Abstract
The Bmp4 signaling molecule is expressed in ventral splanchnic and branchial-arch mesoderm and outflow-tract (OFT) myocardium, suggesting a role for Bmp4 in OFT development. Inactivation of Bmp4 in the caudal branchial arch and splanchnic mesoderm and OFT myocardium by using a conditional null allele of Bmp4 and the Nkx2.5cre recombinase allele resulted in abnormal morphogenesis of branchial-arch arteries (BAAs) and defective OFT septation. Expression of aortic-sac myocardial markers was reduced and expression of the sm22LacZ transgene, a smooth-muscle marker, was attenuated in BAAs and conotruncus of Nkx2.5cre; Bmp4 conditional mutants. Moreover, we found tissue-specific functions for Bmp4 in the regulation of cellular proliferation and apoptosis. We also demonstrate a strong genetic interaction between Bmp4 and Bmp7 in OFT development. Our findings uncover a previously uncharacterized function for Bmp4 in vascular remodeling of the BAAs, and they show definitively that Bmp4, in cooperation with Bmp7, has a central role in OFT septation.
The vertebrate heart can be subdivided into inflow, outflow, and primitive-ventricular regions (1). The cardiac outflow tract (OFT), which develops from the anterior part of the linear heart tube, forms the right-sided conotruncal region after heart looping. Initially unseptated, the OFT divides into the pulmonary trunk (PT) and aorta, and it is critical for separation of postnatal pulmonary and systemic circulation. Congenital OFT malformations are common, making an understanding of the genetic pathways regulating OFT development an important goal in developmental biology and clinical medicine.
At defined areas of the OFT, endocardial cells undergo an epithelial to mesenchymal transformation (perhaps in response to a signal from overlying myocardium) and invade the intervening space to form the endocardial cushions. The cardiac neural crest also invades the forming aorto-pulmonary (AP) septum and OFT cushions (2). The OFT myocardium receives an additional input from splanchnic and branchial-arch mesoderm, the anterior or secondary heart field (SHF), which may be important for OFT lengthening and morphogenesis (3).
Bmp4 is a member of the Bone morphogenetic protein (Bmp) subclass of transforming growth factor type β (TGF-β)-signaling molecules (4). Bmp4 expression in splanchnic and branchial-arch mesoderm (which contributes to OFT myocardium) and within the OFT myocardium itself suggests a role in OFT morphogenesis (ref. 5 and see below). Investigation of Bmp4 function in cardiac development has been hampered by the early embryonic lethality of Bmp4 null mutant embryos (6). Recent work analyzing an allele of the ubiquitously expressed Bmp type 2 receptor (Bmpr2), containing a partial ectodomain deletion, revealed defective proximal OFT septation in mouse embryos, providing insight into Bmp function in the OFT (7). However, because Bmpr2 is broadly expressed, the developmental mechanisms responsible for the cushion defects remain unclear. Overexpression of noggin in chick embryos suggested that Bmp signaling is required for both migration of cardiac neural crest and proliferation of OFT mesenchyme (8). Moreover, inactivation of Bmp4 in the heart by using a cardiac troponin T (cTnT) cre transgene and a conditional allele of Bmp4 that is also a hypomorph (9), revealed that Bmp4 regulated proliferation of atrioventricular cushion mesenchyme (10).
To investigate the tissue-specific requirements for Bmp4 signaling in the OFT, we generated a Bmp4 conditional null allele to analyze Bmp4 function in OFT morphogenesis directly. Inactivation of Bmp4 in splanchnic and branchial-arch mesoderm and OFT myocardium by using the Nkx2.5cre allele resulted in severe defects in OFT septation with AP window. Markers of aortic-sac and OFT myocardium were reduced or absent, suggesting that Bmp4 has a role in myocardial differentiation. Interestingly, cell proliferation was up-regulated in Bmp4 mutant OFT myocardium but reduced in cushion mesenchyme. We also found that recruitment of vascular smooth muscle to forming vessels was reduced in Nkx2.5cre; Bmp4 conditional mutant embryos. Finally, crosses of Bmp4 conditional mutants to Bmp7 mutant embryos uncovered a strong genetic interaction.
Materials and Methods
Whole-Mount and Section in Situ Hybridization. Whole-mount and section in situ hybridization was performed as described (11).
Casting-Dye Injection. Embryos were harvested, and the yellow casting dye (Connecticut Biological Supply, South Hampton, MA) was injected into the ventricle by using pulled glass, fixed in buffered formalin, dehydrated, and cleared in BABB (2:1 benzyl alcohol/benzyl benzoate).
LacZ Staining and Histology. Staining for LacZ and histology were performed as described (11).
Generation of the Bmp4floxneo and Bmp4null Alleles and Other Strains. To generate the Bmp4floxneo allele, a targeting vector was constructed that introduced one LoxP site into an EcoRI site upstream of the Bmp4 fourth exon while another LoxP site followed by a frt flanked PGKneo cassette was introduced into a downstream BamHI site (see Fig. 8, which is published as supporting information on the PNAS web site). To generate the Bmp4null allele, we crossed the Bmp4floxneo allele to the nestin cre transgenic line, directing cre expression in the germline (12). The Bmp7tm1 null allele (The Jackson Laboratory) and the prx1cre line have been described (13, 14).
Proliferation and Apoptosis. Proliferating cell nuclear antigen (PCNA) staining was performed according to the manufacturer's instructions (Zymed), and terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling analysis was performed according to the manufacturer's protocol (Serologicals, Clarkston, GA).
