Abstract
Context
Red meat consumption has been consistently associated with an increased risk of type 2 diabetes. However, whether changes in red meat intake are related to subsequent type 2 diabetes risk remains unknown.
Objective
We evaluated the association between changes in red meat consumption during a 4-year period and subsequent 4-year risk of type 2 diabetes in US adults.
Design, setting and participants
We followed 26,357 men in the Health Professionals Follow-up Study (HPFS, 1986–2006), 48,709 women in the Nurses’ Health Study (NHS, 1986–2006) and 74,077 women in NHS II (1991–2007). Diet was assessed by validated food frequency questionnaires and updated every 4 years. Time-dependent Cox proportional hazard models were used to calculate hazard ratios (HRs) with adjustment for age, family history, race, marital status, initial red meat consumption, initial and changes in other lifestyle factors (physical activity, smoking status, alcohol intake, energy intake, and dietary quality). Results across cohorts were pooled by inverse-variance-weighted fixed-effect meta-analyses.
Main Outcome Measure
Incident T2D cases validated by supplementary questionnaires.
Results
During 1,965,824 person-years of follow-up, we documented 7,540 incident type 2 diabetes cases. In the multivariate-adjusted models, increasing red meat intake during a 4-year interval was associated with an elevated risk of type 2 diabetes over the subsequent four years in each cohort (all P-trend <0.001): compared with the reference group of no change in red meat intake, increasing red meat intake of >0.5 serving/d was associated with a 48% (pooled HR, 1.48; 95% CI, 1.37–1.59) elevated risk in the subsequent 4-year period, and the association was modestly attenuated after further adjustment for initial body mass index and concurrent weight gain (1.30; 95% CI, 1.21–1.41). A reduction of red meat consumption of >0.5 serving/day from baseline to the first four years of follow-up was associated with a 14% (95% CI, 7%–20%) lower risk during subsequent follow-up through 2006/2007.
Conclusions
Increasing red meat consumption over time is associated with an elevated subsequent risk of type 2 diabetes, and the association is partly mediated by body weight. Our results add further evidence that limiting red meat consumption over time confers benefits for diabetes prevention.
Keywords: red meat, diabetes, prospective cohort study
INTRODUCTION
Red meat consumption has been consistently related to an elevated risk of type 2 diabetes. For example, three recent meta-analyses of prospective cohort studies all reported positive associations.1–3 However, most previous studies measured red meat consumption only at baseline with limited follow-up information. In real life, a person’s eating behavior changes over time, and secular trends in red meat intake are also changing dramatically across the globe.4 Because a measurement at a single point in time does not capture the variability of red meat intake during follow-up, it is important to evaluate whether changes in red meat intake over time alter the risk of developing type 2 diabetes. Therefore, we analyzed data from three Harvard cohort studies: the Health Professionals Follow-up Study (HPFS), the Nurses’ Health Study (NHS), and the NHS II, in which we have collected repeated measurements of red meat intake every 4 years, as well as other dietary components, lifestyle factors, and medical history with up to 20 years of follow-up. These repeated measures and long duration of follow-up allow us to investigate the association between dynamic changes in red meat intake and subsequent risk of type 2 diabetes. We conducted two sets of change analysis. In the first analysis, we examined 4-year change in red meat intake in relation to diabetes incidence in the next 4 years of follow-up. In the second analysis, to examine long-term effects of meat intake on diabetes, we analyzed changes in red meat intake from baseline to the first 4-year follow-up with diabetes incidence in the subsequent 12 (NHS II) and 16 (NHS and HPFS) years of follow-up.
SUBJECTS AND METHODS
Study Population
The HPFS was initiated in 1986 when 51,529 US male health professionals, aged 40 to 75 years, returned a baseline questionnaire about detailed medical history, as well as lifestyle and usual diet. The NHS consists of 121,700 registered female nurses, aged 30 to 55 years, who completed a baseline questionnaire about lifestyle and medical history in 1976. The NHS II was established in 1989 and was comprised of 116,671 younger female registered nurses, aged 25 to 42 years, who responded to a baseline questionnaire similar to the NHS questionnaire. Detailed descriptions of the cohorts have been introduced elsewhere.3, 5 In all cohorts, questionnaires were administered at baseline and biennially thereafter to collect and update information on lifestyle practices (e.g., smoking, physical activity) and occurrence of chronic diseases. The cumulative follow-up of the three cohorts all exceed 90% of potential person-times.
In the current analysis, we used 1986 for the HPFS and NHS and 1991 for the NHS II as the baseline, when we assessed detailed information on diet and lifestyle factors. Because we used the changes in red meat consumption every 4 years as the exposure to predict the subsequent 4-year diabetes risk, we excluded men and women who had a history of diabetes (including type 1, type 2, and gestational diabetes), cardiovascular disease, or cancer 4 years after baseline (i.e., 1990 for the HPFS and NHS, and 1995 for the NHS II). In addition, we excluded participants who left more than 10 blank food items on the baseline food frequency questionnaire (FFQ), reported unusual total energy intake levels (i.e., <800 or >4200 kcal/d for men, and <500 or >3500 kcal/d for women), or did not report meat consumption. After exclusions, data from 26,357 HPFS men, 48,710 NHS women, and 74,077 NHS II women were available. Participants who were excluded due to missing baseline FFQ data were similar in age and body mass index (BMI) compared to those included in the analysis (data not shown). The study protocol was approved by the institutional review boards of Brigham and Women’s Hospital and Harvard School of Public Health.
