Abstract
The ability to selectively and efficiently target transgene delivery to specific cell types in vitro and in vivo remains one of the formidable challenges in gene therapy. Lentiviral vectors have several advantages that make them attractive as gene delivery vehicles and their tropism can be altered through pseudotyping, allowing transgene delivery to specific populations of cells. The human interleukin-13 receptor α2 (IL-13Rα2) is uniquely overexpressed in many different human tumors, making it an attractive target for cancer therapy. In this study, we examined whether IL-13Rα2-positive tumor cells can be specifically targeted with lentiviral vector pseudotypes containing a truncated fusion (F) protein derived from measles virus (MV) and a tail-truncated and receptor-blind MV hemagglutinin (H) protein bearing IL-13 at the C terminus. The retargeted lentiviral vector efficiently transduced cells that express high levels of IL-13Rα2, but not cells expressing low levels of IL-13Rα2 in vitro. In vivo, it specifically targeted IL-13Rα2-positive glioma cell xenografts in immunodeficient mice in the context of subcutaneous and intracranial glioma models. Similar lentiviral vectors may be developed for targeting other tumors expressing specific cell surface receptors.
Ou and colleagues develop lentiviral vectors that bear measles virus (MV) glycoproteins and human interleukin (IL)-13 to test whether such vectors can specifically target IL-13 receptor α2 (IL-13Rα2)-positive tumor cells. They show that the retargeted lentiviral vectors efficiently transduce IL-13Rα2-positive cells in vitro, and that they specifically target IL-13Rα2-positive glioma cell xenografts in the context of subcutaneous and intracranial glioma mouse models in vivo.
Introduction
Retroviral vectors based on gammaretroviruses and lentiviruses provide powerful tools for gene delivery in vitro, ex vivo, and in vivo (D'Costa et al., 2009; Matrai et al., 2010; Dropulic, 2011). To exploit the full therapeutic potential of retroviral vectors, they must be capable of transducing target cells while avoiding impacting nontarget cells. A major technical challenge in facilitating the efficient transduction of the desired target cells by viral vectors is that the native tropism of the virus often does not meet the therapeutic need (Waehler et al., 2007). To bypass this issue, the host range of retroviral vectors including that of lentiviral vectors can be altered by a process known as pseudotyping. Pseudotyped retroviral vectors consist of vector particles bearing envelope (Env) glycoproteins derived from other enveloped viruses. Such particles possess the tropism of the virus from which the glycoprotein was originally derived (Cronin et al., 2005).
The development of lentiviral vectors that display a reduced tropism for the natural receptor and an increased specificity for a chosen receptor to allow targeted transduction of specific cell types in vitro and in vivo has been challenging, due to a number of issues such as targeting specificity and vector titers (Frecha et al., 2008). A novel strategy for lentiviral vector targeting based on engineered measles virus (MV) H and F glycoproteins has emerged (Funke et al., 2008). This strategy is promising because of its flexibility and because background transduction in nontarget cells is low (Funke et al., 2008; Anliker et al., 2010; Munch et al., 2011). The native H protein mediates attachment to the viral receptor CD46, signaling lymphocytic activation molecule (SLAM; Yanagi et al., 2006), or Nectin-4 (Muhlebach et al., 2011) on the cell surface, and signals to the F protein to trigger cell fusion. The steps required to retarget this cell fusion reaction are ablation of H protein-mediated CD46 and SLAM receptor recognition and introduction of a new binding specificity in the H glycoprotein (Nakamura et al., 2004). Various ligands such as epidermal growth factor (EGF) (Funke et al., 2008), single-chain antibodies (Anliker et al., 2010), and designed ankyrin repeat proteins (DARPins) (Munch et al., 2011) have been successfully displayed on MV H, allowing retargeted lentiviral vector delivery to the corresponding target cells.
The flexibility of the H glycoprotein to accommodate cell-targeting ligands was also demonstrated in the context of other viruses. For example, Allen and colleagues (2008) successfully made a retargeted recombinant MV displaying interleukin (IL)-13, fused to the C terminus of the H protein, and demonstrated specific targeting to human IL-13 receptor α2 (IL-13Rα2)-positive cells both in vitro and in vivo. IL-13Rα2 is an appealing target because it is overexpressed in various types of tumors, including but not limited to glioblastoma, ovarian cancer, pancreatic cancer, prostate cancer, renal cell carcinoma, head and neck cancer, AIDS-Kaposi's sarcoma, and oral squamous cell carcinoma (Joshi and Puri, 2009).
The purpose of our study was to develop lentiviral vectors that bear MV glycoproteins and human IL-13 to test whether such vectors can specifically target IL-13Rα2-positive tumor cells in vitro and in vivo.
Materials and Methods
Plasmid constructs
The pSLIK-Neo/TRE Pitt-IL-13Rα2 lentiviral vector plasmid was constructed as follows: The TRE Pitt promoter fragment (Pluta et al., 2005) derived from pLitTREPitt (Addgene plasmid #18661) was subcloned into pENTR2B (Invitrogen, Carlsbad, CA). A cDNA fragment encoding human IL-13Rα2, derived from pSPORT6/hIL-13Rα2 (Open Biosystems, Huntsville, AL), was inserted downstream of the TRE Pitt promoter, resulting in pENTR2B/TRE Pitt/IL-13Rα2. The final pSLIK-Neo/TRE Pitt-IL-13Rα2 plasmid was generated by Gateway recombination (Invitrogen) between the pENTR2B/TRE Pitt/IL-13Rα2 entry plasmid and the pSLIK-Neo destination vector (Addgene plasmid #25735) (Shin et al., 2006), using LR Clonase II (Invitrogen). The pNL(CMV)EGFP/CMV/WPREΔU3 plasmid was derived from the pNL-EGFP/CMV/WPREΔU3 plasmid (Ricks et al., 2008). It bears a hybrid 5′ long terminal repeat (LTR) that was derived from pHIV dlκB SP1 CMV IE(a) (Chang et al., 1993). The pNL(CMV)Fluc/CMV/WPREΔU3 plasmid was derived from pNL(CMV)EGFP/CMV/WPREΔU3 by replacing the enhanced green fluorescent protein (EGFP) coding region with a PCR fragment encoding firefly luciferase (Fluc); the PCR fragment was generated with the pGL4.20-Luc2/Puro plasmid (Promega, Madison, WI) as a template. The pNL(CMV)GLuc/CMV/WPREΔU3 lentiviral vector plasmid encoding membrane-bound Gaussia luciferase (GLuc) was generated by Gateway recombination between the pENTR2B/CD8-SP-GLuc-TM entry plasmid and the pNL(CMV)DEST/CMV/WPREΔU3 destination vector. The CD8-SP-TM sequence encoding the CD8 leader peptide and the CD8 transmembrane domain (Santos et al., 2009) was synthesized by GenScript USA (Piscataway, NJ). A fragment encoding humanized GLuc lacking its native signal peptide (NanoLight Technology, Pinetop, AZ) was inserted in-frame between the CD8 leader peptide and the CD8 transmembrane domain.
The pCD/NL-BHΔ1 packaging plasmid was derived from pCD/NL-BH*ΔΔΔ (Zhang et al., 2004). It contains the Gag- and Pol-coding regions but lacks functional Tat-, Rev-, and accessory protein-encoding sequences.
The pCG-FcΔ30 plasmid encoding the MV Edmonston strain F glycoprotein, bearing a 30-amino acid truncation at the cytoplasmic tail, was described previously (Moll et al., 2002). Plasmid pCG-HcΔ20-AA-IL-13 encodes MV virus Edmonston strain-derived hemagglutinin (H) fused to IL-13 (Allen et al., 2008). Amino acids Y481 and R533 of the H protein were mutated to alanine to ablate the ability of the protein to bind to the native MV receptors CD46 and SLAM (Nakamura et al., 2004). The pCG-HcΔ20-AA-IL-13 plasmid was constructed by subcloning a 1774-bp NsiI–SpeI fragment derived from pCG-HAA-IL-13-H6 (Allen et al., 2008) into pCG-HcΔ20 (Moll et al., 2002). Plasmid pCG-HcΔ18-AA-IL-13 was derived from the pCG-HcΔ20-AA-IL-13 plasmid by further truncation of the cytoplasmic tail-encoding sequence, using a synthetic DNA fragment prepared by GenScript USA. The pCG-HcΔ18-AA plasmid lacks the IL-13-coding region. It was derived from pCG-HcΔ18-AA-IL-13 by deletion of the IL-13-encoding XmaI–SpeI fragment and substituting for it a synthetic DNA fragment encoding a hexahistidine (His6) tail. The pCEF-VSV-G plasmid contains a hybrid CEF promoter (human cytomegalovirus enhancer combined with human EF-1α promoter) (Kuroda et al., 2011) and bears a vesicular stomatitis virus G protein (VSV-G)-coding region, derived from pLTR-G (Reiser et al., 1996).