Results
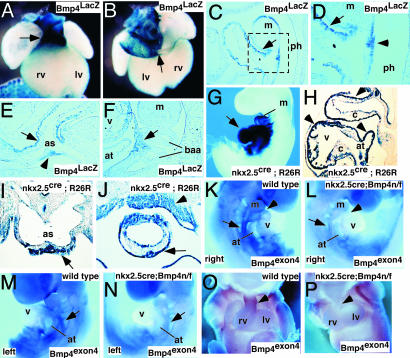
Expression of Bmp4 in the Branchial-Arch and Splanchnic Mesoderm and the OFT Myocardium. We examined Bmp4 expression by using the Bmp4 LacZ knock-in allele, Bmp4LacZ, which had been generated (6). Whole-mount analysis and sectioning revealed that Bmp4 was highly expressed in the OFT of the heart at 9.0 and 10.5 dpc (Fig. 1 A–E). Bmp4 was detected in the coronary sinus and inferior and superior vena cava (Fig. 1B). Bmp4 was expressed in OFT myocardium and in myocardium overlying the junction of the branchial-arch artery (BAA) and the aortic sac (Fig. 1 C–E). Expression was detected also at 9.0 dpc within mesoderm ventral to the BAAs (Fig. 1F). These cells have been proposed to comprise the SHF that migrates and contributes to OFT myocardium (3). Together, these data suggest that Bmp4 functions in the formation of the OFT myocardium at early stages of its formation in the branchial arch and splanchnic mesoderm. Bmp4 may have a role in OFT maturation by signaling to underlying conotruncal cushions. Expression in mesenchyme surrounding BAAs and in the myocardium overlying the junction between the aortic sac and the BAA suggests a function for Bmp4 in BAA remodeling.
Fig. 1.
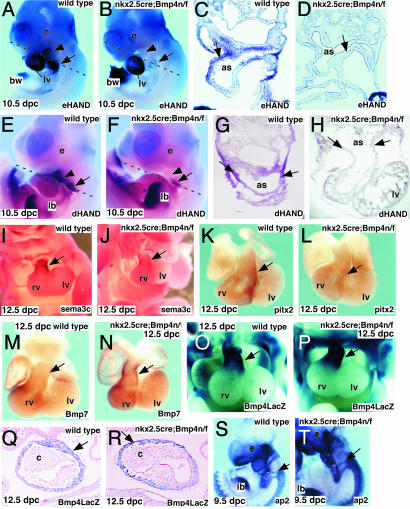
Bmp4 expression and tissue-specific inactivation in mouse embryos. (A and B) Bmp4LacZ expression in 10.5-dpc wild-type embryos. Ventral (A) and dorsal (B) views show Bmp4 expression restricted to the OFT (arrow in A) and in the cardinal veins that drain into the right atrium (arrow in B). (C–F) Parasagittal (C, D, and F) and transverse (E) sections through 9.0-dpc mouse embryos. D is a magnification (×400) of the boxed area in C. Bmp4 expression in OFT myocardium (arrows in C and D) and myocardium near the junction of the aortic sac and the BAA (arrow in E) is shown. Bmp4 expression in the pharyngeal endoderm (arrowhead in D and E) and branchial-arch mesenchyme (arrow in F) is shown. (G–J) Nkx2.5cre;R26R compound mice stained for LacZ to detect cre activity at 9.5 dpc (G and H) and 10.5 dpc (I and J). Parasagittal (H) and coronal (I and J) sections show cre activity in OFT myocardium (arrows in H–J), branchial-arch mesoderm (arrowhead in J), and atrioventricular myocardium (arrowheads in H). (K–P) In situ analysis with Bmp4 exon 4 probe at 9.0 (K–N) and 11.5 (O and P) dpc, comparing hybridization signal in OFT myocardium (arrowhead in K and L) and branchial-arch region (arrows in K–N). as, aortic sac; at, atrium; c, cushion; m, mandible; lv, left ventricle; rv, right ventricle; ph, pharynx; v, ventricle.
Inactivation of Bmp4 in the Branchial Arch Mesenchyme and OFT Myocardium by Using the Nkx2.5cre Allele. To investigate Bmp4 in the OFT, we constructed a Bmp4 conditional null allele, the Bmp4floxneo allele (see Materials and Methods and Fig. 8). This allele of Bmp4 contains LoxP sites surrounding exon 4, encoding the mature Bmp4 peptide, a region of Bmp4 that is essential for its function. Removal of exon 4 would be predicted to result in a Bmp4 null allele.
To inactivate Bmp4 in the branchial arch and splanchnic mesoderm, as well as aortic-sac and OFT myocardium, we used the Nkx2.5cre knock-in allele that directs cre activity to these regions of the heart, the prospective SHF, and pharyngeal endoderm (ref. 15 and Fig. 1 G–J). The Nkx2.5cre allele also directs cre activity to the ventricular and atrial myocardium (Fig. 1H). To determine whether Bmp4 exon 4 had been deleted in the forming hearts of Nkx2.5cre; Bmp4null/flox (n/f) mutant embryos, we performed whole-mount analysis with a Bmp4 exon 4-specific probe. At 9.0 dpc, Bmp4 exon4 was expressed in branchial arch and splanchnic mesoderm, whereas in the Nkx2.5cre; Bmp4 n/f mutant embryos, expression of Bmp4 exon 4 was not detected (Fig. 1 K–N). Similarly, at 11.5 dpc, expression of Bmp4 exon4 was absent from the OFT myocardium of Nkx2.5cre; Bmp4 n/f mutant embryos, (Fig. 1 O and P). From these data, we conclude that Nkx2.5cre; Bmp4 n/f embryos efficiently delete Bmp4 exon4 in branchial-arch and splanchnic mesoderm and in the OFT myocardium.
Bmp4 Is Required for Normal Septation of the OFT and BAA Remodeling. Most Nkx2.5cre; Bmp4 n/f mutant embryos died by 13.5 dpc, although there was a range of lethality, with an occasional Nkx2.5cre; Bmp4 n/f mutant embryo surviving until 18.5 dpc. The variability in expressivity of the Nkx2.5cre; Bmp4 n/f mutant phenotype most likely results from variability of cre activity (data not shown). At time points before lethality, we noted that the Nkx2.5cre; Bmp4 n/f mutant embryos had peripheral edema that was often associated with a pericardial effusion, suggesting that embryonic lethality was secondary to heart failure (data not shown).