Assessment of Meat Consumption
Dietary information was collected by a validated FFQ in 1986 for the HPFS and NHS and in 1991 for the NHS II, and was updated every 4 years with similar FFQs. In all FFQs, we asked participants how often, on average, they consumed each food of a standard portion size. Frequency responses ranged from “never or less than once per month” to “six or more times per day.” Questionnaire items on unprocessed red meat (85g, 3 oz) included “beef, pork, or lamb as main dish,” “hamburger,” and “beef, pork, or lamb as a sandwich or mixed dish.” Items on processed red meat included “bacon” (2 slides, 13 g), “hot dogs” (one, 45 g), and “sausage, salami, bologna, and other processed red meats” (1 piece, 28 g). The reproducibility and validity of FFQs have been demonstrated in detail elsewhere.6–8 Correlation coefficients between FFQ and multiple diet records ranged from 0.38 to 0.70 for various red meat items.7
Assessment of Covariates
In the follow-up questionnaires, we obtained updated information on risk factors for type 2 diabetes, such as body weight, cigarette smoking, physical activity, and a history of hypertension and hypercholesterolemia. We also ascertained menopausal status and postmenopausal hormone use in women. Alcohol intake was asked on the FFQ and updated every 4 years. We also collected information on family history of diabetes, race, and marital status. To assess overall diet quality, a diet score was calculated based on the 2010 Alternative Healthy Eating Index (AHEI-2010),9 which was designed to reflect food choices and nutrients associated with reduced chronic disease risk. For the current analysis, we constructed the AHEI score without the meat and alcohol components, because they were separately included in the models.
Assessment of Diabetes
Incident type 2 diabetes cases were identified by self-report on the main questionnaires every 2 years and confirmed by a validated supplementary questionnaire regarding symptoms, diagnostic tests, and treatment. The diagnosis was confirmed if at least one of the following was reported according to the National Diabetes Data Group criteria:10 1) one or more classic symptoms (excessive thirst, polyuria, weight loss, or hunger) plus fasting glucose levels ≥7.8 mmol/L or random glucose levels ≥11.1 mmol/L; 2) at least two elevated glucose concentrations on different occasions (fasting levels ≥7.8 mmol/L, random glucose levels ≥11.1 mmol/L, and/or concentrations ≥11.1 mmol/L after two hours or more by oral glucose tolerance testing) in the absence of symptoms; or 3) treatment with hypoglycemic medication (insulin or oral hypoglycemic agent). For cases diagnosed in 1998 and later, the fasting glucose threshold was lowered to 7.0 mmol/L according to the American Diabetes Association criteria.11
The validity of the supplementary questionnaire for diagnosis of type 2 diabetes has been documented previously: of 59 cases in HPFS and 62 cases in NHS confirmed by supplementary questionnaires, 57 (97%) and 61 (98%) cases were re-confirmed by medical records.12, 13 In another substudy to assess the prevalence of undiagnosed diabetes in NHS, only 1 out of 200 randomly-selected women had elevated fasting glucose or fructosamine levels barely above the diagnostic cutoffs.14 We exclude false-positive cases and only included incident cases confirmed by the supplemental questionnaires.
Statistical Analysis
We calculated each individual’s person-years from the date of return of baseline questionnaire to the date of diagnosis of diabetes, death, or the end of follow-up (January 31, 2006 for HPFS, June 30, 2006 for NHS, and June 30, 2007 for NHS II), whichever came first. We used change in red meat consumption updated every 4 years as a time-varying exposure, and time-dependent Cox proportional hazard regression was used to estimate the hazard ratio (HR) for diabetes risk in the subsequent 4 years. For example, we used changes in red meat consumption between 1986 and 1990 questionnaires to predict diabetes risk during the period from 1990 to 1994, and the changes between 1990 and 1994 questionnaires for the period from 1994 to 1998, so on so forth. In the multivariate analysis, in addition to age and calendar time, we simultaneously controlled for various potential confounding factors, including race (white, non-white), family history of diabetes (yes, no), marital status (with spouse, yes or no; updated every 4 years), history of hypertension and hypercholesterolemia (yes, no; updated every 4 years), and simultaneous changes in other lifestyle factors: smoking status (never to never, never to current, past to past, past to current, current to past, current to current, missing indicator), as well as initial and changes (all in quintiles) in alcohol intake, physical activity, energy intake, and diet quality (AHEI score). In NHS and NHS II, we also adjusted for postmenopausal status and menopausal hormone use. We have recently reported that increasing red meat consumption was related to weight gain in the three cohorts;5 therefore, body weight and weight gain could be mediators. We adjusted for initial BMI (<23, 23–24.9, 25–29.9, 30–34.9, ≥35 kg/m2) and changes in body weight (quintiles) in each 4-year period as time-varying covariates in an additional model. We also analyzed processed and unprocessed red meat separately.
In the second analysis, to examine long-term effects of red meat intake on diabetes, we analyzed changes in intake from baseline to the first 4-year follow-up and diabetes incidence in the subsequent follow-up years. Specifically, we used changes in red meat consumption between 1986 and 1990 to predict diabetes risk from 1990 to 2006 for the NHS and HPFS, and we used changes in red meat consumption between 1991 and 1995 to predict diabetes risk from 1995 to 2007 for the NHS II.
To minimize missing values during follow-up, we replaced missing values with carried-forward values for continuous variables, and added a missing indicator for categorical variables. Stratified analyses were performed a priori by initial BMI categories (<30.0 and ≥30.0 kg/m2), and the interaction was tested by including cross-product terms in the models. An inverse-variance-weighted, fixed-effect meta-analysis was used to combine the results across cohorts because no significant heterogeneity was found.
We conducted a series of sensitivity analyses to test the robustness of our results: 1) we stopped updating the dietary information after self-report of incident cardiovascular disease or cancer during the follow-up; 2) we censored participants at the time when they did not answer FFQs during the follow-up; 3) we used a multiple imputation procedure with 20 rounds of imputation and included all covariates to account for missing dietary and covariate data. All analyses were performed using SASsoftware, version 9.2 (SAS Institute, North Carolina), at a two-tailed P value of 0.05.