Cell lines
Human embryonic kidney 293T (CRL-11268; American Type Culture Collection [ATCC], Manassas, VA) cells and human osteosarcoma (HOS) (CRL-1543; ATCC) cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Lonza, Walkersville, MD) containing 10% heat-inactivated fetal bovine serum (FBS), 2.5 mM l-glutamine, penicillin (100 U/ml), and streptomycin (100 μg/ml) (all from Invitrogen, Grand Island, NY). HOS-IL-13Rα2 cells were generated by transduction of HOS cells with the pSLIK-Neo/TRE Pitt-IL-13Rα2 lentiviral vector and selection in medium containing G418 (500 μg/ml). Pooled cell clones were induced with doxycycline (Dox, 1 μg/ml), stained with a phycoerythrin (PE)-conjugated mouse anti-human IL-13Rα2 antibody (Diaclone, Besançon, France), and sorted for cells expressing high levels of IL-13Rα2.
Human U251 glioblastoma cells (National Cancer Institute [NCI], Frederick, MD) were cultured in RPMI 1640 medium. U87 glioma cells (U87; ATCC) and T98G glioblastoma multiforme cells (CRL-1690; ATCC) were cultured in Eagle's minimal essential medium (EMEM). Both RPMI 1640 and EMEM were supplemented with 10% FBS, 25 mM HEPES, 0.1 mM nonessential amino acids (NEAAs), 2 mM glutamine, 1 mM sodium pyruvate, penicillin (100 U/ml), and streptomycin (100 μg/ml) (Invitrogen). DU145 human prostate carcinoma cells (HTB-81; ATCC) were cultured in EMEM supplemented with 10% heat-inactivated FBS, 2 mM glutamine, penicillin (100 U/ml), and streptomycin (100 μg/ml) (Invitrogen).
Cytotoxicity assay
Cytotoxicity mediated by chimeric fusion immunotoxin consisting of IL-13 linked to Pseudomonas aeruginosa exotoxin (IL-13-PE) was determined as described previously (Joshi et al., 2002; Joshi and Puri, 2005).
Lentiviral vector production
Lentiviral vector production was carried out with 6-well plates, 15-cm dishes, or HYPERFlask cell culture vessels (Corning, Lowell, MA), as described previously (Kuroda et al., 2009, 2011; Kutner et al., 2009a,b). For transfection using 6-well plates (4×105 HEK 293T cells per well), 0.53 μg of the pNL(CMV)EGFP/CMV/WPREΔU3, pNL(CMV)Fluc/CMV/WPREΔU3, or pNL(CMV)GLuc/CMV/WPREΔU3 vector plasmid, 0.32 μg of the pCD/NL-BHΔ1 packaging plasmid, 0.76 μg of the pCMV-rev plasmid (Lewis et al., 1990) (obtained from M.-L. Hammarskjöld and D. Rekosh through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH), 0.066 μg of the pCG-FcΔ30 plasmid, and 0.033 μg of the pCG-HcΔ18-AA-IL-13 plasmid were used per well. For experiments involving VSV-G pseudotypes, the pCEF-VSV-G plasmid (0.18 μg/well) was used. For 15-cm dishes and HYPERFlask vessels, the number of cells and the quantities of the various plasmids were proportionally increased according to the cell culture surface area. The cell culture media were changed the day after transfection and the vector-containing supernatants were harvested on the third day (about 60 hr posttransfection), filtered through a 0.45-μm pore size filter (Millipore, Billerica, MA), and concentrated by ultracentrifugation through a 20% sucrose cushion. The vector pellets were resuspended in phosphate-buffered saline (PBS) supplemented with Polybrene (3 μg/ml), and stored at −80°C. Vector stocks were titrated by qPCR using genomic DNA from U251 cells transduced using woodchuck hepatitis virus posttranscriptional regulatory element (WPRE)-specific primers (Kutner et al., 2009b). Vector titers are expressed as transducing units (TU)/ml. Relative vector particle numbers were determined with a p24 ELISA kit (Kutner et al., 2009b).
Transduction in vitro and vector neutralization
HOS-IL-13Rα2, U251, or DU145 cells were seeded in 12-well plates at a density of 1×105 cells per well the day before transduction. When needed, Dox was added to the cell culture medium at a final concentration of 1 μg/ml. The next day, cell culture medium was removed from each well and a 0.5-ml aliquot of unconcentrated lentiviral vector sample and 0.5 ml of cell culture medium supplemented with Polybrene (16 μg/ml) were added. After overnight incubation in a CO2 incubator, the vector–Polybrene mixture was replaced with 1 ml of fresh medium.
For vector neutralization, polyclonal goat anti-human IL-13Rα2 antibodies (R&D Systems, Minneapolis, MN) were used. Cells were preincubated with the antibody for 1 hr at room temperature. The antibody was also present during the 12-hr transduction step. Afterward, the vector–antibody mixture was replaced with 1 ml of fresh medium.
For vector neutralization using the recombinant IL-13Rα2 extracellular domain (R&D Systems), vectors were first preincubated with the recombinant protein at 37°C in a humidified 5% CO2 incubator for 1 hr. Twelve hours later, the vector-containing medium was removed, and the cells were washed once with PBS and replenished with fresh complete medium. Three days posttransduction, cells were harvested with 0.25% trypsin–EDTA (Invitrogen), fixed for 10 min with an equal volume of PBS supplemented with 2% paraformaldehyde (Sigma-Aldrich, St. Louis, MO), and washed once with PBS. The percentage of EGFP-positive cells was determined by fluorescence-activated cell sorting (FACS) with a BD FACSCalibur or FACSCanto II system (BD Biosciences, San Jose, CA). GLuc activity was measured with the Dual Luciferase assay system from Promega, and Fluc activity was measured with the regular luciferase assay system from Promega.
Determination of IL-13Rα2 levels by FACS
U251, U87, T98G and HOS-IL-13Rα2 cells were washed twice with plain PBS (Ca2+ and Mg2+ free), detached with PBS supplemented with 5 mM EDTA and 2.5% FBS, centrifuged at 500×g for 5 min, and resuspended in staining solution (CO2-independent medium [Invitrogen], supplemented with 2.5% FBS and 2.5 mM EDTA) at a concentration of 5 million cells/ml. For each sample, 100-μl aliquots of the cells were used. The cells were stained with a biotin-labeled goat anti-IL-13Rα2 antibody (10 μg/ml) or biotin-labeled normal goat IgG (R&D Systems), for 1 hr at 4°C. The cells were washed once with plain PBS, resuspended in staining solution containing Alexa 647-labeled streptavidin (10 μg/ml; Invitrogen), incubated for 1 hr at 4°C, washed twice with plain PBS, and resuspended in 0.5 ml of staining solution containing YO-PRO-1 viability dye (1 μl/ml; Invitrogen). YO-PRO-1-negative (live) cells were analyzed with the FACSCalibur system (BD Biosciences).
Assessment of vector targeting in vivo with a subcutaneous model
The animal protocol and procedures were approved by Institutional Animal Care and Use Committee at the Center for Biologics Evaluation and Research (Bethesda, MD) (protocol 2011–02). The animal facilities are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All experiments were performed according to institutional guidelines. Five million U251 glioma cells or DU145 prostate carcinoma cells in 100 μl of PBS were grafted subcutaneously into 6- to 8-week-old male athymic nu/nu mice (NCI). One to 3 weeks later, when tumor volumes had reached about 100 mm3, 30-μl aliquots (3×106 TU) of GLuc-encoding lentiviral vectors (MV HcΔ18-AA-IL-13/FcΔ30) were injected into the tumors. Three days after vector injection, mice were imaged with the IVIS system (Caliper Life Sciences, Hopkinton, MA) immediately after 100 μl of the coelenterazine substrate (Nanolight Technology) was injected into each tumor.
