Abstract
Terminally differentiated post-mitotic cells are generally considered irreversibly developmentally locked, i.e. incapable of being reprogrammed in vivo into entirely different cell types. We found that brief expression of a single transcription factor, the ELT-7 GATA factor, can convert the identity of fully differentiated, highly specialized non-endodermal cells of the pharynx into fully differentiated intestinal cells in intact larvae and adult Caenorhabditis elegans. Stable expression of intestine-specific molecular markers parallels loss of markers for the original differentiated pharynx state; hence, there is no apparent requirement for a dedifferentiated intermediate during the transdifferentiation process. Based on high-resolution morphological characteristics, the transdifferentiated cells become remodeled to resemble typical intestinal cells at the level of both the cell surface and internal organelles. Thus, post-mitotic cells, though terminally differentiated, remain plastic to transdifferentiation across germ layer lineage boundaries and can be remodeled to adopt the characteristics of a new cell identity without removal of inhibitory factors. Our findings establish a simple model to investigate how cell context influences forced transdifferentiation of mature cells.
Keywords: Transdifferentiation, Cellular reprogramming, C. elegans
INTRODUCTION
Cell fate is progressively restricted during development such that most cells ultimately become post-mitotic, highly specialized and terminally differentiated, with a fixed morphology and pattern of gene expression. Early embryonic cells in C. elegans are capable of being reprogrammed into cell types of all three germ layers by appropriate transcription factors, but lose this ability during mid-embryogenesis, as they commit to particular pathways of differentiation (Horner et al., 1998; Zhu et al., 1998; Gilleard and McGhee, 2001; Quintin et al., 2001; Fukushige and Krause, 2005). Eliminating polycomb complex (Yuzyuk et al., 2009) or Notch signaling (Djabrayan et al., 2012) components extends the period for multipotency during embryogenesis; however, post-mitotic somatic cells in late embryos, larvae or adults cannot be reprogrammed by the transcription factors reported to date (Horner et al., 1998; Zhu et al., 1998; Gilleard and McGhee, 2001; Quintin et al., 2001; Fukushige and Krause, 2005). Developing and proliferating germline cells can prematurely adopt somatic cell fates upon removal of translational regulators (Ciosk et al., 2006) or inhibitory chromatin remodeling factors (Tursun et al., 2011; Patel et al., 2012). However, germline cells are uncommitted pluripotent cells that are poised to differentiate shortly after gametogenesis, whereas most somatic cells become terminally differentiated.
We report here that a transcription factor that regulates terminal intestine differentiation violates the embryonic multipotency to commitment transition (MCT) and can reprogram and remodel differentiated cells in C. elegans larvae and adults into intestine-like cells without removal of inhibitory factors. We found that a brief pulse of the ELT-7 GATA transcription factor activates intestine-specific gene expression in diverse non-intestinal cells. Post-mitotic pharyngeal cells maintain intestine gene expression, lose pharynx gene expression, and become remodeled to resemble intestinal cells at the fine ultrastuctural level. Our results suggests that post-mitotic, terminally differentiated cells can be reprogrammed and remodeled without prior removal of the initial cell identity and demonstrate that susceptibility to in vivo reprogramming is influenced by transcription factor identity and cellular context.
RESULTS AND DISCUSSION
ELT-7 activates and maintains intestine-specific gene expression in differentiated pharyngeal cells at all stages of development
Although early embryonic cells in C. elegans are multipotent, cells become refractory to reprogramming later in embryogenesis, following the MCT (Horner et al., 1998; Zhu et al., 1998; Gilleard and McGhee, 2001; Quintin et al., 2001; Fukushige and Krause, 2005). In contrast to other regulators of cell fate specification that have been reported, we found that ELT-7, a GATA transcription factor that regulates terminal intestinal differentiation (Maduro and Rothman, 2002; Sommermann et al., 2010), activates an intestinal marker (elt-2::lacZ::GFP) in non-intestinal cells when briefly ectopically expressed via a heat-shock promoter at any embryonic, larval, or adult stage (Fig. 1A-C; supplementary material Fig. S1). We also detected IFB-2, an intermediate filament protein that is a component of the terminal web of the fully differentiated intestine (Bossinger et al., 2004), outside of the intestine in such animals (Fig. 1D; supplementary material Fig. S2). By contrast, the END-1 GATA transcription factor, which specifies the endoderm progenitor (Zhu et al., 1997), does not activate widespread intestinal gene expression after the MCT (Fig. 1; supplementary material Fig. S2). Thus, ELT-7 may violate the well-documented restriction to developmental reprogramming that occurs during embryogenesis.