RESULTS
We documented a total of 7,540 incident type 2 diabetes cases during the follow-up (1,561 in the HPFS, 3,482 in the NHS, and 2,497 in the NHS II). Table 1 describes the distribution of baseline characteristics according to change in total red meat consumption. Compared to people with relatively stable intake, individuals who decreased or increased their intake were generally younger, had higher BMI levels, had a lower diet quality score, and were more likely to be smokers. Those who decreased intake were also more likely to report a diagnosis of hypertension or hypercholesterolemia. As expected, increasing red meat intake was related to concurrent weight gain, increases in energy intake, and decreases in dietary quality scores, while the associations with decreasing red meat intake were in the opposite directions.
Table 1.
Characteristics according to baseline 4-year changes in total red meat intake
| Variable | Changes in frequency of red meat consumption (categories in serving/d)
|
||||
|---|---|---|---|---|---|
| Moderate to large decrease (>0.50) | Small to moderate decrease (0.15–0.50) | No change/relatively stable (±0.14) | Small to moderate increase (0.15–0.50) | Moderate to large increase (>0.50) | |
| HPFS | |||||
| Number of participants, n | 6145 | 5427 | 6728 | 4484 | 3573 |
| Initial red meat intake, serving/d | 1.93 ± 0.89 | 1.17 ± 0.62 | 0.73 ± 0.63 | 0.89 ± 0.62 | 1.07 ± 0.67 |
| Age, year | 55.9 ± 8.9 | 56.2 ± 9.1 | 56.6 ± 9.2 | 56.0 ± 9.1 | 55.9 ± 9.0 |
| Initial body mass index, kg/m2 | 25.6 ± 3.1 | 25.2 ± 3.0 | 24.9 ± 2.9 | 25.2 ± 2.9 | 25.6 ± 3.2 |
| Weight change, kg | 0.23 ± 3.8 | 0.54 ± 3.4 | 0.68 ± 3.3 | 0.95 ± 3.4 | 1.2 ± 3.6 |
| Initial physical activity, MET-hr/wk | 19.6 ± 29.3 | 22.3 ± 30.6 | 24.3 ± 30.9 | 22.4 ± 32.8 | 19.1 ± 26.3 |
| Changes in physical activity, MET-hr/wk* | −0.8 ± 33.5 | −0.4 ± 32.4 | −0.8 ± 32.0 | −1.3 ± 33.2 | −0.9 ± 34.0 |
| Initial alcohol intake, g/d | 12.3 ± 16.0 | 11.8 ± 15.2 | 10.2 ± 13.8 | 12.0 ± 15.6 | 12.3 ± 16.3 |
| Changes in alcohol intake, g/d | −1.8 ± 9.7 | −1.5 ± 9.2 | −1.0 ± 8.5 | −1.1 ± 9.0 | −0.7 ± 10.1 |
| Race, white (%) | 96.0 | 95.9 | 95.3 | 96.4 | 95.7 |
| Marital status, with spouse (%) | 89.2 | 89.7 | 89.6 | 89.6 | 88.5 |
| Current smoker (%) | 7.8 | 6.7 | 5.9 | 8.1 | 11.1 |
| Hypertension (%) | 16.6 | 15.7 | 14.3 | 14.8 | 15.1 |
| High cholesterol (%) | 25.0 | 20.6 | 19.6 | 16.4 | 15.6 |
| Family history of diabetes (%) | 21.3 | 20.3 | 20.6 | 20.1 | 20.9 |
| Total energy intake, kcal/d | 2251 ± 603 | 2004 ± 571 | 1858 ± 558 | 1914 ± 571 | 2009 ± 592 |
| Change in energy intake, kcal/d | −361 ± 488 | −158 ± 440 | −47 ± 438 | 61 ± 439 | 278 ± 504 |
| AHEI score | 46.6 ± 9.5 | 49.2 ± 9.9 | 51.8 ± 10.5 | 50.1 ± 10.5 | 47.8 ± 10.2 |
| Change in AHEI score | 2.2 ± 8.4 | 0.5 ± 8.1 | −0.3 ± 8.0 | −1.0 ± 8.1 | −1.5 ± 8.2 |
|
| |||||
| NHS | |||||
| Number of participants, n | 11401 | 10965 | 12841 | 8450 | 5052 |
| Initial red meat intake, serving/d | 1.70 ± 0.69 | 1.04 ± 0.49 | 0.71 ± 0.49 | 0.77 ± 0.48 | 0.87 ± 0.49 |
| Age, year | 55.6 ± 7.1 | 55.8 ± 7.1 | 56.3 ± 7.0 | 56.0 ± 7.1 | 55.4 ± 7.1 |
| Initial body mass index, kg/m2 | 25.2 ± 4.8 | 24.9 ± 4.4 | 24.6 ± 4.3 | 24.9 ± 4.4 | 25.4 ± 4.8 |
| Weight change, kg | 0.6 ± 5.0 | 1.0 ± 4.5 | 1.2 ± 4.5 | 1.5 ± 4.5 | 1.9 ± 4.9 |
| Initial physical activity, MET-hr/wk | 12.9 ± 18.3 | 14.1 ± 20.1 | 15.8 ± 22.8 | 15.0 ± 22.3 | 13.1 ± 18.2 |
| Changes in physical activity, MET-hr/wk | 1.5 ± 15.2 | 1.5 ± 15.5 | 1.3 ± 16.3 | 1.1 ± 15.9 | 1.2 ± 15.6 |