Assessment of vector targeting in vivo using an intracranial model
The animal protocol and procedures were approved by the Institutional Animal Care and Use Committees at the Center for Biologics Evaluation and Research (protocol 2003–13). One million U251 glioma cells in 10 μl of PBS were grafted intracranially by a bolus injection (Kawakami et al., 2004) into 6- to 8-week-old female athymic nu/nu mice (NCI), using a model 900 small animal stereotaxic instrument from David Kopf Instruments (Tujunga, CA). Five days later, 10-μl aliquots (7×105 TU) of Fluc-encoding lentiviral vector (MV HcΔ18-AA-IL-13/FcΔ30) were injected at the same location where the tumor cells were originally injected. At various times after vector injection, mice were imaged with the IVIS system (Caliper Life Sciences) 15 min after intraperitoneal injection of the d-luciferin substrate (150 mg/kg; Caliper Life Sciences).
Results
Design of lentiviral vectors for targeted transgene delivery into IL-13Rα2-positive cells
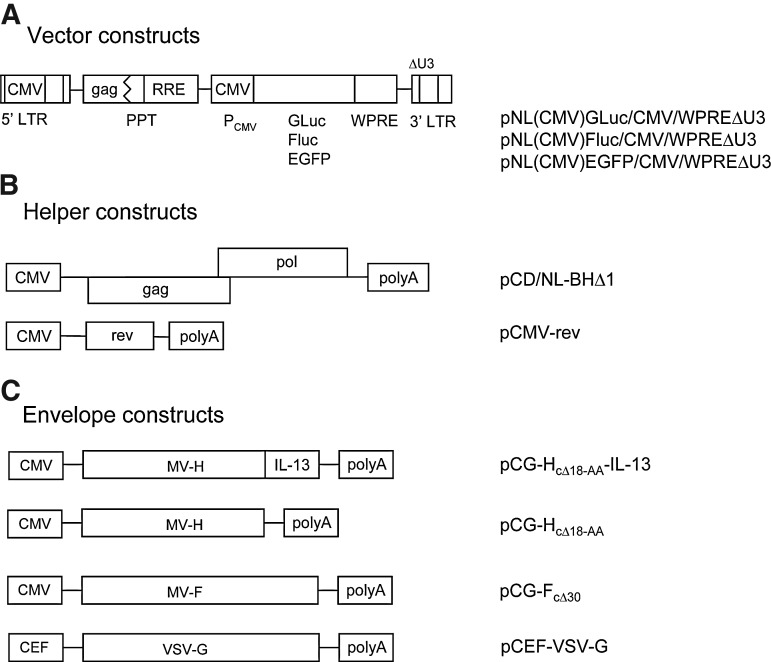
For targeted transgene delivery into IL-13Rα2-positive cells in vitro and in vivo, safety-improved lentiviral vectors, based on a previously described second-generation lentiviral vector packaging system (Zhang et al., 2004; Ricks et al., 2008), were developed (Fig. 1). The improved packaging system is Tat independent and includes the pCD/NL-BHΔ1 helper plasmid encoding the HIV-1 Gag and Pol functions; it also includes the pCMV-rev plasmid (Lewis et al., 1990) that encodes Rev (Fig. 1B). The vector plasmids used encode either Gaussia luciferase (GLuc), firefly luciferase (Fluc), or EGFP (Fig. 1A) and bear a hybrid 5′ LTR (Chang et al., 1993) containing a CMV-IE promoter sequence. The Env glycoprotein constructs used include the MV-based pCG-HcΔ18-AA-IL-13, pCG-HcΔ18-AA, and pCG-FcΔ30 plasmids, as well as the pCEF-VSV-G plasmid encoding the vesicular stomatitis virus G glycoprotein (Fig. 1C).
FIG. 1.
Representation of the lentiviral vector system. (A) Vector constructs. pNL(CMV)GLuc/CMV/WPREΔU3, pNL(CMV)Fluc/CMV/WPREΔU3, and pNL(CMV)EGFP/CMV/WPREΔU3 bear Gaussia luciferase (GLuc), firefly luciferase (Fluc), or enhanced green fluorescent protein (EGFP) transgene sequences, respectively. CMV, human cytomegalovirus immediate-early promoter; RRE, Rev response element; LTR, long terminal repeat; PPT, central polypurine tract; WPRE, woodchuck hepatitis virus posttranscriptional element. (B) Helper (packaging) constructs. pCD/NL-BHΔ1 encodes the Gag and Pol proteins and pCMV-rev (Lewis et al., 1990) encodes Rev. (C) Env glycoprotein constructs. The pCG-HcΔ18-AA-IL-13 plasmid encodes a fusion protein consisting of a receptor-blind variant of the measles virus (MV) H protein that lacks 18 residues of the cytoplasmic tail fused to interleukin (IL)-13. The pCG-HcΔ18-AA plasmid lacks the IL-13 coding sequence. Plasmid pCG-FcΔ30 encodes the MV F-protein lacking 30 amino acids of the cytoplasmic tail. Plasmid pCEF-VSV-G expresses the vesicular stomatitis virus G glycoprotein.
Targeted transduction of IL-13Rα2-positive cells in vitro
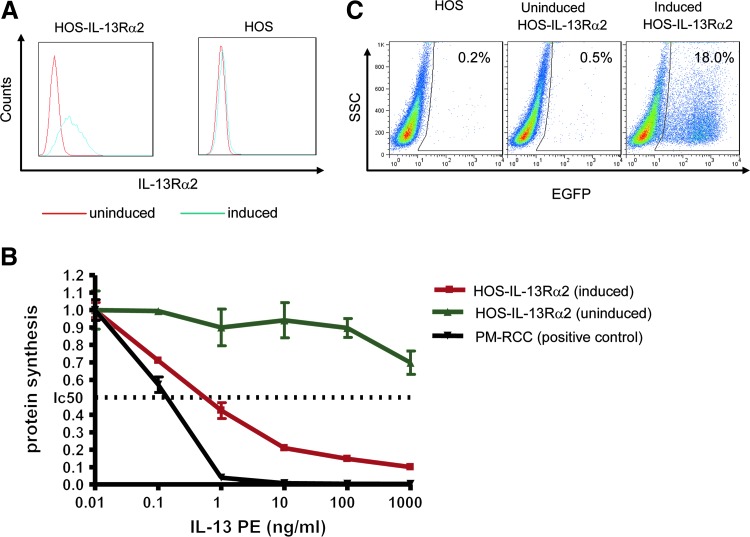
To test the ability of lentiviral vectors pseudotyped with the MV HcΔ18-AA-IL-13 and FcΔ30 glycoproteins to selectively transduce target cells in vitro, an engineered human osteosarcoma (HOS) cell line (HOS-IL-13Rα2) that expresses human IL-13Rα2 in response to Dox addition was developed. Consistent with results reported previously (Saito et al., 2005), unmodified HOS cells and HOS-IL-13Rα2 cells in the absence of Dox did not reveal any IL-13Rα2-positive cells above background by FACS (Fig. 2A); however, in the presence of Dox, a substantial fraction of the cells were positive after staining with an anti-IL-13Rα2 antibody. This indicates that IL-13Rα2 overexpression in HOS-IL-13Rα2 cells is strictly dependent on Dox addition.
FIG. 2.
Regulated overexpression of IL-13Rα2 in human osteosarcoma (HOS) cells. (A) FACS analysis of IL-13Rα2 overexpression in HOS cells. Left: HOS-IL-13Rα2 cells with or without doxycycline (Dox). Right: Unmodified HOS cells with or without Dox. Blue line, cells exposed to Dox (1 μg/ml); red line, cells without Dox. (B) Protein synthesis inhibition in HOS-IL-13Rα2 cells, using IL-13 linked to Pseudomonas aeruginosa exotoxin (IL-13-PE). HOS-IL-13Rα2 cells induced with Dox or uninduced cells were treated with IL-13-PE; the concentrations of IL-13-PE used varied from 0.01 to 1000 ng/ml; renal carcinoma cells (PM-RCCs), which are positive for IL-13Rα2, served as a positive control. (C) HOS cells and HOS-IL-13Rα2 cells treated with or without Dox were transduced with an EGFP-encoding lentiviral vector pseudotyped with the MV HcΔ18-AA-IL-13/FcΔ30 glycoproteins and analyzed for EGFP expression 3 days later with a FACSCalibur. Color images available online at www.liebertpub.com/hgtb
To test whether IL-13Rα2 receptors expressed by HOS-IL-13Rα2 cells in the presence of Dox are functional, we treated the cells with various concentrations of a chimeric fusion immunotoxin consisting of IL-13 linked to Pseudomonas aeruginosa exotoxin (IL-13-PE). Uptake of IL-13-PE and inhibition of protein synthesis have been shown previously to correlate with IL-13Rα2 levels (Obiri et al., 1996; Joshi et al., 2002; Joshi and Puri, 2005). As shown in Fig. 2B, treatment with IL-13-PE inhibited protein synthesis in HOS-IL-13Rα2 cells exposed to Dox; renal cell carcinoma cells (PM-RCCs; Obiri et al., 1996) were used as a positive control. In contrast, protein synthesis was not affected in HOS-IL-13Rα2 cells lacking Dox (uninduced cells) when using IL-13-PE up to 100 ng/ml. These findings are consistent with the results shown in Fig. 2A; they indicate that the levels of functional IL-13 receptors in uninduced HOS-IL-13Rα2 cells are low and that the production of functional IL-13Rα2 receptors in HOS-IL-13Rα2 cells can be induced by Dox addition.