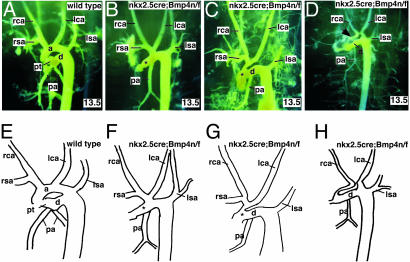
We performed casting-dye injections at two developmental time points to visualize the OFT and BAAs in Nkx2.5cre; Bmp4 n/f mutant embryos. At 12.0 dpc, both wild-type and Nkx2.5cre; Bmp4 n/f mutant embryos had well formed BAAs, revealing that the initial assembly of the endothelial tubes was intact in the Nkx2.5cre; Bmp4 n/f mutant embryos (data not shown). In contrast, at 13.5 dpc, after remodeling of the OFT and BAAs, the Nkx2.5cre; Bmp4 n/f mutant embryos had severe defects in the architecture of the BAAs (Fig. 2 A–D).
Fig. 2.
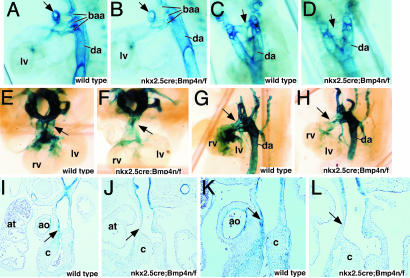
Casting-dye analysis of vascular morphogenesis in Nkx2.5cre; Bmp4 n/f mutant embryos. (A–D) Yellow casting-dye injections at 13.5 dpc, photographed in left-oblique orientation. Wild-type (A) and three classes of phenotypes (B–D) are shown. In B and C, areas of defective septation are indicated (by an asterisk), and undersized aorta (D) is indicated by an arrow. (E–H) Diagrams of wild-type and mutant dye injections from A–D. Each diagram is a representation of the cast image above it. a, ascending aorta; d, ductus arteriosus; lca, left carotid artery; lsa, left subclavian artery; rca, right carotid artery; rsa, right subclavian artery.
In wild-type embryos, the right brachiocephalic, left carotid, and left subclavian arteries branch directly from the ascending aorta (Fig. 2. A and E). In Nkx2.5cre; Bmp4 n/f mutant embryos, the left carotid branched either from the right brachiocephalic artery in the most severe embryos or directly from the aorta in more mildly affected embryos (Fig. 2). In all of the mildly affected embryos, there was an aortic interruption between the left carotid and the left subclavian, known as a type B interruption (Fig. 2. C and G) and (16). In all Bmp4 mutant embryos, only one pulmonary artery (PA), originating from the ductus arteriosus in the injected embryos, was identified (Fig. 2. B–D and F–H). The origin of the second PA is unclear, although, in human patients, the PA is known to originate from many places, including the right ventricle (17).
Defects were also observed in septation of the OFT. The most severe defect, observed in two embryos, was persistent truncus arteriosus, although we cannot rule out the alternative classification of total AP window (17, 18) (Fig. 2 B and F). The most common OFT anomaly in Nkx2.5cre; Bmp4 n/f mutant embryos was a proximal, AP window in which the most proximal aspect of the OFT septum failed to form (17). This defect was found in the majority of Nkx2.5cre; Bmp4 n/f mutant embryos (Fig. 2 C and G). The final abnormality, observed in three Nkx2.5cre; Bmp4 n/f mutants, was failure of proximal septation with unequal sizes of the PT and ascending aorta (Fig. 2 D and H).
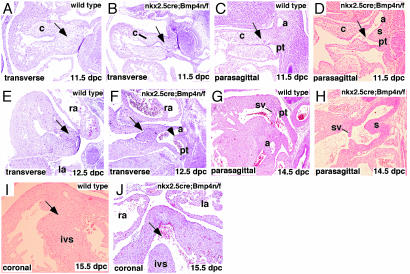
Microscopic Analysis Revealed Defects in Conotruncal and Atrioventricular Septation in Nkx2.5cre; Bmp4 n/f Mutants. Sections of embryos revealed that at 11.5 dpc, septation within the distal OFT of wild-type embryos was progressing, whereas in Nkx2.5cre; Bmp4 n/f mutant embryos, cushion growth was delayed (Fig. 3 A–D). At 11.5 dpc, the separation of the aorta from the PT had initiated in wild-type embryos, but in Nkx2.5cre; Bmp4 n/f mutant embryos, the distal OFT remained unseptated with hypoplastic cushions (Fig. 3 C and D). At 12.5 dpc, the distal septum of wild-type embryos was extensive, whereas in the mutant embryos, the communication between the aorta and PT was clearly visible (Fig. 3 E and F). By 14.5 dpc, the OFT had undergone substantial remodeling and septation in wild-type embryos, but in the Bmp4 mutant, septation failed to progress, resulting in the proximal AP window defect that was also observed in the casting-dye injections. Moreover, the semilunar valves in the mutant were clearly hypoplastic in the 14.5-dpc embryos when compared with wild type (Fig. 3 G and H).
Fig. 3.
Histologic analysis of Nkx2.5cre; Bmp4 n/f mutant embryos. (A–D) Transverse (A and B) and parasagittal (C and D) sections through the OFT of wild-type (A and C) and Bmp4 conditional mutant (B and D) embryos at 11.5 dpc. Distal septal cushions are reduced in Bmp4 mutants (compare arrows in A–D). Communication between PT and aorta is evident (arrow in D). (E and F) Transverse sections of wild-type (E) and Bmp4 mutant (F) at 12.5 dpc reveal communication between PT and aorta (arrowhead in F) and abnormal cushions (compare arrows in E and F). (G and H) Parasagittal sections reveal septated and remodeled OFT septum in wild-type (G) and AP window in mutant with valve hypoplasia in mutants (H). (I and J) The ventricular septum has fused in the wild-type 15.5-dpc embryo (arrow in I) but a membranous ventricular septal defect in mutant (arrow in J). a, aorta; c, cushion; ivs, interventricular septum; left atrium; ra, right atrium; s, AP septum; sv, semilunar valve.