| Initial alcohol intake, g/d | 6.5 ± 11.0 | 6.3 ± 10.4 | 6.3 ± 10.5 | 6.4 ± 10.7 | 6.5 ± 11.5 |
| Changes in alcohol intake, g/d | −1.4 ± 6.7 | −1.1 ± 6.2 | −1.0 ± 6.4 | −0.9 ± 6.4 | −0.6 ± 6.6 |
| Race, white (%) | 98.2 | 98.1 | 98.2 | 98.5 | 98.0 |
| Marital status, with spouse (%) | 94.8 | 94.4 | 93.4 | 94.4 | 94.9 |
| Current smoker (%) | 17.2 | 15.7 | 14.1 | 16.5 | 19.6 |
| Hypertension (%) | 22.4 | 21.9 | 21.7 | 22.6 | 24.1 |
| High cholesterol (%) | 35.8 | 35.5 | 33.9 | 30.9 | 28.8 |
| Family history of diabetes (%) | 29.0 | 27.9 | 28.0 | 28.3 | 29.0 |
| Menopausal status and postmenopausal hormone use | |||||
| Premenopausal (%) | 32.1 | 31.1 | 28.6 | 30.5 | 33.5 |
| Postmenopausal + never users (%) | 27.5 | 27.3 | 27.7 | 26.9 | 26.4 |
| Postmenopausal + past users (%) | 13.5 | 13.8 | 14.5 | 14.0 | 13.2 |
| Postmenopausal + current users (%) | 24.8 | 25.9 | 27.3 | 26.7 | 24.5 |
| Missing information (%) | 2.2 | 1.8 | 2.0 | 1.9 | 2.4 |
| Total energy intake, kcal/d | 1990 ± 519 | 1759 ± 492 | 1646 ± 488 | 1678 ± 493 | 1750 ± 506 |
| Change in energy intake, kcal/d | −273 ± 442 | −80 ± 396 | 26 ± 390 | 137 ± 398 | 320 ± 443 |
| AHEI score | 45.6 ± 9.4 | 47.7 ± 9.8 | 49.6 ± 10.2 | 48.2 ± 10.1 | 46.9 ± 9.8 |
| Change in AHEI score | 1.8 ± 8.6 | 0.7 ± 8.3 | −0.3 ± 8.0 | −0.8 ± 8.1 | −1.5 ± 8.4 |
|
| |||||
| NHS II | |||||
| Number of participants, n | 16532 | 18900 | 21667 | 10954 | 6024 |
| Initial red meat intake, serving/d | 1.61 ± 0.66 | 0.92 ± 0.45 | 0.60 ± 0.63 | 0.69 ± 0.47 | 0.78 ± 0.48 |
| Age, year | 40.1 ± 4.6 | 40.3 ± 4.6 | 40.3 ± 4.6 | 40.0 ± 4.7 | 39.8 ± 4.7 |
| Initial body mass index, kg/m2 | 25.0 ± 5.4 | 24.3 ± 4.9 | 23.9 ± 4.7 | 24.4 ± 5.1 | 25.2 ± 5.6 |
| Weight change, kg | 2.6 ± 6.1 | 2.9 ± 5.8 | 3.2 ± 5.7 | 3.9 ± 6.0 | 4.7 ± 6.8 |
| Initial physical activity, MET-hr/wk | 18.6 ± 24.0 | 20.2 ± 25.6 | 23.5 ± 30.6 | 20.3 ± 26.5 | 18.9 ± 24.9 |
| Changes in physical activity, MET-hr/wk | −1.1 ± 20.8 | −1.7 ± 21.1 | −2.8 ± 23.2 | −2.5 ± 21.8 | 3.1 ± 21.3 |
| Initial alcohol intake, g/d | 3.0 ± 6.0 | 3.2 ± 6.0 | 3.2 ± 6.0 | 3.2 ± 6.1 | 3.0 ± 6.2 |
| Changes in alcohol intake, g/d | 0.2 ± 4.4 | 0.3 ± 4.4 | 0.5 ± 4.5 | 0.5 ± 4.6 | 0.5 ± 4.9 |
| Race, white (%) | 96.9 | 97.3 | 96.9 | 96.9 | 96.4 |
| Marital status, with spouse (%) | 86.8 | 85.9 | 81.6 | 83.5 | 83.2 |
| Current smoker (%) | 11.4 | 10.0 | 9.2 | 10.6 | 13.2 |
| Hypertension (%) | 9.5 | 8.1 | 8.1 | 9.1 | 10.4 |
| High cholesterol (%) | 21.3 | 19.7 | 19.3 | 21.3 | 23.3 |
| Family history of diabetes (%) | 36.5 | 34.9 | 33.4 | 34.2 | 36.1 |
| Menopausal status and postmenopausal hormone use | |||||
| Premenopausal (%) | 90.9 | 91.6 | 91.3 | 91.0 | 90.3 |
| Postmenopausal + never users (%) | 0.5 | 0.4 | 0.5 | 0.4 | 0.5 |
| Postmenopausal + past users (%) | 0.7 | 0.7 | 0.7 | 0.9 | 0.8 |
| Postmenopausal + current users (%) | 5.9 | 5.4 | 5.4 | 5.7 | 6.3 |
| Missing information (%) | 2.1 | 2.0 | 2.0 | 2.0 | 2.1 |
| Total energy intake, kcal/d | 2045 ± 537 | 1773 ± 503 | 1659 ± 508 | 1674 ± 504 | 1730 ± 512 |
| Change in energy intake, kcal/d | −255 ± 481 | −46 ± 432 | 69 ± 439 | 236 ± 441 | 476 ± 488 |
| AHEI score | 41.7 ± 8.8 | 44.0 ± 9.1 | 46.1 ± 9.7 | 44.4 ± 9.5 | 43.3 ± 9.4 |
| Change in AHEI score | 1.9 ± 7.9 | 0.6 ± 7.8 | −0.2 ± 7.9 | −0.6 ± 7.9 | −1.6 ± 8.0 |
Abbreviations: AHEI, Alternate Healthy Eating Index; HPFS, Health Professional Follow-up Study; MET, Metabolic Equivalent of Task; NHS, Nurses’ Health Study.
