Abstract
Inactivating mutations of the pro-opiomelanocortin (POMC) gene in both mice and humans leads to hyperphagia and obesity. To further examine the mechanisms whereby POMC-deficiency leads to disordered energy homeostasis, we have generated mice lacking all POMC-derived peptides. Consistent with a previously reported model, Pomc-/- mice were obese and hyperphagic. They also showed reduced resting oxygen consumption associated with lowered serum levels of thyroxine. Hypothalami from Pomc-/- mice showed markedly increased expression of melanin-concentrating hormone mRNA in the lateral hypothalamus, but expression of neuropeptide Y mRNA in the arcuate nucleus was not altered. Provision of a 45% fat diet increased energy intake and body weight in both Pomc-/- and Pomc+/- mice. The effects of leptin on food intake and body weight were blunted in obese Pomc-/- mice whereas nonobese Pomc-/- mice were sensitive to leptin. Surprisingly, we found that Pomc-/- mice maintained their acute anorectic response to peptide-YY3-36 (PYY3-36). However, 7 days of PYY3-36 administration had no effect on cumulative food intake or body weight in wild-type or Pomc-/- mice. Thus, POMC peptides seem to be necessary for the normal response of energy balance to high-fat feeding, but not for the acute anorectic effect of PYY3-36 or full effects of leptin on feeding. The finding that the loss of only one copy of the Pomc gene is sufficient to render mice susceptible to the effects of high fat feeding emphasizes the potential importance of this locus as a site for gene-environment interactions predisposing to obesity.
Hypothalamic neurons expressing pro-opiomelanocortin (POMC) are involved in the maintenance of energy homeostasis through the integration of a number of peripheral and central signals related to energy status (1-3). POMC is highly expressed in neuronal cell bodies of the arcuate nucleus, with POMC-expressing neurons innervating other hypothalamic regions known to regulate feeding behavior, including the paraventricular nucleus, lateral hypothalamus, and dorsomedial hypothalamic nucleus (DMH) (4). Pharmacological and genetic studies have revealed that POMC-derived melanocortin peptides or synthetic agonists suppress feeding through activation of the melanocortin 4-receptor (MC4R) whereas the endogenous antagonist agouti-related protein (AgRP) or synthetic antagonists stimulate food intake (5).
Approximately 40% of POMC-expressing neurons colocalize with the long isoform of the leptin receptor (6). Leptin activates POMC neurons directly and indirectly, through hyperpolarization of neuropeptide Y (NPY)/AgRP neurons, thereby reducing their tonic inhibitory γ-aminobutyric acid (GABA) input to POMC neurons (1). Recent data suggest that the gut peptide PYY3-36 (peptide-YY3-36) reduces food intake, at least in part, through direct hyperpolarization of NPY/AgRP neurons and subsequent disinhibition of POMC neurons (3).
Given the critical role that hypothalamic POMC plays in the regulation of energy balance, it is not surprising that mice and humans lacking POMC develop hyperphagia and obesity (7, 8). To further examine the mechanisms whereby POMC deficiency leads to hyperphagic obesity, we have generated an independent line of mice lacking all POMC-derived peptides. In a previous model of murine POMC deficiency, the design of the targeting construct left open the possibility that some N-terminal POMC-derived peptides might be retained. The possibility that N-terminal peptides of POMC might have biological activity has recently been reinforced by studies of Bicknell et al. (9) on adrenal growth. We used this new model to examine the effects of complete POMC deficiency on body composition and energy balance, as well as the hypothalamic expression of key orexigenic neuropeptides. In addition, we examined the extent to which POMC peptides are required for the known anorectic effects of leptin and the gut peptide PYY3-36. Finally, we have explored whether partial or complete loss of Pomc influences the response to the provision of a high-fat diet.
Materials and Methods
Targeting Vector. DNA clones spanning the Pomc gene were isolated from a 129/SVJ mouse genomic library. A targeting construct was generated by PCR using the following primers: the 3′ arm was amplified by using 3′ armF (5′-TTTGGCGCGCCTGAGGGTGCAGGGGTCTTCTCAT-3′) and 3′ armR (5′-TTTGGCGCGCCGCTACTGGAAGCAGGTTGGTTAGTGGA-3′). The resulting 1.1-kb amplicon was cloned into the AscI site. The 5′ arm was generated in two steps by using a strategy that introduced a point mutation at the start codon. The external primers used for the amplification of the 5′ arm were as follows: 5′ armF (5′-TTTCTCGAGTAAGTGCAGATTGTTTCCTTTGG-3′) and 5′ armR (5′-AAAGGCCGGCCAGAGGTCGAGTTTGCAAGCCCGGAT-3′). The amplicon was cloned into the XhoI and FseI sites. Two internal primers were used to create a specific point mutation within the start site, resulting in the introduction of an MluI site, which was used to facilitate cloning of the 4.1-kb 5′ arm and identification of the correctly mutated allele. The wild-type and mutated regions are shown below (introns in lowercase and exons in uppercase, with underlined bases denoting those that were mutated): wild-type, cgtggaagATGCCGAGATTCTGC; knockout, cgtggaagACGCGTAGATTCTGC. The next ATG is not in frame with the peptide and would create a nonsense peptide if used. There are no other ATGs before the deletion point. The first 54 bp of exon 3 were retained to allow splicing to a tau-lacZ cassette, which also contained a neomycin resistance gene (neor) driven by the PGK gene promoter and flanked by 34-bp-long Cre-recombinase (Cre) recognition (loxP) sites oriented in the same direction such that a Cre-mediated recombination event would remove the PGK promoter and neor cassette.