We also observed ventricular septal defects in all mutant embryos (Fig. 3 I and J). Histologic analysis showed that the ventricular septal defect likely results from a deficiency in the contruncal mesenchyme, which is in proximity to the AV-cushion mesenchyme and is thought to be important for septation and for walling off the left AV canal from the right ventricle (19). Together with the casting-dye data, these data show that Bmp4 function is necessary for growth of the conotruncal cushions and OFT septation, as well as BAA remodeling. These data also suggest that Bmp4 has a more important function in proximal OFT septation.
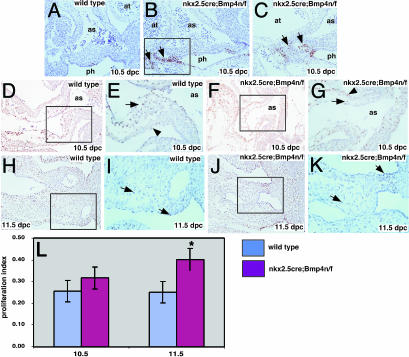
Bmp4 Regulates Cellular Proliferation in the OFT. To investigate the hypothesis that Bmp4 regulated proliferation and/or apoptosis in the forming OFT, we used terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling studies on serial sections. These studies revealed a modest up-regulation of apoptotic cells in the pharyngeal endoderm and surrounding mesenchyme in proximity to the aortic sac of Nkx2.5cre; Bmp4 n/f mutant embryos (Fig. 4 A–C).
Fig. 4.
Defects in proliferation and apoptosis in Nkx2.5cre; Bmp4 n/f mutant embryos. (A–C) Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling in transverse sections of 10.5-dpc embryos. Stages and genotypes are labeled. Arrows in B indicate areas of up-regulated apoptosis in the Nkx2.5cre; Bmp4 n/f mutants in both mesenchyme and pharyngeal endoderm. C is a higher magnification (×400) of the boxed area in B.(D–K) PCNA staining at two stages: 10.5 (D–G) and 11.5 (H–K) dpc. The data for the OFT myocardium are also presented in L. At 10.5 dpc, the Nkx2.5cre; Bmp4 n/f mutants have severely reduced OFT cushions with fewer PCNA-positive cells (compare arrows in E and G). The 10.5-dpc OFT myocardium has similar numbers of PCNA-positive cells in wild-type and Nkx2.5cre; Bmp4 n/f mutants (compare arrowheads in E and G). At 11.5 dpc, the Nkx2.5cre; Bmp4 n/f mutant OFT cushion (J and K) has enlarged but lags behind wild type (H and I). (L) Proliferative index in OFT myocardium of wild-type and Nkx2.5cre; Bmp4 n/f mutants at two stages. as, aortic sac; at, atrium; ph, pharynx. At 11.5 dpc, the mutant has a statistically significant increase in proliferation. *, P < 0.05 (χ2 test).
PCNA immunostaining experiments revealed that at 10.5 dpc, cell proliferation in the Nkx2.5cre; Bmp4 n/f mutant OFT cushions was severely reduced. Fewer proliferating cells were found in the hypoplastic cushions of the Nkx2.5cre;Bmp4 n/f mutants (Fig. 4 D–G). At 11.5 dpc, when more Bmp4 mutant OFT-cushion mesenchyme had formed, cell counting of PCNA-positive cells revealed a significant reduction in the number of proliferating cells in the mutant embryos. At 11.5 dpc, the proliferative index of OFT cushion mesenchyme in wild-type embryos was 43.3 ± 1.0%, whereas in the Bmp4 mutant the proliferative index was 33.6 ± 2.7% (P < 0.05, Student's t test).
We also noted that the number of PCNA-positive cells in the OFT myocardium of Nkx2.5cre; Bmp4 n/f mutants was greater than in the wild-type controls. At both 10.5 and 11.5 dpc, the proliferative index in Nkx2.5cre; Bmp4 n/f mutants was greater than in the wild-type controls, although this difference was statistically significant only at 11.5 dpc (Fig. 4 H–L). Taken together, these data reveal that Bmp4 functions in both the regulation of apoptosis and proliferation in OFT development. The role of Bmp4 in regulation of apoptosis is likely a minor one with regard to OFT morphogenesis because elevated apoptosis was observed in only a few sections of our serial section analysis. In contrast, regulation of proliferation, with an interesting difference between the myocardium and mesenchyme, appears to be a major function for Bmp4 in the OFT. Bmp4 promotes proliferation of cushion mesenchyme and concurrently represses cell proliferation in the OFT myocardium.
HAND Genes are Down-Regulated in the Aortic-Sac Myocardium of Nkx2.5cre; Bmp4 n/f Mutant Embryos. We next examined markers of the aortic-sac and OFT myocardium. Expression of the basic helix–loop–helix genes, eHAND and dHAND, was severely reduced in the Nkx2.5cre; Bmp4 n/f mutant embryos (Fig. 5 A, B, E, and F). Sections through wild-type and Nkx2.5cre; Bmp4 n/f mutant embryos revealed that eHAND expression was reduced or absent in the myocardium of the aortic sac and posterior pericardial wall (Fig. 5 C and D), whereas dHAND was reduced in the aortic-sac myocardium (Fig. 5 G and H). Expression of eHAND was maintained at normal levels in the left ventricle of the Nkx2.5cre; Bmp4 n/f mutant embryos (Fig. 5 A and B). Expression of sema3c and pitx2 was also reduced in the OFT myocardium of 11.5-dpc mutant embryos (Fig. 5 I–L). In contrast, Bmp7, which is normally expressed in the OFT and right ventricular myocardium, was expanded in the OFT of Nkx2.5cre; Bmp4 n/f mutant embryos (Fig. 5 M and N). Expression of LacZ from the Bmp4LacZ allele in Nkx2.5cre; Bmp4 n/f mutants was intact, suggesting that anterior myocardium moved correctly into the OFT. This observation also reveals that Nkx2.5cre; Bmp4 n/f mutant cells had maintained their fate as Bmp4 expressing cells (Fig. 5 O–R). These data suggest that Bmp4 signaling functions in differentiation of the aortic-sac and OFT myocardium.