Table 2 shows the HRs of diabetes according to changes in total red meat consumption. Compared to individuals whose intake remained relatively stable in each 4-year period, those who increased their red meat intake were at elevated risks (P <0.001 in all three cohorts): increasing red meat intake of >0.5 serving/d was associated with a 48% (pooled HR, 1.48; 95% CI, 1.37–1.59) elevated risk in the subsequent 4-year period, and the association was modestly attenuated after further adjustment for initial BMI and concurrent weight gain (pooled HR, 1.30; 95% CI, 1.21–1.41). A moderate increase (0.15–0.50 serving/d) in red meat intake was also associated with an elevated risk: the corresponding pooled HR was 1.21 (95% CI, 1.13–1.30) and 1.15 (95% CI, 1.07–1.23) before and after adjustment for initial BMI and concurrent weight gain, respectively. The associations were greater for processed than unprocessed red meat (eTable 1).
Table 2.
Hazard ratios of type 2 diabetes according to updated 4-year changes in total red meat intakea
| Changes in frequency of red meat consumption (categories in serving/d)
|
P for trendb | |||||
|---|---|---|---|---|---|---|
| Moderate to large decrease (>0.50) | Small to moderate decrease (0.15–0.50) | No change/relatively stable (±0.14) | Small to moderate increase (0.15–0.50) | Moderate to large increase (>0.50) | ||
| HPFS | ||||||
| Cases/person-years | 336/69097 | 255/74221 | 458/133862 | 248/60190 | 264/44372 | |
| Multivariate model 1c | 1.06 (0.89–1.27) | 0.92 (0.78–1.09) | 1.00 | 1.21 (1.03–1.43) | 1.59 (1.34–1.88) | <0.001 |
| Multivariate model 2d | 1.08 (0.90–1.29) | 0.94 (0.80–1.12) | 1.00 | 1.20 (1.01–1.41) | 1.48 (1.25–1.75) | 0.001 |
|
| ||||||
| NHS | ||||||
| Cases/person-years | 658/128173 | 770/168022 | 1061/246411 | 587/117130 | 406/62021 | |
| Multivariate model 1c | 0.90 (0.80–1.01) | 0.96 (0.87–1.06) | 1.00 | 1.16 (1.05–1.28) | 1.36 (1.21–1.53) | <0.001 |
| Multivariate model 2d | 0.95 (0.84–1.07) | 0.98 (0.89–1.08) | 1.00 | 1.10 (0.99–1.21) | 1.22 (1.08–1.38) | <0.001 |
|
| ||||||
| NHS II | ||||||
| Cases/person-years | 466/141889 | 433/179136 | 682/296201 | 452/146734 | 464/98366 | |
| Multivariate model 1c | 1.00 (0.87–1.15) | 0.98 (0.86–1.11) | 1.00 | 1.30 (1.15–1.47) | 1.55 (1.37–1.76) | <0.001 |
| Multivariate model 2d | 1.04 (0.90–1.19) | 0.98 (0.86–1.11) | 1.00 | 1.20 (1.06–1.36) | 1.31 (1.16–1.49) | <0.001 |
|
| ||||||
| Poolede | ||||||
| Multivariate model 1c | 0.96 (0.89–1.04) | 0.96 (0.89–1.03) | 1.00 | 1.21 (1.13–1.30) | 1.48 (1.37–1.59) | <0.001 |
| Multivariate model 2d | 1.00 (0.92–1.09) | 0.97 (0.91–1.04) | 1.00 | 1.15 (1.07–1.23) | 1.30 (1.21–1.41) | <0.001 |
Data are based on 20 years of follow-up (1986–2006) in the Health Professionals Follow-up Study (HPFS), 20 years of follow-up (1986–2006) in the Nurses’ Health Study (NHS), and 16 years of follow-up (1991–2007) in the Nurses’ Health Study II (NHS II). The exposure was change in red meat intake in each 4-year period, and the outcome was the incidence of type 2 diabetes in the subsequent 4 years.
P value for trend was derived from tests of linear trend across categories of changes in red meat consumption by treating the median value of the each category as a continuous variable.
Multivariate Model 1: adjusted for age, initial red meat intake (quintiles), race (white, non-white), marital status (with spouse, yes or not), family history of diabetes (yes or not), history of hypertension (yes or not), history of hypercholesterolemia (yes or not), and simultaneous changes in other lifestyle factors: smoking status (never to never, never to current, past to past, past to current, current to past, current to current, missing indicator), initial and change in alcohol intake (quintiles), initial and change in physical activity (quintiles), initial and change in total energy intake (quintiles), as well as initial and change in diet quality (Alternative Healthy Eating Index, quintiles). Among nurses, postmenopausal status and menopausal hormone use (NHS and NHS II) were also included.
Multivariate Model 2: model 1 plus initial BMI (<23, 23–24.9, 25–29.9, 30–34.9, ≥35 kg/m2) and weight change (quintiles) during the 4-year period.
The results across the three cohorts were pooled using fixed-effect meta-analysis.
No significant decreased diabetes risk was found with reduction of red meat intake within a 4-year period (Table 2). However, when we used reduction in red meat consumption from baseline to the first 4-year follow-up as the exposure to predict future risk of diabetes during the whole follow-up (instead of just the subsequent 4 years), we observed that a reduction of red meat intake >0.5 serving/d was associated with a 14% (pooled HR, 0.86; 95% CI, 0.80–0.93) and 10% (pooled HR, 0.90; 95% CI, 0.83–0.97) lower risk before and after adjustment for initial BMI plus concurrent weight gain, respectively (Table 3).