Generation of Pomc-/- Mice. A screening primer (3′ screen, 5′-AAATTGGGCCATGGGACTGACAAGCTC-3′) situated just downstream of the region used as the 3′ arm was used with a neo primer to identify targeted clones derived from 129S6/SvEv embryonic stem (ES) cells. A further primer pair was used to amplify an external 5′ probe for Southern blot verification of homologous recombination at the Pomc locus. One correctly targeted ES cell clone was injected into C57BL/6J blastocysts to produce chimeric animals that were subsequently crossed with 129S2/SVHsd mice to generate offspring with the mutant allele. Genotype analysis of large numbers of mice was facilitated by PCR. Two separate PCR reactions were performed by using oligonucleotide primers specific for the wild-type POMC exon 3 allele (POMC Fwd, 5′-GCTTGCAAACTCGACCTCTCG-3′; POMC Rev, 5′-CACTGGCCCTTCTTGTGCG-3′) and oligonucleotides specific for the PGK-neo cassette (Neo Fwd, 5′-GGGTGGAGAGGCTATTCGGCTA-3′; Neo Rev, 5′-GAAGAACTCGTCAAGAAGGCGATAGAA-3′).
Animal Care and Maintenance. All animal protocols used in these studies were approved by the United Kingdom Home Office. Mice were group-housed or individually housed in cages. Mice were maintained on either a 4.5 kcal % fat diet (1 kcal = 4.18 kJ) (Special Diet Services, Witham, U.K.) or 45 kcal % fat diet (Research Diets, New Brunswick, NJ) with ad libitum access to water and chow unless stated otherwise.
Histochemistry. Male Pomc+/- and wild-type mice were terminally anesthetized with i.p.-administered pentobarbitone (50 mg/kg of body weight) and perfused transcardially with heparinized saline followed by 4% paraformaldehyde. Brains were removed, placed in 15% sucrose/fixative solution, and immersed in 30% sucrose/phosphate buffer solution at 4°C. Forty micrometer coronal sections were cut by using a sliding microtome and collected in 1× PBS. Sections were incubated with X-gal staining buffer (5 mM potassium ferricyanide/5 mM potassium ferrocyanide/2 mM MgCl2/1× PBS/1 mg/ml 5-bromo-4-chloro-3-indolyl β-d-galactoside) (pH 7.3) in darkness at 37°C for 8-12 h.
Body Weight and Food Intake. Body weight was measured starting on postnatal day 22. For measurement of food consumption, 8-week-old male mice were housed individually in cages, and food intake was measured every 24 h for 5 days from preweighed portions of food dispensed from wire cage tops. Average daily food intake of both regular chow (4.5 kcal % fat) and the high-fat diet (45 kcal % fat) was determined. Cages were carefully monitored for evidence for food wastage, which was negligible.
Plasma Composition. Plasma levels of corticotropin (ACTH) precursors were measured by using an enzyme-linked immunoassay derived from the immunoradiometric assay of Crosby et al. (10). The assay, which is specific for POMC and ProACTH, had a sensitivity of 12 pmol/liter and was validated for compatibility with mouse plasma. Blood for corticosterone was taken between 1600 and 1700 hours. Total thyroid hormone was measured by using DELFIA assay (Wallac/Perkin-Elmer) following manufacturer's instructions. Blood was taken at 0900 hours after ad libitum feeding. In all situations, blood was taken within 1 min of handling mice.
Oxygen Consumption Measurements. Oxygen consumption was simultaneously determined for multiple animals by indirect calorimetry by using an Oxymax 4.4 System (Columbus Instruments, Columbus, OH). The experimental animals were allowed to acclimatize to the chambers for 2 h, and measurements were taken subsequently for 3 h during the light cycle (1100 to 1600 hours). Samples were recorded every 4 min, with the room air reference taken every 16 min and the air flow to chambers 500 ml/min. Data represent the mean oxygen consumption per minute over the 3-h testing period.
Body Composition. Fat and lean body mass were determined by using dual-energy x-ray absorptiometry (Lunar PIXImus2 mouse densitometer, General Electric Medical Systems) as described by the manufacturer.
In Situ Hybridization. In situ hybridization was performed as described (11) on brain sections from 3-month-old male mice by using antisense oligonucleotide probes designed for NPY and melanin-concentrating hormone (MCH) mRNA. The duration of exposure to the x-ray film varied according to the mRNA transcript under investigation. For quantification of NPY and MCH, mRNA sections were placed in x-ray cassettes and then exposed to autoradiographic film for 5-7 days. The sections were analyzed and compared against a 14C-labeled standard of known radioactivity (Amersham Biosciences). The optical density of the autoradiographic images was measured by using a computerized Macintosh-based image analysis system (nih image). The optical densities were obtained in six consecutive sections per mouse, and the average value for each animal was used to calculate group means.
Responses to Leptin and PYY3-36. Mice fasted for 24 h were i.p. injected with 100 μl of PYY3-36 (Bachem) (10 μg per 100 grams of body weight) or saline at the onset of the dark cycle (1900 hours), and 4-h cumulative food intake was measured. To test the effects of chronic PYY3-36 administration, freely feeding mice were given two daily (0800 and 1800 hours) i.p. injections of either PYY3-36 or saline for 7 days. Food intake and body weight were measured daily during the treatment period. To study the effects of leptin, freely feeding obese and preobese mice received i.p. injections twice daily (0800 and 1800 hours) of either saline or recombinant murine leptin (2.5 μg/g of body weight) (Amgen Biologicals) for 4 days. Average daily food consumption during the treatment period was compared between wild-type and Pomc-/- mice.
Statistical Analysis. Values are reported as means ± SEM. All data sets were analyzed for statistical significance by using Student's t test, with the exception of body weights, which were analyzed by repeated-measures ANOVA. The prism software package (GraphPad, San Diego) was used for all analyses.
