., Albert I., Munemitsu S., Polakis P. The APC protein and E-cadherin form similar but independent complexes with alpha-catenin, beta-catenin, and plakoglobin. J Biol Chem. 1995 Mar 10;270(10):5549–5555. doi: 10.1074/jbc.270.10.5549. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B., Souza B., Albert I., Müller O., Chamberlain S. H., Masiarz F. R., Munemitsu S., Polakis P. Association of the APC gene product with beta-catenin. Science. 1993 Dec 10;262(5140):1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Reed J. C. Anchorage dependence, integrins, and apoptosis. Cell. 1994 May 20;77(4):477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- Smith K. J., Johnson K. A., Bryan T. M., Hill D. E., Markowitz S., Willson J. K., Paraskeva C., Petersen G. M., Hamilton S. R., Vogelstein B. The APC gene product in normal and tumor cells. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2846–2850. doi: 10.1073/pnas.90.7.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. J., Levy D. B., Maupin P., Pollard T. D., Vogelstein B., Kinzler K. W. Wild-type but not mutant APC associates with the microtubule cytoskeleton. Cancer Res. 1994 Jul 15;54(14):3672–3675. [PubMed] [Google Scholar]
- Su L. K., Kinzler K. W., Vogelstein B., Preisinger A. C., Moser A. R., Luongo C., Gould K. A., Dove W. F. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992 May 1;256(5057):668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- Su L. K., Vogelstein B., Kinzler K. W. Association of the APC tumor suppressor protein with catenins. Science. 1993 Dec 10;262(5140):1734–1737. doi: 10.1126/science.8259519. [DOI] [PubMed] [Google Scholar]
- Trahair J. F., Neutra M. R., Gordon J. I. Use of transgenic mice to study the routing of secretory proteins in intestinal epithelial cells: analysis of human growth hormone compartmentalization as a function of cell type and differentiation. J Cell Biol. 1989 Dec;109(6 Pt 2):3231–3242. doi: 10.1083/jcb.109.6.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestweber D., Kemler R. Identification of a putative cell adhesion domain of uvomorulin. EMBO J. 1985 Dec 16;4(13A):3393–3398. doi: 10.1002/j.1460-2075.1985.tb04095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe M., Nagafuchi A., Tsukita S., Takeichi M. Induction of polarized cell-cell association and retardation of growth by activation of the E-cadherin-catenin adhesion system in a dispersed carcinoma line. J Cell Biol. 1994 Oct;127(1):247–256. doi: 10.1083/jcb.127.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]