Table 3.
Hazard ratios of type 2 diabetes during 12 (NHS II) and 16 years (NHS and HPFS) of follow-up, according to initial 4-years changes in total red meat categoriesa
| Changes in frequency of red meat consumption (categories in serving/d)
|
P for trendb | |||||
|---|---|---|---|---|---|---|
| Moderate to large decrease (>0.50) | Small to moderate decrease (0.15–0.50) | No change /relatively stable (±0.14) | Small to moderate increase (0.15–0.50) | Moderate to large increase (>0.50) | ||
| HPFS | ||||||
| Cases/person-years | 410/88993 | 320/79063 | 326/98626 | 277/64893 | 274/50940 | |
| Multivariate model 1c | 0.91 (0.77–1.09) | 1.06 (0.90–1.24) | 1.00 | 1.24 (1.05–1.47) | 1.40 (1.17–1.66) | <0.001 |
| Multivariate model 2d | 0.94 (0.78–1.11) | 1.06 (0.90–1.25) | 1.00 | 1.22 (1.03–1.44) | 1.30 (1.09–1.55) | <0.001 |
|
| ||||||
| NHS | ||||||
| Cases/person-years | 894/168326 | 769/162814 | 840/191145 | 585/125816 | 455/73919 | |
| Multivariate model 1c | 0.82 (0.73–0.91) | 0.92 (0.83–1.01) | 1.00 | 1.03 (0.92–1.14) | 1.25 (1.11–1.41) | <0.001 |
| Multivariate model 2d | 0.86 (0.77–0.97) | 0.91 (0.83–1.01) | 1.00 | 0.98 (0.88–1.09) | 1.12 (1.00–1.27) | 0.002 |
|
| ||||||
| NHS II | ||||||
| Cases/person-years | 658/191832 | 579/220505 | 592/253202 | 397/127237 | 303/69634 | |
| Multivariate model 1c | 0.90 (0.79–1.03) | 0.95 (0.84–1.07) | 1.00 | 1.21 (1.06–1.38) | 1.34 (1.16–1.55) | <0.001 |
| Multivariate model 2d | 0.93 (0.82–1.07) | 0.97 (0.86–1.09) | 1.00 | 1.12 (0.99–1.28) | 1.16 (1.01–1.34) | 0.01 |
|
| ||||||
| Poolede | ||||||
| Multivariate model 1c | 0.86 (0.80–0.93) | 0.95 (0.89–1.02) | 1.00 | 1.13 (1.05–1.21) | 1.31 (1.21–1.42) | <0.001 |
| Multivariate model 2d | 0.90 (0.83–0.97) | 0.96 (0.89–1.03) | 1.00 | 1.07 (0.99–1.15) | 1.17 (1.08–1.27) | <0.001 |
Data are based on 20 years of follow-up (1986–2006) in the Health Professionals Follow-up Study (HPFS), 20 years of follow-up (1986–2006) in the Nurses’ Health Study (NHS), and 16 years of follow-up (1991–2007) in the Nurses’ Health Study II (NHS II). The exposure was change in red meat intake in the baseline 4-year period (1986 to 1990 in the HPFS and NHS, and 1991 to 1995 in the NHS II), and the outcome was the incidence of type 2 diabetes in the subsequent follow-up years (1990 to 2006 in the HPFS and NHS, and 1995 to 2007 in the NHS II).
P value for trend was derived from tests of linear trend across categories of changes in red meat consumption by treating the median value of the each category as a continuous variable.
Multivariate Model 1: adjusted for age, initial red meat intake (quintiles), race (white, non-white), marital status (with spouse, yes or not), family history of diabetes (yes or not), history of hypertension (yes or not), history of hypercholesterolemia (yes or not), and simultaneous changes in other lifestyle factors: smoking status (never to never, never to current, past to past, past to current, current to past, current to current, missing indicator), initial and change in alcohol intake (quintiles), initial and change in physical activity (quintiles), initial and change in total energy intake (quintiles), as well as initial and change in diet quality (Alternative Healthy Eating Index, quintiles). Among nurses, postmenopausal status and menopausal hormone use (NHS and NHS II) were also included.
Multivariate Model 2: model 1 plus initial BMI (<23, 23–24.9, 25–29.9, 30–34.9, ≥35 kg/m2) and weight change (quintiles) during the 4-year period.
The results across the three cohorts were pooled using fixed-effect meta-analysis.
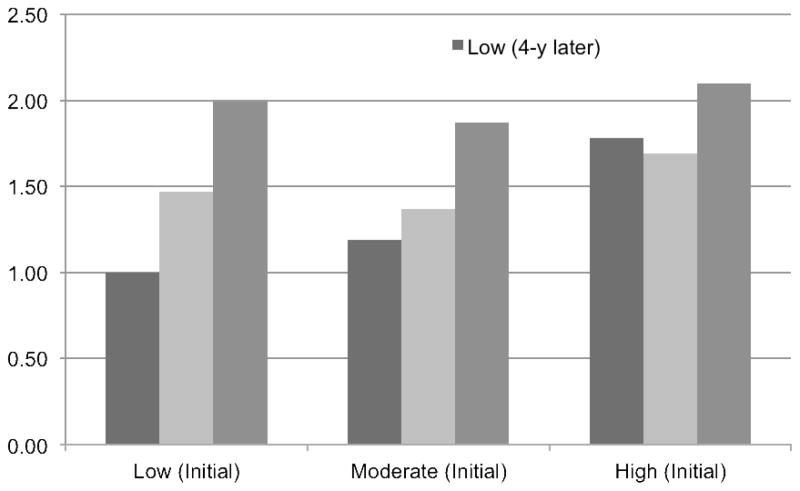
Figure 1 and eTable 2 show results based on the initial and 4-year later intake of red meat. Compared with stable low-level consumers (<2 servings/wk; reference group), individuals who increased their red meat intake from low to high levels had an almost two-fold risk (pooled HR, 1.99; 95% CI, 1.53–2.58). The pooled HR was 1.37 (95% CI, 1.22–1.53) for stable moderate-level consumers, and it was elevated to 1.87 (1.65–2.12) if increasing intake from moderate to high levels, while decreased to 1.19 (1.02–1.38) if reducing intake from moderate to low levels. Compared with the reference group, the pooled HR was 2.10 (95% CI, 1.87–2.37) for stable high-level consumers, and it was decreased to 1.69 (1.49–1.92) and 1.78 (1.40–2.27) if intake was reduced from high to moderate or low levels 4 years later, respectively. All estimates were attenuated after adjustment for initial BMI and concurrent weight changes (eTable 2).