Results
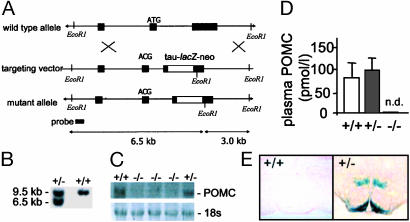
Generation of Pomc-Deficient Mice. The Pomc gene was selectively inactivated by homologous recombination with a targeting vector construct that replaced the majority of exon 3 with a tau-lacZ reporter and neor cassette driven by a PGK gene promoter (Fig. 1A). In addition, point mutations were introduced at the initiator ATG to create a stop codon. A single ES cell clone was detected that carried a correctly targeted Pomc allele (data not shown). Chimeras of the ES cell clone transmitted the mutation through the germ line when mated with 129S2/SVHsd females. F1 heterozygous mice were intercrossed to generate F2 wild-type, Pomc+/- or Pomc-/- mice. Southern blotting of genomic DNA confirmed correct targeting of the Pomc locus (Fig. 1B). Northern hybridization analysis demonstrated that Pomc-/- mice did not express POMC mRNA in the brain, and, by using a two-site immunoassay, no POMC-derived peptides were detected in plasma from Pomc-/- mice (Fig. 1 C and D). Finally, histochemical staining of Pomc+/- mouse brains demonstrated β-galactosidase activity in brain regions that express Pomc including the hypothalamic arcuate nucleus (Fig. 1E) and nucleus of the solitary tract (data not shown). Pomc-/- mice were born at a frequency of 8% rather than the 25% frequency expected for a recessive disorder from a heterozygous mating, suggesting that partial embryonic lethality is associated with POMC deficiency. This result was also observed with the previous model of POMC deficiency (7). The partial lethality was not corrected on glucocorticoid administration (25 mg/liter) to the drinking water of pregnant dams (data not shown).
Fig. 1.
Targeted deletion of the murine Pomc locus. (A) Schematic diagrams and partial restriction maps of the mouse wild-type Pomc locus, Pomc targeting vector, and mutant Pomc allele. The filled boxes represent Pomc coding sequence or 5′ probe. The open boxes represent the tau-lacZ-PGK-neor cassette. The loxP sites (not shown) are in direct orientation such that a Cre-mediated recombination event would cause removal of the PGK promoter and neor coding sequence. (B) Southern blot analysis of tail DNA from wild-type and Pomc+/- mice digested with EcoRI. The 9.5-kb band represents the wild-type allele whereas the lower, 6.5-kb band represents the targeted allele. (C) Northern blot analysis showing an absence of POMC mRNA expression in brains from Pomc-/- mice. (Lower) UVL photograph of 18s RNA as loading control. (D) Two-site immunoassay showing that peripheral POMC peptides are not detectable (n.d.) in plasma from Pomc-/- mice. (E) Histochemical staining for β-galactosidase activity in brains from Pomc+/- mice demonstrated POMC-expressing neurons projecting from the arcuate nucleus to hypothalamic and extra-hypothalamic sites. No staining is observed in brains from wild-type mice.
Phenotype of Pomc-/- Mice. Consistent with the known effects of POMC-derived peptides and the previous POMC-deficient model (7), Pomc-/- mice had undetectable corticosterone and a subtle pigmentation phenotype consisting of a lighter ventral coat color compared with wild-type mice (Fig. 2A). Pomc+/- mice had no discernible pigmentation phenotype. Interestingly, however, Pomc+/- mice had lower corticosterone levels than wild-type mice (84 ± 10 vs. 242 ± 16 ng/ml, respectively; P < 0.01) despite normal adrenal gland structure (data not shown).
Fig. 2.
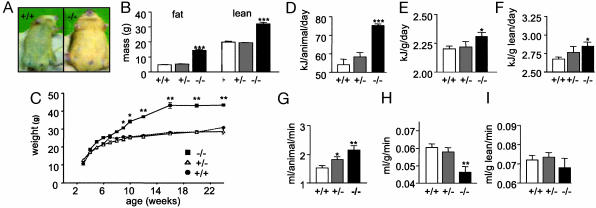
Phenotype of Pomc-/- mice. (A) Pomc-/- mice develop obesity and have a lighter coat color compared with a sex- and age-matched 4-month-old wild-type littermate. (B) Fat and lean mass were determined by dual-energy x-ray absorptiometry (DEXA) of group-housed 2- to 3-month-old male mice. The data represent the means ± SEM. n = 5 for each genotype. ***, P < 0.001 compared with wild-type mice. (C) Body weights of group-housed male mice on low-fat diet (wild-type, n = 10; Pomc+/-, n = 10; Pomc-/-, n = 4). Body weight of Pomc-/- mice is statistically different from wild type (*, P < 0.05 at 10 weeks, reaching **, P < 0.01 at 24 weeks). There is no difference between growth curves of wild-type and Pomc+/- mice. (D) Food intake of individually housed 2-month-old male mice maintained on a low-fat diet (n = 8 for each genotype). Data were corrected for total body mass (E) and lean body mass (F). Pomc-/- vs. wild-type mice, *, P < 0.05, ***, P < 0.001. (G) Oxygen consumption in 3-month-old male mice was determined by indirect calorimetry as described (n = 5 for each genotype). Data were corrected for total body mass (H) and lean body mass (I). Pomc-/- vs. wild-type mice, *, P < 0.05, **, P < 0.01.