Fig. 5.
Defects in markers of aortic sac and OFT myocardium in Nkx2.5cre; Bmp4 n/f mutant embryos. (A–H) In situ analysis with eHAND (A–D) and dHAND (E–H) probes (A, B, E, and F) and transverse sections (C, D, G, and H). Plane of section is indicated by dotted line (A, B, E, and F). Expression is reduced in the mutant aortic sac (arrow in A–H) and in branchial arch (arrowhead in A, B, E, and F). The body wall that expresses eHAND has been partially removed (A and B). Expression of dHAND in the branchial pouches is reduced (E and F). (I–N) In situ analysis with sema3c (I and J), pitx2 (K and L), and Bmp7 (M and N) probes. Signal is indicated by arrows. (O–R) LacZ staining directed by Bmp4 LacZ. Whole-mount LacZ staining reveals LacZ-positive cells in OFT of wild-type and Bmp4 mutant embryos (arrows). Coronal sections (Q and R) reveal LacZ-positive cells in OFT myocardium (arrows). (S and T) In situ analysis with ap2 probe on 9.5-dpc wild-type (S) and Bmp4 mutant (T) embryos. Arrows indicate the migrating cardiac neural-crest cells. as, aortic sac; bw, body wall; c, cushions; e, eye; rv, right ventricle; lb, limb bud; lv, left ventricle.
Because defects in cardiac neural crest may also result in similar phenotypes of defective vascular remodeling, we analyzed markers of cardiac neural crest in Nkx2.5cre; Bmp4 n/f mutant embryos. Expression of crabp1, ap2 and hoxa2 was detected at normal levels in the migrating neural crest of Nkx2.5cre; Bmp4 n/f mutant embryos. These data suggested that the initial migration of the cardiac neural crest was intact in the Nkx2.5cre; Bmp4 n/f mutant embryos (Fig. 5 S and T, and data not shown).
Bmp4 Signaling Is Required to Recruit Vascular Smooth Muscle to Forming BAAs and the Conotruncal Region of the Heart. One possible explanation for defective vascular remodeling in Nkx2.5cre; Bmp4 n/f mutant embryos was a deficiency in smooth-muscle recruitment to the formed endothelial tubes in the Nkx2.5cre; Bmp4 n/f mutant embryos. We used the sm22LacZ transgene, which marks vascular smooth muscle (20), to visualize smooth muscle in Nkx2.5cre; Bmp4 n/f mutant embryos. Because smooth muscle is a neural-crest derivative, this experiment would also determine whether neural-crest development was defective in the Nkx2.5cre; Bmp4 n/f mutants (21). At 11.5 dpc, recruitment of smooth-muscle cells to the BAAs was detected in the wild-type embryos, but in Nkx2.5cre; Bmp4 n/f mutant embryos fewer smooth-muscle cells were observed surrounding the BAAs (Fig. 6 A–D).
Fig. 6.
Smooth-muscle recruitment is defective in the absence of Bmp4 signaling. (A–D) sm22LacZ expression at 10.5 dpc expression in aortic sac is reduced in mutant oblique lateral (A and B) and frontal (C and D) views. Compare arrows in A and B as well as in C and D.(E–H). Whole-mount view of sm22LacZ expression at 12.5 dpc in wild-type and Bmp4 mutant embryos. Arrows indicate areas of reduced contribution of LacZ-positive cells in mutant. (I–L) Sections through 12.5-dpc embryos. Arrows indicate areas of reduced LacZ staining in the Bmp4 mutant in whole-mount views and sections. ao, aorta; at, atrium; c, cushion; da, dorsal aorta; rv, right ventricle; lv, left ventricle.
By 12.5 dpc, the difference in smooth-muscle recruitment was exacerbated in the Nkx2.5cre; Bmp4 n/f mutant embryos. Fewer LacZ-positive cells were found in the OFT, as well as, the BAAs of Nkx2.5cre; Bmp4 n/f mutant embryos. Nkx2.5cre; Bmp4 n/f mutants also had fewer LacZ-positive cells in the proximal OFT and ventricles (Fig. 6 E–H). Sections revealed that in wild-type embryos, LacZ-positive smooth-muscle cells contributed to the proximal OFT, whereas in Nkx2.5cre; Bmp4 n/f mutants, LacZ-positive cells were only detected distally (Fig. 6 I–L). Taken together, these data suggest that Bmp4 signaling has a central role in stabilization of forming vessels by promoting the recruitment or differentiation of vascular smooth muscle and supports the notion that neural-crest derivatives fail to expand in the Bmp4 mutants.
Bmp7 Cooperates with Bmp4 in Morphogenesis of the OFT. The Nkx2.5cre; Bmp4 n/f mutant embryos had a delay in formation of the OFT cushions, suggesting that other Bmp family members functioned in cushion morphogenesis. Moreover, in the Nkx2.5cre; Bmp4 n/f mutant embryos we found that expression of Bmp7 was up-regulated in OFT myocardium. To test the hypothesis that Bmp7 had overlapping function with Bmp4 in OFT morphogenesis, we generated compound Bmp4;Bmp7 mutant embryos.