The capacity of EGFP-encoding lentiviral vectors pseudotyped with the MV HcΔ18-AA-IL-13 and FcΔ30 glycoproteins to selectively transduce Dox-induced HOS-IL-13Rα2 cells was investigated next. As shown in Fig. 2C, EGFP-positive cells emerged among cells that were treated with Dox before and during transduction but not among parental HOS cells or HOS-IL-13Rα2 cells that lacked Dox, indicating that transduction was IL-13Rα2 dependent.
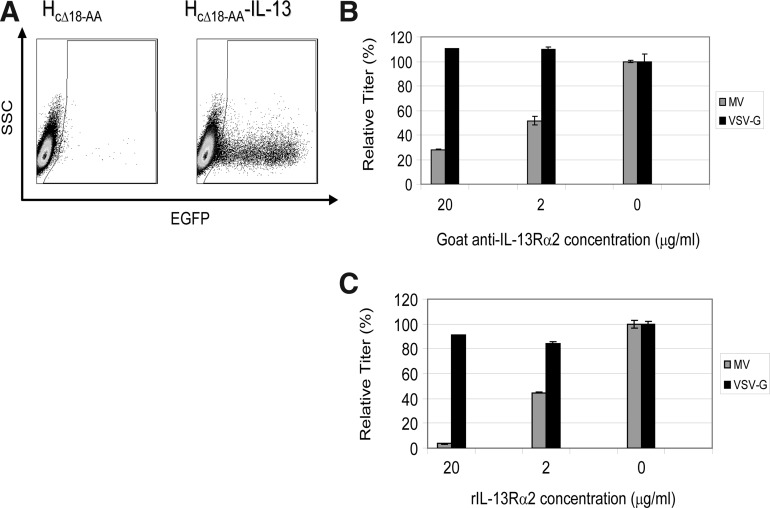
Three additional control experiments were performed to further confirm that efficient transduction of HOS-IL-13Rα2 cells was dependent on IL-13 and IL-13Rα2 (Fig. 3). In the first control experiment, IL-13Rα2-expressing target cells were transduced with an otherwise identical lentiviral vector except that the chimeric MV H protein did not contain IL-13. The results presented in Fig. 3A show that there was no transduction of HOS-IL-13Rα2 cells in the absence of the IL-13 ligand. In the second control experiment, IL-13Rα2-expressing target cells were treated with a polyclonal goat anti-IL-13Rα2 antibody 1 hr before and during transduction. Figure 3B shows that transduction was blocked in a dose-dependent manner for the vector pseudotyped with MV chimeric H glycoprotein, but not for the vector pseudotyped with VSV-G. The third control experiment involved treatment of the vector with the soluble extracellular domain of IL-13Rα2 (rIL-13Rα2) 1 hr before and during transduction. Transduction using the MV chimeric H glycoprotein-containing vector was blocked in a dose-depended manner, whereas the VSV-G-containing vector was unaffected (Fig. 3C). Taken together, these experiments indicate that transduction of HOS-IL-13Rα cells was specifically mediated by IL-13 and IL-13Rα2.
FIG. 3.
Specificity of in vitro transduction. (A) IL-13Rα2-expressing HOS cells were transduced with EGFP-encoding lentiviral vectors pseudotyped either with HcΔ18-AA-IL-13/FcΔ30 glycoprotein (right) or FcΔ30/HcΔ18-AA glycoprotein (left). (B) IL-13Rα2-expressing HOS cells were treated with a goat anti-IL-13Rα2 polyclonal antibody before and during transduction; MV, MV HcΔ18-AA-IL-13/FcΔ30-pseudotyped lentiviral vector; VSV-G, VSV-G-pseudotyped lentiviral vector. (C) The pseudotyped lentiviral vectors were treated with recombinant rIL-13Rα2, the soluble extracellular domain of IL-13Rα2, before and during transduction; MV, MV HcΔ18-AA-IL-13/FcΔ30-pseudotyped lentiviral vector; VSV-G, VSV-G-pseudotyped lentiviral vector.
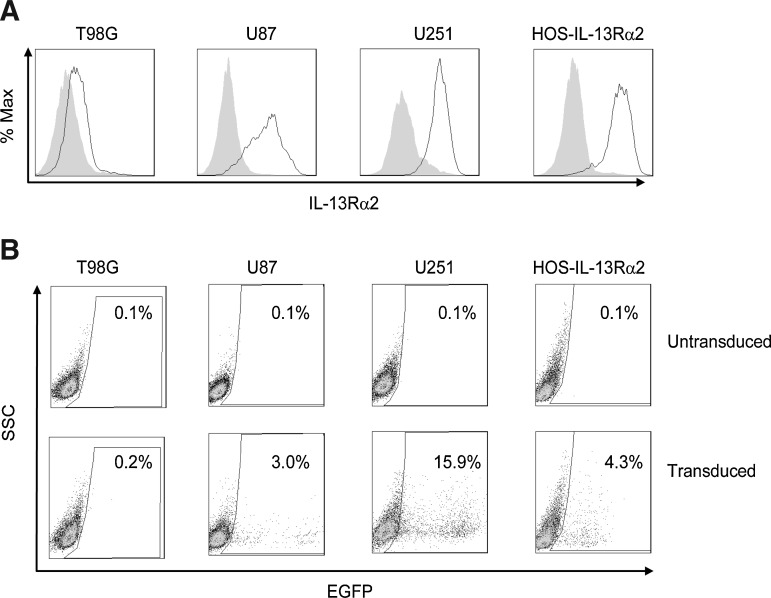
We next tested whether pseudotyped lentiviral vectors containing the MV HcΔ18-AA-IL-13 and FcΔ30 glycoproteins could transduce tumor cells that naturally express IL-13Rα2. To that end, we transduced the IL-13Rα2-positive U251 and U87 glioma cell lines; HOS-IL-13Rα2 cells treated with Dox and T98G glioblastoma multiforme cells were used as positive and negative controls, respectively. Figure 4B shows that all cell lines could be transduced successfully except for T98G cells, consistent with the finding that IL-13Rα2 expression in T98G cells was close to background levels, but positive for the other cell lines (Fig. 4A).
FIG. 4.
Targeted transduction of IL-13Rα2-positive human tumor cell lines in vitro. (A) Surface expression of IL-13Rα2 on human glioma cells (T98G, U87, and U251) and on IL-13Rα2-expressing HOS cells. Cells were stained with a polyclonal goat-anti IL-13Rα2 antibody (black lines) or a control goat antibody (shaded areas) and an Alexa 647-conjugated secondary antibody and were analyzed by FACS, using a FACSCalibur instrument. (B) Human glioma cells (T98G, U87, and U251) and IL-13Rα2-expressing HOS cells were transduced with an EGFP-encoding lentiviral vector and analyzed as described for Fig. 2C.