When weaned onto standard lab chow (4.5% fat) Pomc-/- showed a statistically significant increase in body weight from 10 weeks whereas Pomc+/- mice were similar in weight to wild-type mice throughout (Fig. 2B). Dual-energy x-ray absorptiometry was used to examine body composition in 2- to 3-month-old male mice. Pomc-/- mice had a significantly higher fat and lean tissue mass compared with wild-type mice (14.5 ± 0.7 vs. 4.0 ± 0.2 g and 32.1 ± 1.0 vs. 19.9 ± 0.6, respectively; P < 0.001 for both) (Fig. 2B). In absolute terms, energy intake per animal was significantly elevated in Pomc-/- mice compared with wild-type mice (76.6 ± 5.7 vs. 60.3 ± 7.1 kJ/day, respectively; P < 0.05) (Fig. 2D). This finding is not unexpected, given that at this age Pomc-/- mice are already significantly heavier than wild-type littermates. However, when corrected for both total and lean mass, Pomc-/- mice were still significantly hyperphagic when compared with wild-type mice (intake per g of total mass: 2.31 ± 0.02 vs. 2.21 ± 0.03 kJ, respectively; P < 0.05; intake per g of lean mass per day: 2.85 ± 0.05 vs. 2.67 ± 0.03 kJ, respectively; P < 0.05). Indirect calorimetry was used to investigate the effect of POMC deficiency on oxygen consumption in 3- to 4-month-old mice. Again, in absolute terms, Pomc-/- mice had higher resting oxygen consumption than wild-type mice (Fig. 2G). However, after correction for total body mass, Pomc-/- mice had a significantly lower resting oxygen consumption (ROC) than wild-type mice (0.0463 ± 0.003 vs. 0.0599 ± 0.002 ml/g per min, respectively; P < 0.01) (Fig. 2H). In keeping with this reduced ROC, total plasma thyroxine was also significantly reduced in freely feeding Pomc-/- mice compared with wild-type and Pomc+/- mice (55.7 ± 5.1 vs. 55 ± 5.1 vs. 35 ± 4.4 nmol/liter, wild-type, Pomc+/-, and Pomc-/-, respectively; P < 0.05).
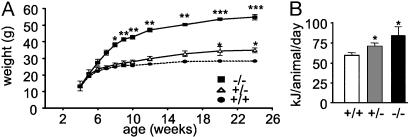
Effects of High-Fat Feeding. High-fat feeding augmented the obese phenotype in Pomc-/- mice, with weight at 6 months significantly higher that that attained on the low-fat chow (54.9 ± 1.7 vs. 43.6 ± 0.3 g, respectively; P = 0.02) (Figs. 2B and 3A). Interestingly, Pomc+/- mice also developed obesity on the high-fat diet, becoming significantly heavier than low-fat chow-fed Pomc+/- mice (weight at 6 months: 34.9 ± 1.5 vs. 28.5 ± 1.3 g, respectively; P < 0.05). Provision of a high-fat diet had no effect on body weight of wild-type mice (Fig. 3A). These results were confirmed in an independent cohort of wild-type mice (data not shown) and are consistent with the known resistance of the 129 strain to the obesogenic effects of high-fat feeding. Energy intake was examined in mice fed the high-fat diet. Energy intake in both Pomc-/- and Pomc+/- mice was significantly elevated compared with wild-type mice (84.0 ± 11.1, 70.9 ± 4.1, and 59.6 ± 3.6 kJ/day, respectively; P < 0.05) (Fig. 3B).
Fig. 3.
Effects of high-fat feeding in Pomc-/-, Pomc+/-, and wild-type mice. (A) On a high-fat diet, Pomc-/- mice are significantly heavier at 8 weeks (*, P < 0.05) and attain a greater adult body weight compared with wild-type mice (wild-type, n = 10, Pomc+/-, n = 10; Pomc-/-, n = 4). ***, P < 0.001. Pomc+/- mice also develop obesity on a high-fat diet compared with wild-type mice by 20 weeks (*, P < 0.05). (B) Food intake of individually housed 2-month-old male mice maintained on a high-fat diet (n = 4 for each group). Pomc-/- and Pomc+/- vs. wild-type mice, *, P < 0.05.
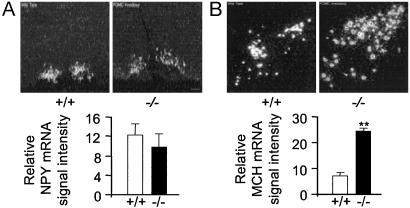
NPY and MCH mRNA Expression Levels in the Hypothalamus of Pomc-/- Mice. To determine whether the hyperphagia in Pomc-/- mice could be attributed to altered hypothalamic neuropeptide expression, in situ hybridization was used to examine the mRNA levels of two potent orexigenic neuropeptides, NPY and MCH in 3-month-old male mice. Quantitation of NPY mRNA expression in the arcuate nucleus revealed no significant differences between wild-type and Pomc-/- mice (Fig. 4). The DMH has been previously identified as a site of increased NPY expression in Mc4R-/- and Ay/a mice (12). In the present study, however, NPY mRNA expression was not observed in the DMH of Pomc-/- mice (Fig. 4A). MCH is an appetite-stimulating peptide that is expressed in the lateral hypothalamus (4). MCH mRNA levels were elevated ≈4-fold (P < 0.01) in the lateral hypothalamus of Pomc-/- mice compared with wild-type controls (Fig. 4 C and D).
Fig. 4.
Hypothalamic NPY and MCH mRNA expression in Pomc-/- and wild-type mice. (A) Pomc-/- mice have normal levels of NPY mRNA in the arcuate nucleus but an absence of NPY in the DMH. Shown are representative darkfield photomicrographs of the arcuate nucleus from male wild-type and Pomc-/- mice. (Scale bar = 150 μm.) Graphed data represent mean of four consecutive brain sections taken from a single brain. Four brains of each genotype were analyzed. (B) MCH mRNA levels are elevated in the lateral hypothalamus of Pomc-/- mice. Shown are representative darkfield photomicrographs of the lateral hypothalamus in a wild-type and Pomc-/-. (Scale bar = 200 μm.) Graphed data represent mean of six consecutive brain sections taken from a single brain. Five brains of each genotype were analyzed. **, P < 0.01.