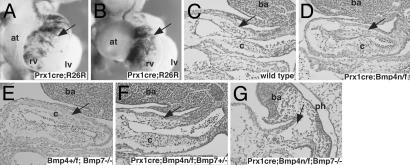
The prx1cre transgene directs low levels of cre activity to the OFT myocardium (Fig. 7 A and B). As a result of low cre activity in OFT myocardium, the prx1cre;Bmp4 n/f embryos have no obvious OFT defect (Fig. 7 C and D). However, because Bmp4 dosage is reduced, these embryos provide a sensitized background to test for a genetic interaction with Bmp7 in the OFT myocardium (Fig. 7 E and F). Although the Bmp7tm1–/– embryos had normal formation of the OFT cushions, the prx1cre; Bmp4 n/f; Bmp7tm1+/– mice had slightly hypoplastic OFT cushions (Fig. 7 D–F). Moreover, the prx1cre; Bmp4 n/f; Bmp7tm1–/– embryos had severe defects in OFT morphogenesis in which OFT cushions were severely reduced and the OFT was shortened (Fig. 7G). These data strongly suggest that Bmp4 and Bmp7 have overlapping functions in OFT morphogenesis.
Fig. 7.
Coordinate regulation of OFT septation by Bmp4 and Bmp7.(A and B) LacZ staining of prx1cre; rosa 26 reporter (R26R) compound heterozygotes at 10.5 dpc in frontal (A) and right-oblique (B) views. LacZ staining identifies cells that have cre activity (arrow). (C–G) Parasaggital sections (hematoxylin/eosin) of 10.5-dpc compound Bmp4; Bmp7 mutant and controls. Area of abnormality in prx1cre; Bmp4 n/f; Bmp7tm1–/– embryos and corresponding area in controls (arrows). at, atrium; ba, branchial arch; c, cushion; rv, right ventricle; lv, left ventricle.
Discussion
Our data reveal that Bmp4 function is important for differentiation of OFT myocardium and for expansion of the OFT-cushion mesenchyme. Our results also show that Bmp4 is required for recruitment of vascular smooth muscle to forming BAAs and, therefore, provide insight into these events. Last, our data uncover a strong genetic interaction between Bmp4 and Bmp7, suggesting that these genes have overlapping functions in the OFT.
Bmp4 Functions to Recruit Vascular Smooth Muscle to the BAAs. Our results uncover a role for Bmp4 in the development of the BAAs. We propose that the defect involves a delay in recruitment of neural crest-derived vascular smooth muscle to the formed endothelial tubes of the BAAs. Formation of the BAAs, and later remodeling into adult aortic-arch arteries, involves paracrine signaling (22). The forming BAA endothelial tubes are located within the branchial-arch mesenchyme in close proximity to surface ectoderm and the endoderm of the branchial pouches and pharynx. Moreover, signaling from endothelium to mesenchyme functions in the recruitment of supporting cells, such as smooth-muscle precursors and pericytes, which are important for vessel stabilization (22). The Nkx2.5cre knock-in allele directs cre activity in both the branchial-arch mesenchyme, as well as the pharyngeal endoderm. This finding suggests that Bmp4 may cooperate with endothelial-derived signals in vascular smooth-muscle development. In the future, it will be important to dissect the exact source of Bmp4 that is important for BAA development.
In vasculature remodeling, there is a local disruption of the interaction between endothelium and supporting cells. This disruption results in regression of the endothelium that likely involves programmed cell death (23). Cell-ablation studies in chick embryos and loss-of-function experiments performed in mice have defined a role for the cardiac crest in maintaining the integrity of the BAAs (21, 24). Moreover, recent fate-mapping experiments using the wnt1 cre and rosa 26 reporter mice confirmed the importance of the cardiac crest in mouse BAA formation (2).
Our results show that recruitment of smooth muscle to the BAAs is reduced in Nkx2.5cre; Bmp4 n/f mutants. Therefore, Bmp4 may be necessary for migration, local proliferation, survival, or differentiation of the vascular smooth muscle. Although we found evidence for elevated apoptosis in the branchial-arch mesenchyme of Nkx2.5cre; Bmp4 n/f, the apoptosis was very restricted and unlikely to be the mechanism for the severe BAA phenotype. Moreover, because we did not detect a major proliferation defect in Bmp4 mutant branchial arch mesenchyme, we favor the hypothesis that Bmp4 is required for differentiation of neural crest-derived mesenchyme into vascular smooth muscle.
The Function of Bmp4 in OFT Septation. There is disagreement regarding the relative contributions of the conotruncal cushions and AP septum in the septation process (19). Our work reveals that Bmp4 provides a signal to the conotruncal cushions that regulates cushion growth. Although cushions still form in the Bmp4 mutant OFT as a result of the overlapping function of Bmp7 (see below), there is a severe proliferation defect in the hypoplastic Bmp4 mutant OFT cushions. Our marker analysis suggested that the initial migration of the cardiac crest was intact in the Nkx2.5cre; Bmp4 n/f mutants but that neural crest-derived smooth-muscle cells, marked by sm22LacZ, failed to populate the proximal OFT of Bmp4 mutant embryos. The deficiency in neural crest-derived smooth muscle suggests that the cardiac neural crest that normally contributes to OFT cushions and the AP septum is also reduced in the proximal OFT of Nkx2.5cre; Bmp4 n/f mutant embryos.
We found that cell proliferation was reduced in the cushion mesenchyme of Nkx2.5cre; Bmp4 n/f mutants. One possibility is that Bmp4 has a direct role in promoting local proliferation of neural-crest cells after they have reached the OFT. Bmp4 might also be important for promoting migration of neural crest destined for the proximal OFT. In support of this notion, overexpression of noggin in chick embryos suggested that Bmp signaling was important for both the migration of cardiac neural crest into the OFT and the proliferation of cushion mesenchyme (8). However, because OFT cushions derive from both endocardium and cardiac neural crest, it is also possible that Bmp4 signals to the endocardium and promotes the mesenchymal transformation important to OFT-cushion development.