Optimized production of lentiviral vectors displaying IL-13
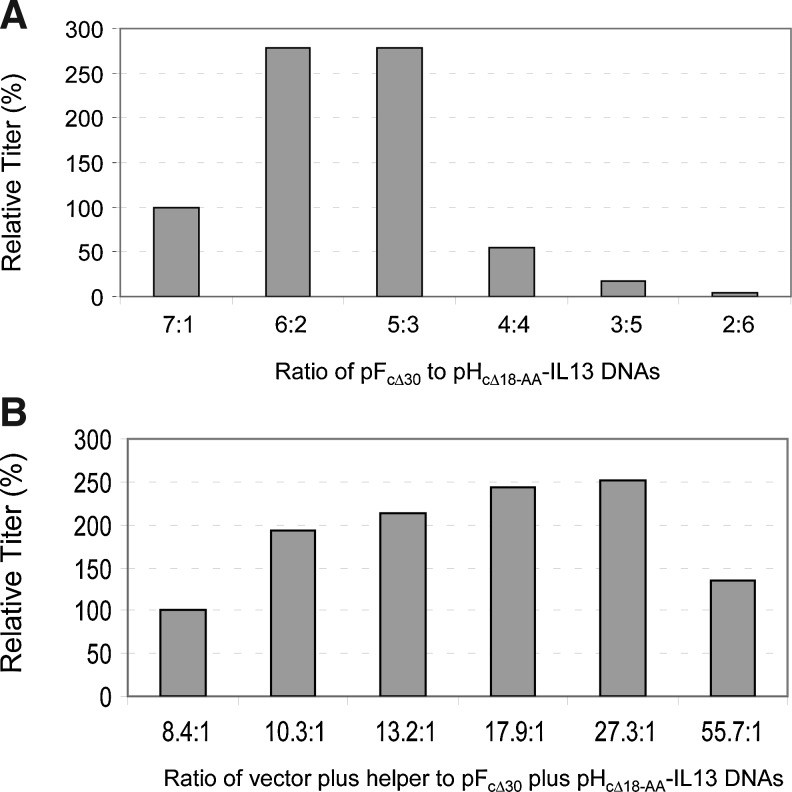
To facilitate the investigation of the performance of lentiviral vectors pseudotyped with MV HcΔ18-AA-IL-13 and FcΔ30 glycoproteins in vivo, vector production was optimized. Ratios of the FcΔ30 and HcΔ18-AA-IL-13 glycoprotein-encoding plasmids used during vector production were optimized first. As shown in Fig. 5A, the optimal ratio was between 3:1 and 1.67:1. A ratio of 2:1 was repeatedly found to be the best (data not shown) and was used for the rest of the study. In addition, the ratio of the vector and helper plasmids in relation to the sum of the FcΔ30 and HcΔ18-AA-IL-13 glycoprotein plasmids was optimized. As shown in Fig. 5B, the optimal ratio of the vector and helper to Env glycoprotein plasmids (pCG-HcΔ18-AA-IL-13 and pCG-FcΔ30) was 27:1. The final conditions used for transfecting HEK 293T cells in 6-well plates were as follows: 0.53 μg of pNL(CMV)EGFP/CMV/WPREΔU3, pNL(CMV)GLuc/CMV/WPREΔU3, or pNL(CMV)Fluc/CMV/WPREΔU3 plasmid, 0.32 μg of pCD/NL-BHΔ1 plasmid, 0.76 μg of pCMV-rev plasmid, and 0.066 μg of pCG-FcΔ30 plasmid and 0.033 μg of pCG-HcΔ18-AA-IL-13 plasmid per well. For large-scale production of lentiviral vectors pseudotyped with MV HcΔ18-AA-IL-13 and FcΔ30 glycoproteins, the conditions used for the 6-well plate format were scaled up proportionally.
FIG. 5.
Optimization of vector production for targeted transduction in vivo. (A) Optimization of ratios of pCG-HcΔ18-AA-IL-13 and pCG-FcΔ30 plasmids. Samples containing 0.53 μg of pNL(CMV)EGFP/CMV/WPREΔU3, 0.32 μg of pCD/NL-BHΔ1, 0.76 μg of pCMV-rev, and various amounts of pCG-FcΔ30 and pCG-HcΔ18-AA-IL-13 plasmid DNAs were prepared to transfect HEK 293T cells in 6-well plates. The combined amounts of pCG-FcΔ30 and pCG-HcΔ18-AA-IL-13 plasmid DNAs used were 0.18 μg; this amount was kept the same for each sample. Sixty hours posttransfection, vector-containing supernatants were harvested and used to transduce U251 cells. Transduced cells were analyzed by FACS 48 hr posttransduction. Vector titers were calculated on the basis of the percentage of EGFP-positive cells. (B) Optimization of vector/helper to Env glycoprotein plasmid ratios. The ratio of the vector to the helper plasmids (1.5:1) was the same for each sample; the ratio of pCG-FcΔ30 to pCG-HcΔ18-AA-IL-13 Env glycoprotein plasmid (2:1) was kept the same; the total amount of plasmid DNA in each sample (1.08 μg) was kept the same but the ratios of the combined amounts of vector and helper plasmids to the Env glycoprotein plasmid varied as indicated.
Targeted transduction in vivo
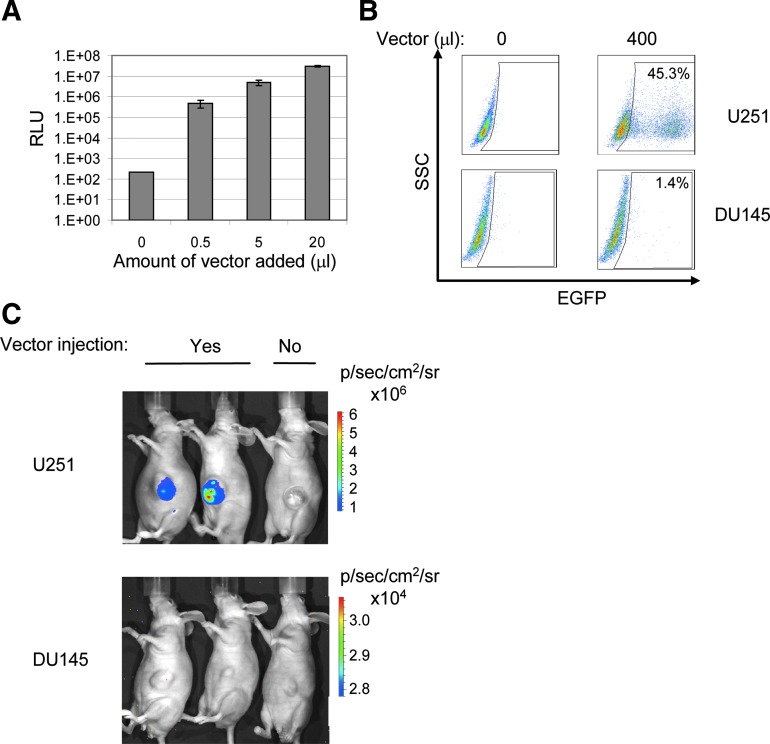
The capacity of lentiviral vectors pseudotyped with the MV HcΔ18-AA-IL-13 and FcΔ30 glycoproteins to selectively transduce target cells in vivo in an IL-13/IL-13Rα2-dependent manner was investigated next. To do this, we first established subcutaneous tumors in nude mice, using the IL-13Rα2-positive U251 cell line. The DU145 prostate carcinoma cell line, which lacks IL-13Rα2 expression (Kawakami et al., 2002), was used as a negative control. The in vitro analysis presented in Fig. 6B confirms that DU145 cells were not transduced above background levels whereas U251 cells were transduced efficiently. To monitor the specificity of vector targeting in the context of subcutaneous tumors, an MV HcΔ18-AA-IL-13- and FcΔ30-pseudotyped lentiviral vector encoding GLuc (Tannous et al., 2005) was used. Figure 6A documents the production of GLuc activity in transduced U251 cells in vitro. As shown in Fig. 6C (top), subcutaneous U251 glioma tumors injected with 3×106 transducing units (TU) of the vector and the coelenterazine substrate were positive, using the IVIS imaging system, whereas tumors that did not receive any vector were negative. DU145 tumors treated in the same way did not produce a detectable signal (Fig. 6C, bottom). Taken together, these results indicate that the targeting specificity in situ of IL-13-containing lentiviral vectors was maintained in the context of the subcutaneous tumor model.
FIG. 6.