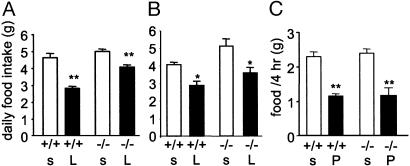
Effects of Leptin and PYY3-36 on Food Intake in Pomc-/- Mice. The effects of leptin on food intake are thought to involve both suppression of arcuate NPY/AGRP neurons and activation of POMC/cocaine and amphetamine regulated transcript (CART) neurons (5). To examine the extent to which POMC peptides are necessary for mediating the effects of leptin on food intake, leptin was administered i.p., twice daily for 4 days to 12- to 15-week-old Pomc-/- and age-matched wild-type controls. Leptin significantly reduced food intake in wild-type mice by 37% (sham-treated, 4.4 ± 0.2 vs. leptin-treated, 2.8 ± 0.1 g/day, P < 0.001) (Fig. 5A). In obese, 12- to 15-week-old Pomc-/- mice, leptin reduced food intake to a lesser degree (19%) than that seen in wild-type mice (Pomc-/- sham-treated, 5.0 ± 0.1 vs. Pomc-/- leptin-treated, 4.1 ± 0.1 g/day, P < 0.001). In addition, after leptin treatment, wild-type mice lost significantly more weight than Pomc-/- mice (body weight as a percentage of pretreatment weight, wild-type, 92 ± 0.9% vs. Pomc-/-, 95.8 ± 0.7%, P = 0.03). To determine whether the reduced ability of leptin to diminish food intake and cause weight loss in older, obese Pomc-/- mice was also seen in younger, nonobese mice, leptin was administered to age- and weight-matched 4-week-old mice. The percentage reduction in food intake in wild-type and Pomc-/- mice was identical (wild-type, 29.3% vs. Pomc-/-, 29.4%) (Fig. 5B), and the magnitude of weight loss after leptin treatment was not significantly different between genotypes (data not shown). Thus, young, nonobese Pomc-/- mice retain full sensitivity to the effects of peripherally administered leptin.
Fig. 5.
Effects of leptin and PYY3-36 on feeding in Pomc-/- and wild-type mice. (A and B) Response of 12- to 15-week-old (A; n = 6 for all groups) and 4-week-old (B; n = 4-7) freely feeding, male wild-type and Pomc-/- mice to peripherally administered leptin. Data represent mean ± SEM daily food intake over 4 days of saline (s) or leptin (L) treatment. Leptin vs. saline, *, P < 0.05, **, P < 0.01. (C) Acute feeding response of 12- to 15-week-old, fasted, male wild-type and Pomc-/- mice to PYY3-36 or saline administered i.p. Data represent mean ± SEM food intake 4 h after saline or PYY3-36 treatment. n = 8 for all groups. PYY3-36 (P) vs. saline (s), **, P < 0.01.
The acute and chronic effects of PYY3-36 treatment on food intake were investigated in 8- to 12-week-old wild-type and Pomc-/- mice. In the acute study, mice were fasted for 24 h and administered a single i.p. injection of either saline or PYY3-36, and total food intake after 4 h was measured. PYY3-36 was capable of suppressing short-term food intake similarly in wild-type and Pomc-/- mice (Fig. 5C). In contrast, we found that the effect on cumulative food intake of chronically administered PYY3-36 (2 daily i.p. injections for 7 days) was indistinguishable from that of saline in both wild-type and Pomc-/- mice (Fig. 6, which is published as supporting information on the PNAS web site). Similarly, chronic PYY3-36 treatment had no effect on body weight in both wild-type (body weight percentage as pretreatment weight: sham, 99.9 ± 0.9%; PYY3-36, 102.2 ± 0.5%) and Pomc-/- mice (sham, 97.3 ± 2.5%; PYY3-36, 95.0 ± 1.6%).
Discussion
Yaswen et al. (7) originally described a model of murine POMC deficiency on a 129/SvEv genetic background. Their strategy involved targeted deletion of exon 3 with exception of the first 18 aa of the POMC protein. The authors found that these animals developed hyperphagic obesity, lacked identifiable adrenal glands, had no detectable levels of circulating corticosterone, and exhibited an increase in the yellow pigment, phaeomelanin, in melanocytes. Subsequently, Smart and Low (13) described the phenotype of these mice back-crossed onto a C57BL/6 genetic background. These animals developed an obesity phenotype that was indistinguishable from the 129 strain of mice. However, pigmentation was unaltered on the C57BL/6 background, and these mice had identifiable, although atrophied and severely dysfunctional, adrenal glands. We have created an independent model of POMC deficiency on the 129/Sv background that differs somewhat from that of Yaswen et al. in that all POMC-derived peptides are ablated. Furthermore, the use of the tau-lacZ reporter construct has enabled us to produce a model that will greatly facilitate future studies to determine the effects of experimental manipulations on the neuroanatomy of POMC-expressing neurons.
In this study, we have confirmed that POMC plays a crucial role in the long-term maintenance of energy homeostasis. Furthermore, and in agreement with Smart and Low, our Pomc-/- mice have identifiable, although atrophic and hypofunctional, adrenal glands (unpublished observations). Having established that POMC deficiency results in a hyperphagic obesity syndrome, we have gone on to explore the basis of the altered energy balance in greater detail.
When raised on a low-fat diet, Pomc-/- mice develop severe, early-onset obesity. This phenotype results from increased food intake and a reduction in resting basal metabolic rate. These data are consistent with what has been reported previously in other mouse models of melanocortin dysfunction (14-16). For example, Ste. Marie et al. (14) found that, in addition to hyperphagia, Mc4R-/- mice consumed 20% less oxygen then wild-type mice. The reduction in metabolic rate may be explained, in part, by hypothyroidism because freely feeding Pomc-/- mice have a significantly lower plasma thyroxine level than their wild-type littermates. These results are in agreement with previous studies implicating central melanocortin signaling in the regulation of the hypothalamic-pituitary-thyroidal axis (17, 18).