Figure 1. Hazard ratios of type 2 diabetes according to updated 4-year changes in total red meat intake.
The low intake level was defined as <2 servings/wk, and moderate intake level was defined as 2–6 servings/wk, and high intake level was defined as ≥7 servings/wk. The reference group (hazard ratio=1.00) was the low intake level both at initial and 4-y later follow-up visit.
The analysis adjusted for age, race (white, non-white), marital status (with spouse, yes or not), family history of diabetes (yes or not), history of hypertension (yes or not), history of hypercholesterolemia (yes or not), and simultaneous changes in other lifestyle factors: smoking status (never to never, never to current, past to past, past to current, current to past, current to current, missing indicator), initial and change in alcohol intake (quintiles), initial and change in physical activity (quintiles), initial and change in total energy intake (quintiles), as well as initial and change in diet quality (Alternative Healthy Eating Index, quintiles). Among nurses, postmenopausal status and menopausal hormone use (NHS and NHS II) were also included.
The results across the three cohorts were pooled using fixed-effect meta-analysis.
We observed significant interaction between initial BMI and changes in red meat intake in relation to risk of type 2 diabetes (eTable 3). Compared with stable consumption, increasing intake >0.5 serving/d within a 4-year period was associated with a 65% (pooled HR, 1.65; 95% CI, 1.48–1.84) elevated risk of developing type 2 diabetes in the subsequent 4-year interval among non-obese individuals; while the corresponding pooled HR was 1.14 (95% CI, 1.02–1.27) among obese individuals.
The results were robust in various sensitivity analyses: compared to stable red meat consumption, the pooled HR was 1.38 (95% CI, 1.28–1.49) for increasing red meat intake >0.5 serving/d within a 4-year period when we stopped updating dietary information after self-reported cardiovascular disease or cancer (eTable 4), 1.45 (95% CI, 1.34–1.57) when we censored participants without dietary information during follow-up (eTable 5), and 1.41 (95% CI, 1.30–1.52) when we used multiple imputation method for the missing data during the follow-up (eTable 6). Again, all estimates were modestly attenuated after adjustment for initial BMI and concurrent weight changes.
DISCUSSION
In these three large prospective cohorts of US men and women, we found that 4-year increases in red meat consumption were positively associated with subsequent 4-year risk of type 2 diabetes, independent of initial red meat intake and changes in other lifestyle factors, including overall dietary quality and body weight. This association was observed for both unprocessed and processed red meat. Decreasing red meat intake was not associated with an acute but rather with a prolonged reduced risk of type 2 diabetes.
Three meta-analyses of prospective cohort studies have reported a positive association between red meat intake and diabetes.1–3 However, most previous studies evaluated the relation between meat intake at baseline and diabetes risk with limited information during follow-up. Because individuals’ eating behaviors may change over time,4 a single time measurement may not capture the variability of red meat intake during follow-up. To the best of our knowledge, this study is the first to investigate the association between changes in red meat intake and subsequent diabetes risk. Our results are largely consistent with previous reports, but extend the findings to suggest that increasing red meat intake is followed by an elevated risk of diabetes in a short-term (4 years) as well as long-term period (12–16 years).
Our previous analysis in the three cohorts found that red meat intake was associated with an increased risk of type 2 diabetes.3 However, that analysis did not take into account changes in red meat intake.3 An important finding from our analysis is that both initial red meat intake (data not shown, but results were similar to our previous paper3) and changes in red meat intake were independently related to an elevated risk of diabetes. Joint analysis of initial and 4-year later red meat intake confirmed that consistent high intake was related to a greater risk of type 2 diabetes compared to a consistent low level, and diabetes risk was elevated quickly and substantially (almost two-fold) when increasing intake from low to high levels. Changing from high to low levels did not completely mitigate the increased risk within 4 years for people with initial high red meat intake; however, the analysis of change during the first four years in relation to diabetes during the entire follow-up period suggests that reduction of red meat intake still has a long-term benefit. The absence of a short-term reduction in risk of type 2 diabetes may be the result of higher-risk patients (those with lipid disorders, hypertension, or other cardiometabolic risk factors) being most likely to be counseled by their healthcare providers to reduce red meat consumption.
In the present study, adjustment for BMI modestly attenuated the association between red meat intake and diabetes risk, suggesting that the association between red meat intake and diabetes risk may be partly mediated through obesity and weight gain. In our cohorts5 and a large European cohort,15 red meat intake was positively associated with future risk of weight gain. Furthermore, we observed a significant interaction with initial BMI, and the association was much stronger among non-obese individuals compared with obese people. This is consistent with the recent EPIC-InterAct study in European populations,16 although that study only used baseline information. It is possible that obese individuals are already at a very high risk of diabetes because of their body weight and higher initial red meat intake (data not shown), and increasing red meat intake has only a modestly deleterious impact on the relative scale. However, the absolute risk associated with red meat intake among obese individuals is much greater, and thus limiting red meat intake among obese individuals is still beneficial.