Targeting IL-13Rα2-positive tumors cells in vivo, using a subcutaneous tumor model. (A) U251 cells were transduced with various amounts of the GLuc-encoding lentiviral vector pseudotyped with MV HcΔ18-AA-IL-13 and FcΔ30 glycoproteins and assayed for luciferase activity 3 days after transduction. (B) U251 cells and DU145 cells were transduced with an EGFP-encoding lentiviral vector pseudotyped with MV HcΔ18-AA-IL-13/FcΔ30 glycoproteins and analyzed as described for Fig. 2C. (C) Mice bearing subcutaneous U251 glioma cell-induced tumors or DU145 prostate carcinoma cell-induced tumors were injected with GLuc-encoding lentiviral vectors pseudotyped with the MV HcΔ18-AA-IL-13/FcΔ30 glycoproteins; vectors were administered directly into the tumors. Control injections consisted of PBS. Three days later, the mice were imaged with the IVIS system after 100 μl (2.5 mg/ml) of the coelenterazine substrate was injected into the tumors. Color images available online at www.liebertpub.com/hgtb
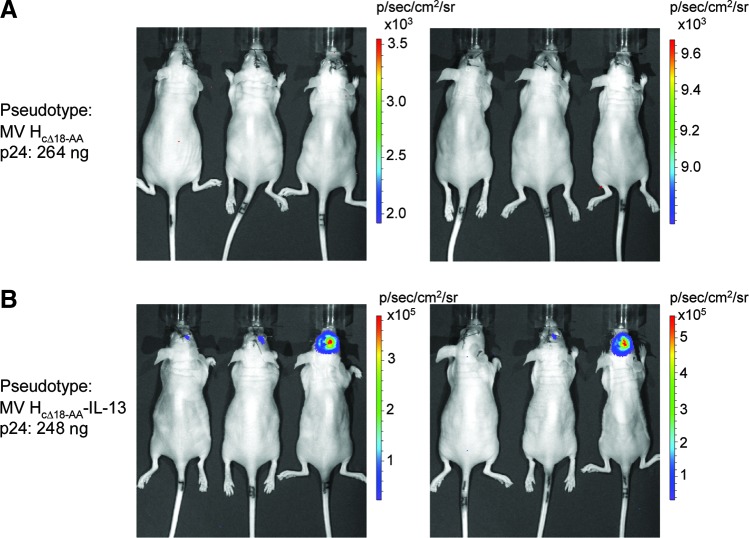
To test the targeting specificity of MV HcΔ18-AA-IL-13 glycoprotein-pseudotyped lentiviral vectors in the context of an orthotopic glioblastoma model, U251 cells were stereotaxically implanted into the brains of nude mice as described previously (Kawakami et al., 2004; Allen et al., 2008). A lentiviral vector pseudotyped with the MV HcΔ18-AA glycoprotein lacking IL-13 was used as a negative control. The vector amount was normalized on the basis of p24 present in the vector samples. A transgene encoding Fluc, the substrate of which, d-luciferin, can cross the blood–brain barrier (Deroose et al., 2006), was used. The results presented in Fig. 7A show that no Fluc activity above background levels was detected in animals that were injected with the control vector bearing the MV HcΔ18-AA glycoprotein lacking IL-13. However, in animals injected with vector pseudotyped with the MV HcΔ18-AA-IL-13 glycoprotein, Fluc activity was detected in five of six animals injected, 2 days after vector injection (Fig. 7B). These results are consistent with the view that transduction of U251 tumor cells in vivo in the context of intracranial glioblastomas was mediated by IL-13/IL-13Rα2.
FIG. 7.
Targeting of IL-13Rα2-positive tumors in an intracranial model. One million U251 cells in 10 μl of PBS were injected into the brains of 6- to 8-week-old athymic nu/nu mice. Five days later, 10-μl aliquots of Fluc-encoding lentiviral vectors pseudotyped with (A) MV HcΔ18-AA/FcΔ30 or (B) MV HcΔ18-AA-IL-13/FcΔ30 were injected through the same burr hole. The amount of p24 injected for the animals shown in (A) was 264 ng, and for the animals shown in (B) it was 248 ng. Two days after vector injection, mice were imaged with the IVIS imaging system 15 min after intraperitoneal injection of the luciferin substrate (150 mg/kg body weight). Each group contained 6 mice and 3 mice were imaged together. Color images available online at www.liebertpub.com/hgtb
Discussion
Efficient transgene delivery in vivo using retargeted lentiviral vectors has been challenging because of a number of issues including low titers. A number of approaches aimed at improving the safety and efficiency of transgene delivery in vivo, involving retargeted lentiviral vectors, have been described. One such approach uses pseudotyped lentiviral vectors involving engineered versions of the Sindbis virus E2 glycoprotein, allowing ligand-mediated cell targeting in the presence of the Sindbis virus E1 fusion protein (Morizono et al., 2001; Yang et al., 2006). In vivo applications of this approach have involved the use of lentiviral vectors to target P glycoprotein-expressing melanoma cells in the lungs of a mouse model of metastatic melanoma (Morizono et al., 2005). A drawback of this approach is that background transduction levels in the absence of a targeting ligand or with cells lacking the corresponding receptors can be substantial. Also, cell entry of retargeted Sindbis virus is pH dependent (Morizono and Chen, 2011); this may limit this approach to receptors that are endocytosed.
Zhang and colleagues (2010) described an alternative targeting strategy involving a cell-specific ligand that is incorporated into lentiviral vector particles containing a truncated version of the VSV-G glycoprotein (VSV-GS) that promotes cell fusion (Jeetendra et al., 2002). In one study, Goyvaerts and colleagues (2012) used this approach to target antigen-presenting cells (APCs) both in vitro and in vivo. To do this, an APC-specific camelid-derived heavy chain antibody and the VSV-GS fusion protein were displayed on lentiviral vector particles. Antibody-dependent transduction of mouse dendritic cells and macrophages was demonstrated both in vitro and in situ. An attractive feature of this particular approach is that vectors can be concentrated to high titers (Zhang et al., 2010; Goyvaerts et al., 2012).
The targeting strategy for lentiviral vectors involving engineered MV H and F glycoproteins is promising because of its flexibility and because background transduction levels are low (Funke et al., 2008; Anliker et al., 2010; Munch et al., 2011). Also, because MV enters cells through direct fusion at the cell membrane, it allows for vector entry in a pH-independent manner. To test the strategy in vivo, Munch and colleagues used lentiviral vectors bearing chimeric H proteins containing DARPins to target HER2/neu-positive tumors in SCID mice (Munch et al., 2011), and Anliker and colleagues used a single-chain antibody to target GluA-expressing neurons in mice (Anliker et al., 2010).
Here, our work focuses on IL-13-displaying lentiviral vectors bearing MV H and F glycoproteins to target IL-13Rα2-expressing cells in vitro and in vivo. IL-13 display to retarget DNA viruses such as herpes simplex virus 1 and replication-defective adenoviral (Ad5) vectors has been described previously (Zhou and Roizman, 2006; Ulasov et al., 2007). To selectively target Ad5 to IL-13Rα2-bearing cells, Ulasov and colleagues (2007) constructed a replication-defective adenoviral vector that possesses an IL-13 ligand presented by a bacteriophage T4-derived fibrin shaft. To assess the ability of the Ad5 vector to effectively transduce glioma cell in vivo, a subcutaneous flank xenograft model of human glioma in nude mice was established. In this model, IL-13-displaying vectors showed a 300-fold increase in transgene expression as compared with adenoviral vectors lacking IL-13. IL-13Rα2-directed targeting has also been achieved with RNA-containing viruses, and Allen and colleagues (2008) demonstrated that retargeting of replicating measles virus vectors by IL-13 display is feasible. The titer of the retargeted virus was unchanged relative to that of the unmodified virus, and the in vivo antitumor activity was comparable to that of the unmodified MV strain. Attempts to target IL-13Rα2-positive cells with murine leukemia virus-based vectors bearing ecotropic Env glycoproteins with IL-13 inserted at various positions were unsuccessful, due in part to blocks in membrane fusion (Ryu et al., 2008).
The results presented in this paper show that IL-13Rα2-positive tumor cells can be targeted selectively both in vitro and in vivo, using IL-13-displaying lentiviral vectors. An advantage of lentiviral vectors is their ability to transduce actively dividing cells as well as nondividing cells (Reiser et al., 1996). This is an attractive feature given that tumors typically consist of a heterogeneous mixture of cells including slowly dividing cells as well as nondividing cells. For example, it was estimated that only a fraction (1–38%) of all glioma cells are in mitosis at any given time (Hoshino et al., 1989).