Provision of a high-fat diet greatly exaggerated the obese phenotype in Pomc-/- mice whereas wild-type mice maintained a body weight similar to that of mice reared on the low-fat diet. Interestingly, Pomc+/- mice fed the high-fat diet exhibited an intermediate obese phenotype, thus demonstrating that, under certain environmental conditions, a single functional copy of the Pomc gene is not sufficient for maintaining normal energy homeostasis. Given the evidence implicating the region of human chromosome 2 containing the POMC gene as a susceptibility locus for common human obesity, it is interesting to note that haploinsufficiency of this gene can interact with dietary factors to increase body weight (19-21).
Previous studies have shown that, in response to an increase in dietary fat, wild-type mice increase diet-induced thermogenesis and physical activity while reducing energy intake to levels isocaloric to those observed on a low-fat diet, thereby maintaining a stable body weight (22). Butler et al. (22) demonstrated that Mc4R-/- mice are defective in this response and consequently exhibit hyperphagia and exaggerated weight gain whereas, under the same circumstances, Lepob/ob and Mc3R-/- mice regulated their metabolism similarly to wild-type mice. In view of the accelerated weight gain and increased caloric intake observed in Pomc-/- mice, our data demonstrate that these mice also lack the ability to coordinate their metabolism in response to changes in dietary fat.
NPY potently stimulates feeding after intracerebroventricular administration (23) and is up-regulated in several rodent obesity models including Lepob/ob and db/db mice (24). Consistent with the findings in Ay/a and Mc4R-/- mice (12), arcuate NPY mRNA levels were not elevated in Pomc-/- mice compared with wild-type mice. In other melanocortin obesity models, such as the Ay/a and Mc4R-/- mice, NPY mRNA expression is induced in the DMH of obese, but not young nonobese, mutant mice (12). These observations suggest that altered melanocortinergic signaling within this brain region may be involved in the development of obesity in these mice. Interestingly, however, NPY mRNA expression was absent in the DMH of obese Pomc-/- mice. The discrepancy between these mouse models may be explained by several possibilities. First, the MC4R may possess constitutive activity in vivo that may be adequate to maintain downstream NPY signaling in the DMH of Pomc-/- mice, without protecting against the development of obesity. Indeed, the mild pigmentation phenotype in Pomc-/- mice, compared with the marked yellow coat color observed in mice lacking Mc1R, suggests that in vivo constitutive activity may be a property of the melanocortin receptor family (25). Alternatively, the absence of circulating glucocorticoids may impact on hypothalamic NPY signaling, and studies are currently ongoing to evaluate neuropeptide expression in glucocorticoid-replete Pomc-/- mice.
Centrally administered MCH potently stimulates food intake (26) and, when infused chronically, causes obesity in mice (27). Furthermore, Mch-null mice are hypophagic and lean (28) whereas transgenic mice overexpressing MCH become obese (29), thus confirming an important role for MCH in energy balance. Hanada et al. (30) previously demonstrated that MCH expression is augmented in Ay/a mice and in rats treated intracerebroventricularly with AgRP or MC3/4-R antagonists. Here, we demonstrated that MCH mRNA is up-regulated in Pomc-/- mice, thus providing further evidence that MCH neuronal activity is negatively regulated by melanocortin peptides. Data from neuroanatomical studies suggest that this regulation occurs indirectly. For example, using a transgenic mouse model in which GFP expression was under control of the MC4R promoter, Liu et al. (31) failed to detect GFP-IR cells coexpressing MCH. Similarly, Kishi et al. (32) recently reported that MC4R mRNA expression levels are very low in the rat lateral hypothalamus. Nonetheless, our observations suggest that loss of MCH regulation may be involved in the etiology of the melanocortin obesity syndromes. However, the mechanism(s) through which melanocortins regulate MCH gene expression remain to be elucidated.
Arcuate POMC neurons are thought to mediate the satiety effects of a number of circulating peripheral hormones, including leptin and PYY3-36 (1, 3). To determine the extent to which POMC peptides are required for the responsiveness of these hormones, exogenous leptin and PYY3-36 were administered peripherally to wild-type and Pomc-/- mice. The signaling isoform of the leptin receptor is expressed on POMC neurons in the arcuate nucleus, and POMC expression is reduced in Lepob/ob and db/db mice (33). Furthermore, loss-of-function mutations in the genes encoding leptin, leptin receptor, POMC, and MC4R result in severe obesity in rodents and man (34). Taken together, these data suggest that the melanocortin axis is a major pathway by which leptin mediates its effects on energy balance. However, our data demonstrate that leptin can suppress food intake by means of a melanocortin-independent pathway, a result similar to that found in Mc4R-/- mice (35) and supported by the additive weight gain and leptin sensitivity observed in Lepob/ob Ay/a double mutant mice (36). Young, nonobese Pomc-/- mice are completely sensitive to the inhibitory effects of peripherally administered leptin on food intake whereas older, obese Pomc-/- mice demonstrate partial leptin resistance. The defective leptin action seen in the older mice is likely due to the development of leptin resistance as a consequence of obesity.
PYY3-36, a Y2-receptor agonist, is secreted from endocrine L cells of the gastrointestinal tract in response to caloric content of a meal (37). Recently, PYY3-36 has been suggested to be a mediator of postprandial satiety in rodents (3) and man (38) through activation of hypothalamic POMC neurons (3). In light of these previous observations, we were intrigued to find that Pomc-/- mice retained a normal, acute anorectic response to peripherally administered PYY3-36. These data demonstrate that melanocortin peptides are not required for the inhibitory effects of PYY3-36 on feeding and suggest that a reduction of NPY neuronal activity may play a more prominent role in PYY3-36-induced hypophagia. Indeed, PYY3-36 is capable of reducing NPY mRNA levels in the arcuate nucleus (3, 39). Although PYY3-36 elicited a robust suppression of short-term food intake, we did not observe any changes in cumulative food intake or body weight in either Pomc-/- or wild-type mice chronically treated with PYY3-36. These results contrast those reported by Batterham et al. (3) in rats and may reflect a species difference in long-term sensitivity to PYY3-36 treatment. The observation that cumulative food intake was not different between wild-type and Pomc-/- mice treated with either saline or PYY3-36 likely reflects an increased sensitivity of Pomc-/- mice to certain stressors such as handling or i.p. injection, thereby causing stress-induced hypophagia. Indeed, similar findings have been reported in Ay/a mice (40).