Since our study is observational in nature, causality cannot be inferred. Randomized clinical trials may better address the causal relation between red meat and type 2 diabetes, but may not be feasible. Our “change-to-risk” analysis method capitalizes on repeated measurements and long-term follow-up. Our analysis approach is, to some extent, a natural experiment, where individuals choose to change their diet and lifestyles without investigator-initiated interventions, and thus, the results may be more externally generalizable to the real world compared to a well-controlled laboratory setting. We do not know the underlying reasons why people increased or decreased their red meat intake. Some people may decrease intake due to health concerns, particularly if they are at high risk of cardiovascular disease. This may explain the lack of association between reduced red meat intake and diabetes risk in the subsequent 4 years. The analysis using the initial 4-year change in red meat intake as the exposure, however, showed a significantly decreased risk during subsequent long-term follow-up, suggesting that it may take longer for the benefits of reducing red meat intake to be manifested.
The strengths of the current study include a large sample size, high follow-up rates, and repeated assessments of dietary and lifestyle variables during a long-time period. Therefore, our cohorts are among the very few studies that are able to investigate changes in red meat intake and subsequent risk of diabetes. The consistency of the results across all three cohorts indicates that our findings are unlikely due to chance.
We are also aware of several limitations. First, our study populations primarily consisted of white educated US adults. Although the homogeneity of socioeconomic status helps reduce confounding, it may potentially limit generalizability. Second, some measurement errors in dietary assessment are inevitable. However, because of a prospective study design, the measurement errors are more likely to attenuate associations towards the null. Third, the FFQs were administrated every 4 years and we do not know the exact time when the changes in red meat intake occurred within that 4-year period. Lastly, changes in red meat intake may be a marker of lifestyle changes, but we have simultaneously adjusted for initial and changes of multiple diet and lifestyle factors, and our previous analysis suggested a very modest correlation among changes in different dietary and behavioral factors.5 However, residual and unmeasured confounding from other lifestyle behaviors is still possible.
In conclusion, in these three cohorts of US adults, we found that increases in red meat intake within a 4-year period were associated with a higher risk of type 2 diabetes in the subsequent 4-year interval. In addition, reduction in red meat intake was associated with lower incidence of diabetes during subsequent long-term follow-up. Our results confirm the robustness of the association between red meat and type 2 diabetes, and add further evidence that limiting red meat consumption over time confers benefits for diabetes prevention.
Supplementary Material
Acknowledgments
We are indebted to participants in the Health Professionals Follow-up Study, Nurses’ Health Study and Nurses’ Health Study II for their continuing outstanding support and colleagues working in these studies for their valuable help.
Funding/Support: The study was supported by the National Institutes of Health grants (P01CA087969, R01CA050385, U19CA055075, R01DK058845, P30DK046200, and U54CA155626). Dr. Sun was supported by a career development award K99HL098459 from the National Heart, Lung, and Blood Institute. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Role of the Sponsor: None of the funding sponsors was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Disclosures: None of the authors had any financial or personal conflict of interest to disclose.
Author Contributions: Drs. Pan and Hu have full access to the data in this study and take complete responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Pan, Willett, Hu.
Acquisition of data: Pan, Manson, Willett, Hu.
Analysis and interpretation of data: Pan, Sun, Bernstein, Manson, Willett, Hu.
Drafting of the manuscript: Pan.
Critical revision of the manuscript for important intellectual content: Sun, Bernstein, Manson, Willett, Hu.
Statistical analysis: Pan.
Obtaining funding and Study supervision: Manson, Willett, Hu.
Administrative, technical, or material support: Manson, Willett, Hu.
Final approval: Pan, Sun, Bernstein, Manson, Willett, Hu.
References
- 1.Aune D, Ursin G, Veierod MB. Meat consumption and the risk of type 2 diabetes: a systematic review and meta-analysis of cohort studies. Diabetologia. 2009;52(11):2277–2287. doi: 10.1007/s00125-009-1481-x. [DOI] [PubMed] [Google Scholar]
- 2.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation. 2010;121(21):2271–2283. doi: 10.1161/CIRCULATIONAHA.109.924977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan A, Sun Q, Bernstein AM, et al. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr. 2011;94(4):1088–1096. doi: 10.3945/ajcn.111.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daniel CR, Cross AJ, Koebnick C, Sinha R. Trends in meat consumption in the USA. Public Health Nutr. 2011;14(4):575–583. doi: 10.1017/S1368980010002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364(25):2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 7.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18(4):858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 8.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93(7):790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 9.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–1018. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 11.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 12.Manson JE, Rimm EB, Stampfer MJ, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991;338(8770):774–778. doi: 10.1016/0140-6736(91)90664-b. [DOI] [PubMed] [Google Scholar]
- 13.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med. 2001;161(12):1542–1548. doi: 10.1001/archinte.161.12.1542. [DOI] [PubMed] [Google Scholar]
- 14.Field AE, Coakley EH, Must A, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161(13):1581–1586. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- 15.Vergnaud AC, Norat T, Romaguera D, et al. Meat consumption and prospective weight change in participants of the EPIC-PANACEA study. Am J Clin Nutr. 2010;92(2):398–407. doi: 10.3945/ajcn.2009.28713. [DOI] [PubMed] [Google Scholar]
- 16.The InterAct consortium. Association between dietary meat consumption and incident type 2 diabetes: the EPIC-InterAct study. Diabetologia. 2013;56(1):47–59. doi: 10.1007/s00125-012-2718-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.