To test the specificity of the system, a Dox-inducible HOS cell line that conditionally overexpresses IL-13Rα2 on Dox addition was constructed. We found that lentiviral vectors pseudotyped with the MV FcΔ30 and HcΔ18-AA-IL-13 proteins efficiently transduced HOS-IL-13Rα2 cells, but not parental HOS cells, in a Dox-dependent manner. These results indicate that transduction of HOS-IL-13Rα2 cells in the presence of Dox, using vectors pseudotyped with the MV HcΔ18-AA-IL-13 and FcΔ30 glycoproteins, is mediated by IL-13Rα2. Such vectors also transduced human U251 glioma cells, which naturally express high levels of IL-13Rα2, but not T98G glioma cells, which express low levels of IL-13Rα2. Also, transduction was highly specific as it could be blocked with anti-IL-13Rα2 antibody or a soluble recombinant human IL-13 Rα2 Fc chimera.
Our long-term goal is to establish lentivirus-based tumor receptor targeting strategies in vivo. Toward this goal we established an orthotopic glioblastoma model using U251 cells that were implanted in the brain of nude mice (Kawakami et al., 2004; Allen et al., 2008). Lentiviral vectors expressing Fluc were injected 5 days later and a lentiviral vector pseudotyped with the MV HcΔ18-AA glycoprotein lacking IL-13 was used as a negative control. Consistent with the in vitro experiments, mice bearing U251 tumors were positive by luciferase imaging with lentiviral vectors displaying the MV HcΔ18-AA-IL-13 glycoprotein but not with lentiviral vectors containing the MV HcΔ18-AA glycoprotein lacking IL-13. This indicates that that transduction was mediated by IL-13Rα2 present on tumor cells. This view is supported by the observation that IL-13Rα2 levels on nontumor cells are low (Joshi and Puri, 2009).
Successful tumor targeting involving lentiviral vectors injected systemically will be dependent on a number of factors such as increased vector titers and improvements in receptor affinities including binding thresholds for membrane fusion triggering (Hasegawa et al., 2007). Mutant IL-13 proteins that demonstrate a higher affinity for the IL-13Rα2 receptor have been described previously. For example, substitution of a glutamine acid residue at position 13 with a lysine residue resulted in an IL-13 mutant protein (IL-13 E13K) that bound receptor-expressing tumor cells with 3- to 10-fold higher affinity compared with unmodified IL-13 (Debinski et al., 1998; Oshima et al., 2000). Nevertheless, when the IL-13 E13K mutant protein was fused to Pseudomonas exotoxin, the increase in affinity did not translate into superior antitumor efficacy compared with the unmodified IL-13 fusion protein (Kioi et al., 2004). In a different experimental system, an IL-13 mutant protein (IL-13 E13Y) that revealed a 20 times higher affinity for the IL-13Rα2 receptor and a 5 times lower affinity for the IL-13Rα1/IL-4 receptor specifically targeted glioblastoma multiforme cells when displayed on cytotoxic T lymphocytes (Kahlon et al., 2004). The performance of these mutant IL-13 proteins in the context of retargeted lentiviral vectors remains to be determined.
In summary, we have displayed IL-13 on lentiviral vector particles through fusion to the C terminus of the MV H protein, whose binding sites for the native receptors were mutated. The specificity of the in vitro transduction process was demonstrated with specific antibodies against IL-13Rα2 and soluble IL-13Rα2. Also, we were able to demonstrate, using luciferase-based imaging, that lentiviral vectors displaying IL-13 specifically targeted IL-13Rα2-positive U251 cells but not IL-13Rα2-negative DU145 cells in vivo. We are currently working on improved methods for vector production and concentration to increase vector titers for systemic delivery of retargeted lentiviral vectors in the context of in vivo models.
Acknowledgments
The authors are grateful to Ritika Dogra for performing the cytotoxicity assay, and thank Howard Mostowski for help with flow cytometry. The authors thank Gibbes Johnson (FDA/CDER) and Shyh-Ching Lo (FDA/CBER) for helpful comments on the manuscript.
Author Disclosure Statement
The authors have no competing interests to disclose.
References
- Allen C. Paraskevakou G. Iankov I., et al. Interleukin-13 displaying retargeted oncolytic measles virus strains have significant activity against gliomas with improved specificity. Mol. Ther. 2008;16:1556–1564. doi: 10.1038/mt.2008.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anliker B. Abel T. Kneissl S., et al. Specific gene transfer to neurons, endothelial cells and hematopoietic progenitors with lentiviral vectors. Nat. Methods. 2010;7:929–935. doi: 10.1038/nmeth.1514. [DOI] [PubMed] [Google Scholar]
- Chang L.J. McNulty E. Martin M. Human immunodeficiency viruses containing heterologous enhancer/promoters are replication competent and exhibit different lymphocyte tropisms. J. Virol. 1993;67:743–752. doi: 10.1128/jvi.67.2.743-752.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin J. Zhang X.Y. Reiser J. Altering the tropism of lentiviral vectors through pseudotyping. Curr. Gene Ther. 2005;5:387–398. doi: 10.2174/1566523054546224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Costa J. Mansfield S.G. Humeau L.M. Lentiviral vectors in clinical trials: Current status. Curr. Opin. Mol. Ther. 2009;11:554–564. [PubMed] [Google Scholar]
- Debinski W. Gibo D.M. Obiri N.I., et al. Novel anti-brain tumor cytotoxins specific for cancer cells. Nat. Biotechnol. 1998;16:449–453. doi: 10.1038/nbt0598-449. [DOI] [PubMed] [Google Scholar]
- Deroose C.M. Reumers V. Gijsbers R., et al. Noninvasive monitoring of long-term lentiviral vector-mediated gene expression in rodent brain with bioluminescence imaging. Mol. Ther. 2006;14:423–431. doi: 10.1016/j.ymthe.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Dropulic B. Lentiviral vectors: Their molecular design, safety, and use in laboratory and preclinical research. Hum. Gene Ther. 2011;22:649–657. doi: 10.1089/hum.2011.058. [DOI] [PubMed] [Google Scholar]
- Frecha C. Szecsi J. Cosset F.L., et al. Strategies for targeting lentiviral vectors. Curr. Gene Ther. 2008;8:449–460. doi: 10.2174/156652308786848003. [DOI] [PubMed] [Google Scholar]
- Funke S. Maisner A. Muhlebach M.D., et al. Targeted cell entry of lentiviral vectors. Mol. Ther. 2008;16:1427–1436. doi: 10.1038/mt.2008.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyvaerts C. De Groeve K. Dingemans J., et al. Development of the Nanobody display technology to target lentiviral vectors to antigen-presenting cells. Gene Ther. 2012 Jan 12; doi: 10.1038/gt.2011.206. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K. Hu C. Nakamura T., et al. Affinity thresholds for membrane fusion triggering by viral glycoproteins. J. Virol. 2007;81:13149–13157. doi: 10.1128/JVI.01415-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T. Prados M. Wilson C.B., et al. Prognostic implications of the bromodeoxyuridine labeling index of human gliomas. J. Neurosurg. 1989;71:335–341. doi: 10.3171/jns.1989.71.3.0335. [DOI] [PubMed] [Google Scholar]
- Jeetendra E. Robison C.S. Albritton L.M., et al. The membrane-proximal domain of vesicular stomatitis virus G protein functions as a membrane fusion potentiator and can induce hemifusion. J. Virol. 2002;76:12300–12311. doi: 10.1128/JVI.76.23.12300-12311.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi B.H. Puri R.K. Optimization of expression and purification of two biologically active chimeric fusion proteins that consist of human interleukin-13 and Pseudomonas exotoxin in Escherichia coli. Protein Expr. Purif. 2005;39:189–198. doi: 10.1016/j.pep.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Joshi B.H. Puri R.K. IL-13 receptor-α2: A novel target for cancer therapy. Immunotherapy. 2009;1:321–327. doi: 10.2217/imt.09.8. [DOI] [PubMed] [Google Scholar]
- Joshi B.H. Kawakami K. Leland P., et al. Heterogeneity in interleukin-13 receptor expression and subunit structure in squamous cell carcinoma of head and neck: Differential sensitivity to chimeric fusion proteins comprised of interleukin-13 and a mutated form of Pseudomonas exotoxin. Clin. Cancer Res. 2002;8:1948–1956. [PubMed] [Google Scholar]