In conclusion, in a newly derived strain of Pomc-/- mice, we have confirmed the effects of this deficiency on body weight, fat mass, and appetite. We have also demonstrated that Pomc-/- mice have a reduced metabolic rate that may also contribute to the obesity phenotype. In addition, POMC deficiency results in a marked up-regulation of MCH mRNA expression in the lateral hypothalamus but has no effects on arcuate NPY mRNA expression. As has previously been reported for mice lacking Mc4R, Pomc-/- mice show an exaggeration of hyperphagia and obesity in response to a high-fat diet, and such a diet exposes an obesity phenotype in heterozygous mice. In contrast to what might be predicted from its proposed mode of action, Pomc-/- mice retain a normal acute anorectic response to the putative gut-derived satiety hormone PYY3-36, and the chronic administration of PYY3-36 had no effect on body weight or cumulative food intake in either Pomc-/- or wild-type mice.
Supplementary Material
Acknowledgments
We thank Sarah Penning for expert technical assistance. This research was supported by the United Kingdom Medical Research Council, the Bristol-Myers Squibb Foundation, and a Raymond and Beverly Sackler Fellowship (to A.P.C.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: POMC, pro-opiomelanocortin; MCH, melanin-concentrating hormone; NPY, neuropeptide Y; PYY3-36, peptide-YY3-36; MC4R, melanocortin-4 receptor; DMH, dorsomedial hypothalamic nucleus; AgRP, agouti-related protein; ES, embryonic stem.
References
- 1.Cowley, M. A., Smart, J. L., Rubinstein, M., Cerdan, M. G., Diano, S., Horvath, T. L., Cone, R. D. & Low, M. J. (2001) Nature 411, 480-484. [DOI] [PubMed] [Google Scholar]
- 2.Heisler, L. K., Cowley, M. A., Tecott, L. H., Fan, W., Low, M. J., Smart, J. L., Rubinstein, M., Tatro, J. B., Marcus, J. N., Holstege, H., et al. (2002) Science 297, 609-611. [DOI] [PubMed] [Google Scholar]
- 3.Batterham, R. L., Cowley, M. A., Small, C. J., Herzog, H., Cohen, M. A., Dakin, C. L., Wren, A. M., Brynes, A. E., Low, M. J., Ghatei, M. A., et al. (2002) Nature 418, 650-654. [DOI] [PubMed] [Google Scholar]
- 4.Saper, C. B., Chou, T. C. & Elmquist, J. K. (2002) Neuron 36, 199-211. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz, M. W., Woods, S. C., Porte, D., Jr., Seeley, R. J. & Baskin, D. G. (2000) Nature 404, 661-671. [DOI] [PubMed] [Google Scholar]
- 6.Cheung, C. C., Clifton, D. K. & Steiner, R. A. (1997) Endocrinology 138, 4489-4492. [DOI] [PubMed] [Google Scholar]
- 7.Yaswen, L., Diehl, N., Brennan, M. B. & Hochgeschwender, U. (1999) Nat. Med. 5, 1066-1070. [DOI] [PubMed] [Google Scholar]
- 8.Krude, H., Biebermann, H., Luck, W., Horn, R., Brabant, G. & Gruters, A. (1998) Nat. Genet. 19, 155-157. [DOI] [PubMed] [Google Scholar]
- 9.Bicknell, A. B., Lomthaisong, K., Woods, R. J., Hutchinson, E. G., Bennett, H. P., Gladwell, R. T. & Lowry, P. J. (2001) Cell 105, 903-912. [DOI] [PubMed] [Google Scholar]
- 10.Crosby, S. R., Stewart, M. F., Ratcliffe, J. G. & White, A. (1988) J. Clin. Endocrinol. Metab. 67, 1272-1277. [DOI] [PubMed] [Google Scholar]
- 11.Pinnock, S. B. & Herbert, J. (2001) Eur. J. Neurosci. 13, 576-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kesterson, R. A., Huszar, D., Lynch, C. A., Simerly, R. B. & Cone, R. D. (1997) Mol. Endocrinol. 11, 630-637. [DOI] [PubMed] [Google Scholar]
- 13.Smart, J. L. & Low, M. J. (2003) Ann. N.Y. Acad. Sci. 994, 202-210. [DOI] [PubMed] [Google Scholar]
- 14.Ste. Marie, L., Miura, G. I., Marsh, D. J., Yagaloff, K. & Palmiter, R. D. (2000) Proc. Natl. Acad. Sci. USA 97, 12339-12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen, A. S., Marsh, D. J., Trumbauer, M. E., Frazier, E. G., Guan, X. M., Yu, H., Rosenblum, C. I., Vongs, A., Feng, Y., Cao, L., et al. (2000) Nat. Genet. 26, 97-102. [DOI] [PubMed] [Google Scholar]
- 16.Huszar, D., Lynch, C. A., Fairchild-Huntress, V., Dunmore, J. H., Fang, Q., Berkemeier, L. R., Gu, W., Kesterson, R. A., Boston, B. A., Cone, R. D., et al. (1997) Cell 88, 131-141. [DOI] [PubMed] [Google Scholar]