Although further experiments are required to distinguish between these possibilities, our data show that Bmp4 promotes expansion of incoming OFT neural crest.
Our results support the proposal that there are distinct pathways regulating septation of the proximal and distal OFT (7). For example, high levels of Bmp-signaling may be required for proximal OFT septation. In the Nkx2.5cre; Bmp4 n/f mutant embryos, total Bmp signaling may be reduced sufficiently to interfere with proximal, but not distal, septation.
Our data expand on these ideas by showing that the total level of Bmp4 and Bmp7 expression are important in OFT septation. Bmp7 expression was up-regulated in the Nkx2.5cre; Bmp4 n/f mutant OFT suggesting a regulatory mechanism controlling total Bmp levels in OFT myocardium. The severe OFT phenotype of prx1cre;Bmp4 n/f; Bmp7tm1–/– mutant embryos revealed that Bmp4 and Bmp7 have overlapping functions in OFT-cushion morphogenesis. It has also been observed that Bmp7 cooperates with Bmp6 in OFT development (25). Together, these data suggest that total levels of Bmp4 and Bmp7 regulate cushion development and support the hypothesis that proximo-distal patterning of OFT cushions is regulated by total levels of Bmp signaling.
Bmp4 and the Differentiation of the OFT Myocardium and the Prospective SHF. Experiments performed in chick embryos have implicated a Bmp2-mediated pathway in specification of the branchial arch and splanchnic mesoderm that will populate the OFT myocardium (26). Our experiments suggest that, in the mouse, this Bmp-signaling function has been partly assumed by Bmp4. The invasion of cells from this SHF, complete by 9.5 dpc in the mouse, may be important for lengthening and arterialization of the OFT (3).
Our data showing Bmp4LacZ expression in the OFT of Bmp4 mutant embryos suggest that cells from the branchial arch and splanchnic mesoderm still migrate into the OFT in the absence of Bmp4. Rather, Bmp4 functions in the final differentiation of the myocardium as shown by reduction in expression of OFT myocardial markers. Moreover, along with defective OFT myocardial differentiation, we observed excessive proliferation in myocardial precursors of Nkx2.5cre; Bmp4 n/f mutants. This observation suggests that the function of Bmp4 in OFT myocardium (to promote differentiation) is distinct from its role in the OFT-cushion mesenchyme, where it is required for cellular expansion. It will be important to determine the underlying molecular mechanisms responsible for the different roles of Bmp4 in the OFT.
Bmp Signaling and the dHAND and eHAND Genes. Although further analysis of regulatory elements are necessary, our data support the notion that the dHAND and eHAND genes are downstream of Bmp4 signaling. eHAND expression is also regulated by Bmp signaling in Xenopus embryos and dHAND has been shown to be a target of Bmp signaling in neuronal development (27, 28). The vascular phenotype of the dHAND null embryos, more severe than the Bmp4 mutant embryos, is consistent with a down-regulation of dHAND and eHAND expression in the Bmp4 mutants (29). It is interesting to note that dHAND regulation is complex and likely involves many input signals, including the endothelin pathway. Mice that are mutant for components of the endothelin signaling pathway have defects in BAA remodeling, raising the possibility that Bmp and endothelin signaling may interact in BAA development (30).
Comparison with Other Cardiac-Specific Bmp4 Conditional Mutants. It is interesting to note that there are difference between our data and the data presented in the work describing the conditional inactivation of Bmp4 by using the rat cardiac troponin T (cTnT) cre transgene, which reported severe atrioventricular canal defects and only mild OFT abnormalities (10). This work contrasts with the Nkx2.5cre; Bmp4 n/f mutants that had only a mild membranous ventricular septal defect but more severe OFT defects. It is likely that the cTnT cre transgenic driver used by Jiao et al. (10) directs relatively low levels of cre activity in the OFT myocardium (see Fig. 2 c–e in ref. 10).
With regard to the more severe AV canal phenotype observed in the cTnT cre; Bmp4 mutants, it is important to note that the conditional Bmp4 null allele used by Jiao et al. (10) is a hypomorph, as reported by the same group (9). Bmp4 is also known to be expressed in lateral-plate mesoderm at early embryonic stages and has a role in left right asymmetry (31). Because the mice reported by Jiao et al. (10) are hypomorphs, they have reduced levels of Bmp4 in lateral-plate mesoderm and other areas where Bmp4 is expressed. Thus, it is possible that the more severe AV canal phenotype observed by Jiao et al. (10) results from the hypomorphic Bmp4 allele that has early defects in Bmp4 expression before cardiac organogenesis.
Supplementary Material
Acknowledgments
We thank A. Bradley, P. Soriano, and R. Behringer for reagents; B. Hogan, J Epstein, E. Olson, D. Srivistava, P. Chambon, and T. Gridley for in situ probes; B. Hogan and Y. Furuta for the Bmp4 LacZ allele; and E. Olson for the sm22 LacZ transgenic line. This work was supported by National Institutes of Dental and Craniofacial Research Grants 2R01DE/HD12324-06 and R01 DE013509 (to J.F.M.) and National Institutes of Health Grants R01 HL50422 and P01 HL49953 (to R.J.S.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: OFT, outflow tract; BAA, branchial-arch artery; PT, pulmonary trunk; AP, aorto-pulmonary; SHF, secondary heart field; dpc, days postcoitum; PA, pulmonary artery; PCNA, proliferating cell nuclear antigen.