- Kahlon K.S. Brown C. Cooper L.J., et al. Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer Res. 2004;64:9160–9166. doi: 10.1158/0008-5472.CAN-04-0454. [DOI] [PubMed] [Google Scholar]
- Kawakami K. Kawakami M. Puri R.K. IL-13 receptor-targeted cytotoxin cancer therapy leads to complete eradication of tumors with the aid of phagocytic cells in nude mice model of human cancer. J. Immunol. 2002;169:7119–7126. doi: 10.4049/jimmunol.169.12.7119. [DOI] [PubMed] [Google Scholar]
- Kawakami K. Kawakami M. Kioi M., et al. Distribution kinetics of targeted cytotoxin in glioma by bolus or convection-enhanced delivery in a murine model. J. Neurosurg. 2004;101:1004–1011. doi: 10.3171/jns.2004.101.6.1004. [DOI] [PubMed] [Google Scholar]
- Kioi M. Kawakami K. Puri R.K. Analysis of antitumor activity of an interleukin-13 (IL-13) receptor-targeted cytotoxin composed of IL-13 antagonist and Pseudomonas exotoxin. Clin. Cancer Res. 2004;10:6231–6238. doi: 10.1158/1078-0432.CCR-04-0700. [DOI] [PubMed] [Google Scholar]
- Kuroda H. Kutner R.H. Bazan N.G., et al. Simplified lentivirus vector production in protein-free media using polyethylenimine-mediated transfection. J. Virol. Methods. 2009;157:113–121. doi: 10.1016/j.jviromet.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Kuroda H. Marino M.P. Kutner R.H., et al. Production of lentiviral vectors in protein-free media. Curr. Protoc. Cell Biol. 2011;50:26.8.1–26.8.13. doi: 10.1002/0471143030.cb2608s50. [DOI] [PubMed] [Google Scholar]
- Kutner R.H. Puthli S. Marino M.P., et al. Simplified production and concentration of HIV-1-based lentiviral vectors using HYPERFlask vessels and anion exchange membrane chromatography. BMC Biotechnol. 2009a;9:10. doi: 10.1186/1472-6750-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutner R.H. Zhang X.Y. Reiser J. Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat. Protoc. 2009b;4:495–505. doi: 10.1038/nprot.2009.22. [DOI] [PubMed] [Google Scholar]
- Lewis N. Williams J. Rekosh D., et al. Identification of a cis-acting element in human immunodeficiency virus type 2 (HIV-2) that is responsive to the HIV-1 Rev and human T-cell leukemia virus types I and II Rex proteins. J. Virol. 1990;64:1690–1697. doi: 10.1128/jvi.64.4.1690-1697.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrai J. Chuah M.K. Vandendriessche T. Recent advances in lentiviral vector development and applications. Mol. Ther. 2010;18:477–490. doi: 10.1038/mt.2009.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll M. Klenk H.D. Maisner A. Importance of the cytoplasmic tails of the measles virus glycoproteins for fusogenic activity and the generation of recombinant measles viruses. J. Virol. 2002;76:7174–7186. doi: 10.1128/JVI.76.14.7174-7186.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizono K. Chen I.S. Receptors and tropisms of envelope viruses. Curr. Opin. Virol. 2011;1:13–18. doi: 10.1016/j.coviro.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizono K. Bristol G. Xie Y.M., et al. Antibody-directed targeting of retroviral vectors via cell surface antigens. J. Virol. 2001;75:8016–8020. doi: 10.1128/JVI.75.17.8016-8020.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizono K. Xie Y. Ringpis G.E., et al. Lentiviral vector retargeting to P-glycoprotein on metastatic melanoma through intravenous injection. Nat. Med. 2005;11:346–352. doi: 10.1038/nm1192. [DOI] [PubMed] [Google Scholar]
- Muhlebach M.D. Mateo M. Sinn P.L., et al. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature. 2011;480:530–533. doi: 10.1038/nature10639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch R.C. Muhlebach M.D. Schaser T., et al. DARPins: An efficient targeting domain for lentiviral vectors. Mol. Ther. 2011;19:686–693. doi: 10.1038/mt.2010.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T. Peng K.W. Vongpunsawad S., et al. Antibody-targeted cell fusion. Nat. Biotechnol. 2004;22:331–336. doi: 10.1038/nbt942. [DOI] [PubMed] [Google Scholar]
- Obiri N.I. Husain S.R. Debinski W., et al. Interleukin 13 inhibits growth of human renal cell carcinoma cells independently of the p140 interleukin 4 receptor chain. Clin. Cancer Res. 1996;2:1743–1749. [PubMed] [Google Scholar]
- Oshima Y. Joshi B.H. Puri R.K. Conversion of interleukin-13 into a high affinity agonist by a single amino acid substitution. J. Biol. Chem. 2000;275:14375–14380. doi: 10.1074/jbc.275.19.14375. [DOI] [PubMed] [Google Scholar]
- Pluta K. Luce M.J. Bao L., et al. Tight control of transgene expression by lentivirus vectors containing second-generation tetracycline-responsive promoters. J. Gene Med. 2005;7:803–817. doi: 10.1002/jgm.712. [DOI] [PubMed] [Google Scholar]
- Reiser J. Harmison G. Kluepfel-Stahl S., et al. Transduction of nondividing cells using pseudotyped defective high-titer HIV type 1 particles. Proc. Natl. Acad. Sci. U.S.A. 1996;93:15266–15271. doi: 10.1073/pnas.93.26.15266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricks D.M. Kutner R. Zhang X.Y., et al. Optimized lentiviral transduction of mouse bone marrow-derived mesenchymal stem cells. Stem Cells Dev. 2008;17:441–450. doi: 10.1089/scd.2007.0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu B.Y. Zavorotinskaya T. Trentin B., et al. The block to membrane fusion differs with the site of ligand insertion in modified retroviral envelope proteins. J. Gen. Virol. 2008;89:1049–1058. doi: 10.1099/vir.0.83445-0. [DOI] [PubMed] [Google Scholar]
- Saito M. Murata T. Watanabe K., et al. Adenoviral vector-mediated gene transfer of IL-13Rα2 chain followed by IL-13 cytotoxin treatment offers potent targeted therapy for cytotoxin-resistant cancers. Int. J. Cancer. 2005;116:1–8. doi: 10.1002/ijc.20995. [DOI] [PubMed] [Google Scholar]
- Santos E.B. Yeh R. Lee J., et al. Sensitive in vivo imaging of T cells using a membrane-bound Gaussia princeps luciferase. Nat. Med. 2009;15:338–344. doi: 10.1038/nm.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K.J. Wall E.A. Zavzavadjian J.R., et al. A single lentiviral vector platform for microRNA-based conditional RNA interference and coordinated transgene expression. Proc. Natl. Acad. Sci. U.S.A. 2006;103:13759–13764. doi: 10.1073/pnas.0606179103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannous B.A. Kim D.E. Fernandez J.L., et al. Codon-optimized Gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Mol. Ther. 2005;11:435–443. doi: 10.1016/j.ymthe.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Ulasov I.V. Tyler M.A. Han Y., et al. Novel recombinant adenoviral vector that targets the interleukin-13 receptor α2 chain permits effective gene transfer to malignant glioma. Hum. Gene Ther. 2007;18:118–129. doi: 10.1089/hum.2006.146. [DOI] [PubMed] [Google Scholar]
- Waehler R. Russell S.J. Curiel D.T. Engineering targeted viral vectors for gene therapy. Nat. Rev. Genet. 2007;8:573–587. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi Y. Takeda M. Ohno S. Measles virus: Cellular receptors, tropism and pathogenesis. J. Gen. Virol. 2006;87:2767–2779. doi: 10.1099/vir.0.82221-0. [DOI] [PubMed] [Google Scholar]
- Yang L. Bailey L. Baltimore D., et al. Targeting lentiviral vectors to specific cell types in vivo. Proc. Natl. Acad. Sci. U.S.A. 2006;103:11479–11484. doi: 10.1073/pnas.0604993103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.Y. La Russa V.F. Reiser J. Transduction of bone-marrow-derived mesenchymal stem cells by using lentivirus vectors pseudotyped with modified RD114 envelope glycoproteins. J. Virol. 2004;78:1219–1229. doi: 10.1128/JVI.78.3.1219-1229.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.Y. Kutner R.H. Bialkowska A., et al. Cell-specific targeting of lentiviral vectors mediated by fusion proteins derived from Sindbis virus, vesicular stomatitis virus, or avian sarcoma/leukosis virus. Retrovirology. 2010;7:3. doi: 10.1186/1742-4690-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G. Roizman B. Construction and properties of a herpes simplex virus 1 designed to enter cells solely via the IL-13α2 receptor. Proc. Natl. Acad. Sci. U.S.A. 2006;103:5508–5513. doi: 10.1073/pnas.0601258103. [DOI] [PMC free article] [PubMed] [Google Scholar]