- 17.Kim, M. S., Small, C. J., Stanley, S. A., Morgan, D. G., Seal, L. J., Kong, W. M., Edwards, C. M., Abusnana, S., Sunter, D., Ghatei, M. A. & Bloom, S. R. (2000) J. Clin. Invest. 105, 1005-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris, M., Aschkenasi, C., Elias, C. F., Chandrankunnel, A., Nillni, E. A., Bjoorbaek, C., Elmquist, J. K., Flier, J. S. & Hollenberg, A. N. (2001) J. Clin. Invest. 107, 111-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Comuzzie, A. G., Hixson, J. E., Almasy, L., Mitchell, B. D., Mahaney, M. C., Dyer, T. D., Stern, M. P., MacCluer, J. W. & Blangero, J. (1997) Nat. Genet. 15, 273-276. [DOI] [PubMed] [Google Scholar]
- 20.Hager, J., Dina, C., Francke, S., Dubois, S., Houari, M., Vatin, V., Vaillant, E., Lorentz, N., Basdevant, A., Clement, K., et al. (1998) Nat. Genet. 20, 304-308. [DOI] [PubMed] [Google Scholar]
- 21.Hixson, J. E., Almasy, L., Cole, S., Birnbaum, S., Mitchell, B. D., Mahaney, M. C., Stern, M. P., MacCluer, J. W., Blangero, J. & Comuzzie, A. G. (1999) J. Clin. Endocrinol. Metab. 84, 3187-3191. [DOI] [PubMed] [Google Scholar]
- 22.Butler, A. A., Marks, D. L., Fan, W., Kuhn, C. M., Bartolome, M. & Cone, R. D. (2001) Nat. Neurosci. 4, 605-611. [DOI] [PubMed] [Google Scholar]
- 23.Stanley, B. G., Kyrkouli, S. E., Lampert, S. & Leibowitz, S. F. (1986) Peptides 7, 1189-1192. [DOI] [PubMed] [Google Scholar]
- 24.Stephens, T. W., Basinski, M., Bristow, P. K., Bue-Valleskey, J. M., Burgett, S. G., Craft, L., Hale, J., Hoffmann, J., Hsiung, H. M., Kriauciunas, A., et al. (1995) Nature 377, 530-532. [DOI] [PubMed] [Google Scholar]
- 25.Robbins, L. S., Nadeau, J. H., Johnson, K. R., Kelly, M. A., Roselli-Rehfuss, L., Baack, E., Mountjoy, K. G. & Cone, R. D. (1993) Cell 72, 827-834. [DOI] [PubMed] [Google Scholar]
- 26.Qu, D., Ludwig, D. S., Gammeltoft, S., Piper, M., Pelleymounter, M. A., Cullen, M. J., Mathes, W. F., Przypek, R., Kanarek, R. & Maratos-Flier, E. (1996) Nature 380, 243-247. [DOI] [PubMed] [Google Scholar]
- 27.Gomori, A., Ishihara, A., Ito, M., Mashiko, S., Matsushita, H., Yumoto, M., Tanaka, T., Tokita, S., Moriya, M., Iwaasa, H. & Kanatani, A. (2003) Am. J. Physiol. Endocrinol. Metab. 284, E583-E588. [DOI] [PubMed] [Google Scholar]
- 28.Shimada, M., Tritos, N. A., Lowell, B. B., Flier, J. S. & Maratos-Flier, E. (1998) Nature 396, 670-674. [DOI] [PubMed] [Google Scholar]
- 29.Ludwig, D. S., Tritos, N. A., Mastaitis, J. W., Kulkarni, R., Kokkotou, E., Elmquist, J., Lowell, B., Flier, J. S. & Maratos-Flier, E. (2001) J. Clin. Invest. 107, 379-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanada, R., Nakazato, M., Matsukura, S., Murakami, N., Yoshimatsu, H. & Sakata, T. (2000) Biochem. Biophys. Res. Commun. 268, 88-91. [DOI] [PubMed] [Google Scholar]
- 31.Liu, H., Kishi, T., Roseberry, A. G., Cai, X., Lee, C. E., Montez, J. M., Friedman, J. M. & Elmquist, J. K. (2003) J. Neurosci. 23, 7143-7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kishi, T., Aschkenasi, C. J., Lee, C. E., Mountjoy, K. G., Saper, C. B. & Elmquist, J. K. (2003) J. Comp. Neurol. 457, 213-235. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz, M. W., Seeley, R. J., Woods, S. C., Weigle, D. S., Campfield, L. A., Burn, P. & Baskin, D. G. (1997) Diabetes 46, 2119-2123. [DOI] [PubMed] [Google Scholar]
- 34.O'Rahilly, S., Farooqi, I. S., Yeo, G. S. & Challis, B. G. (2003) Endocrinology 144, 3757-3764. [DOI] [PubMed] [Google Scholar]
- 35.Marsh, D. J., Hollopeter, G., Huszar, D., Laufer, R., Yagaloff, K. A., Fisher, S. L., Burn, P. & Palmiter, R. D. (1999) Nat. Genet. 21, 119-122. [DOI] [PubMed] [Google Scholar]
- 36.Boston, B. A., Blaydon, K. M., Varnerin, J. & Cone, R. D. (1997) Science 278, 1641-1644. [DOI] [PubMed] [Google Scholar]
- 37.Neary, N. M., Small, C. J. & Bloom, S. R. (2003) Gut 52, 918-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Batterham, R. L., Cohen, M. A., Ellis, S. M., Le Roux, C. W., Withers, D. J., Frost, G. S., Ghatei, M. A. & Bloom, S. R. (2003) N. Engl. J. Med. 349, 941-948. [DOI] [PubMed] [Google Scholar]
- 39.Challis, B. G., Pinnock, S. B., Coll, A. P., Carter, R. N., Dickson, S. L. & O'Rahilly, S. (2003) Biochem. Biophys. Res. Commun. 311, 915-919. [DOI] [PubMed] [Google Scholar]
- 40.De Souza, J., Butler, A. A. & Cone, R. D. (2000) Neuroendocrinology 72, 126-132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.