References
- 1.Olson, E. N. & Schneider, M. D. (2003) Genes Dev. 17, 1937–1956. [DOI] [PubMed] [Google Scholar]
- 2.Jiang, X., Rowitch, D. H., Soriano, P., McMahon, A. P. & Sucov, H. M. (2000) Development (Cambridge, U.K.) 127, 1607–1616. [DOI] [PubMed] [Google Scholar]
- 3.Kelly, R. G. & Buckingham, M. E. (2002) Trends Genet. 18, 210–216. [DOI] [PubMed] [Google Scholar]
- 4.Hogan, B. L. (1996) Curr. Opin. Genet. Dev. 6, 432–438. [DOI] [PubMed] [Google Scholar]
- 5.Abdelwahid, E., Rice, D., Pelliniemi, L. J. & Jokinen, E. (2001) Cell Tissue Res. 305, 67–78. [DOI] [PubMed] [Google Scholar]
- 6.Lawson, K. A., Dunn, N. R., Roelen, B. A., Zeinstra, L. M., Davis, A. M., Wright, C. V., Korving, J. P. & Hogan, B. L. (1999) Genes Dev. 13, 424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delot, E. C., Bahamonde, M. E., Zhao, M. & Lyons, K. M. (2003) Development (Cambridge, U.K.) 130, 209–220. [DOI] [PubMed] [Google Scholar]
- 8.Allen, S. P., Bogardi, J. P., Barlow, A. J., Mir, S. A., Qayyum, S. R., Verbeek, F. J., Anderson, R. H., Francis-West, P. H., Brown, N. A. & Richardson, M. K. (2001) Dev. Biol. 235, 98–109. [DOI] [PubMed] [Google Scholar]
- 9.Kulessa, H. & Hogan, B. L. (2002) Genesis (New York) 32, 66–68. [DOI] [PubMed] [Google Scholar]
- 10.Jiao, K., Kulessa, H., Tompkins, K., Zhou, Y., Batts, L., Baldwin, H. S. & Hogan, B. L. (2003) Genes Dev. 17, 2362–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu, M. F., Pressman, C., Dyer, R., Johnson, R. L. & Martin, J. F. (1999) Nature 401, 276–278. [DOI] [PubMed] [Google Scholar]
- 12.Trumpp, A., Depew, M. J., Rubenstein, J. L., Bishop, J. M. & Martin, G. R. (1999) Genes Dev. 13, 3136–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo, G., Hofmann, C., Bronckers, A. L., Sohocki, M., Bradley, A. & Karsenty, G. (1995) Genes Dev. 9, 2808–2820. [DOI] [PubMed] [Google Scholar]
- 14.Logan, M., Martin, J. F., Nagy, A., Lobe, C., Olson, E. N. & Tabin, C. J. (2002) Genesis (New York) 33, 77–80. [DOI] [PubMed] [Google Scholar]
- 15.Moses, K. A., De Mayo, F., Braun, R. M., Reecy, J. L. & Schwartz, R. J. (2001) Genesis (New York) 31, 176–180. [DOI] [PubMed] [Google Scholar]
- 16.Backer, C. L. & Mavroudis, C. (2000) Ann. Thorac. Surg. 69, S298–S307. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs, J. P., Quintessenza, J. A., Gaynor, J. W., Burke, R. P. & Mavroudis, C. (2000) Ann. Thorac. Surg. 69, S44–S49. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs, M. L. (2000) Ann. Thorac. Surg. 69, S50–S55. [DOI] [PubMed] [Google Scholar]
- 19.Webb, S., Qayyum, S. R., Anderson, R. H., Lamers, W. H. & Richardson, M. K. (2003) J. Anat. 202, 327–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, L., Miano, J. M., Cserjesi, P. & Olson, E. N. (1996) Circ. Res. 78, 188–195. [DOI] [PubMed] [Google Scholar]
- 21.Etchevers, H. C., Vincent, C., Le Douarin, N. M. & Couly, G. F. (2001) Development (Cambridge, U.K.) 128, 1059–1068. [DOI] [PubMed] [Google Scholar]
- 22.Hanahan, D. (1997) Science 277, 48–50. [DOI] [PubMed] [Google Scholar]
- 23.Meeson, A. P., Argilla, M., Ko, K., Witte, L. & Lang, R. A. (1999) Development (Cambridge, U.K.) 126, 1407–1415. [DOI] [PubMed] [Google Scholar]
- 24.Brown, C. B., Feiner, L., Lu, M. M., Li, J., Ma, X., Webber, A. L., Jia, L., Raper, J. A. & Epstein, J. A. (2001) Development (Cambridge, U.K.) 128, 3071–3080. [DOI] [PubMed] [Google Scholar]
- 25.Kim, R. Y., Robertson, E. J. & Solloway, M. J. (2001) Dev. Biol. 235, 449–466. [DOI] [PubMed] [Google Scholar]
- 26.Waldo, K. L., Kumiski, D. H., Wallis, K. T., Stadt, H. A., Hutson, M. R., Platt, D. H. & Kirby, M. L. (2001) Development (Cambridge, U.K.) 128, 3179–3188. [DOI] [PubMed] [Google Scholar]
- 27.Sparrow, D. B., Kotecha, S., Towers, N. & Mohun, T. J. (1998) Mech. Dev. 71, 151–163. [DOI] [PubMed] [Google Scholar]
- 28.Howard, M. J., Stanke, M., Schneider, C., Wu, X. & Rohrer, H. (2000) Development (Cambridge, U.K.) 127, 4073–4081. [DOI] [PubMed] [Google Scholar]
- 29.Srivastava, D., Thomas, T., Lin, Q., Kirby, M. L., Brown, D. & Olson, E. N. (1997) Nat. Genet. 16, 154–160. [DOI] [PubMed] [Google Scholar]
- 30.Yanagisawa, H., Hammer, R. E., Richardson, J. A., Williams, S. C., Clouthier, D. E. & Yanagisawa, M. (1998) J. Clin. Invest. 102, 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujiwara, T., Dehart, D. B., Sulik, K. K. & Hogan, B. L. (2002) Development (Cambridge, U.K.) 129, 4685–4696. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.