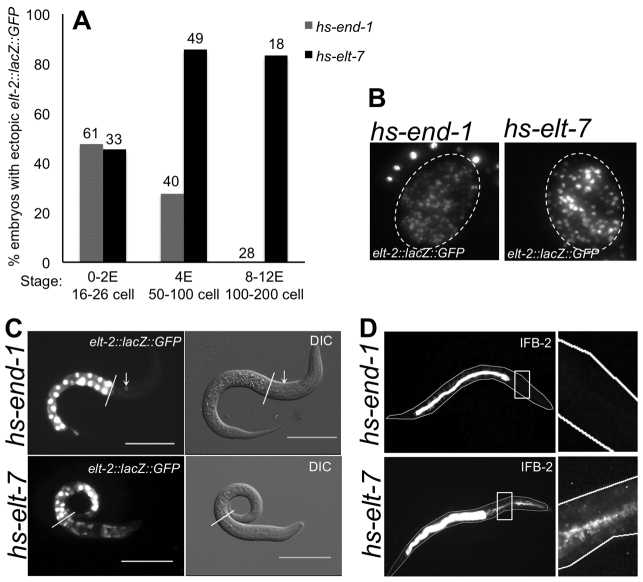
Fig. 1.
ELT-7, but not END-1, activates endoderm differentiation in differentiated non-endodermal cells. (A) Percentage of embryos showing ectopic elt-2::lacZ::GFP expression (>20 GFP+ nuclei) following heat shock at various embryonic stages (based on E cell number). Total number of embryos scored is shown above each bar. (B) Embryos with widespread ectopic elt-2::lacZ::GFP expression after ubiquitous END-1 or ELT-7 induction. (C) Transient ectopic elt-2::lacZ::GFP in one cell outside the pharynx (arrow) after ectopic END-1 expression and in many cells throughout the body after ectopic ELT-7 expression. White line indicates the anterior end of the intestine. Scale bars: 20 μm. (D) Expression of IFB-2 outside the intestine of hs-elt-7 but not hs-end-1 larvae. Insets show region anterior to the endogenous intestine, 24 hours post-heat-shock.
In the endogenous intestine, ELT-7 and ELT-2 expression is maintained throughout larval development and adulthood via cross- and auto-regulatory feedback mechanisms (Zhu et al., 1998; Fukushige et al., 1999; Sommermann et al., 2010). Although intestinal gene expression is never observed outside the intestine in non-heat-shocked hs-elt-7 larvae (n=128) or in heat-shocked larvae that do not contain the hs-elt-7 transgene (n=46), over 50% (n=13) of larvae carrying the hs-elt-7 transgene express the elt-2 intestinal reporter in non-intestinal cells as early as 4 hours after heat shock (Fig. 2C). Expression increases in intensity and cell number rapidly: by 6 hours, broad expression is seen in 100% of animals and remains high over the ensuing 24 hours (n=16). Although expression in most cells is then progressively lost, we found that cells in the neuromuscular feeding organ, the pharynx (Fig. 2A-C), show strong continuous expression throughout the life of the animal (Fig. 2B): by 72 hours, nearly all expression of the intestinal marker is restricted to cells of the pharynx. The C. elegans pharynx comprises seven different cell types that become terminally differentiated and post-mitotic during embryogenesis (Albertson and Thomson, 1976; Mango, 2007); the bulk of the organ consists of muscle and marginal cells. We detected immunoreactive ELT-2 (89% of animals; n=39) and IFB-2 (98% of animals; n=98) in pharyngeal muscle and marginal cells of most worms at 24-48 hours after ectopic ELT-7 expression (Fig. 2D; supplementary material Table S1). Maintained expression of the elt-2 reporter and immunoreactive ELT-2 and IFB-2 suggest that the endogenous intestine ‘lockdown circuitry’ can be activated in post-mitotic pharyngeal cells.
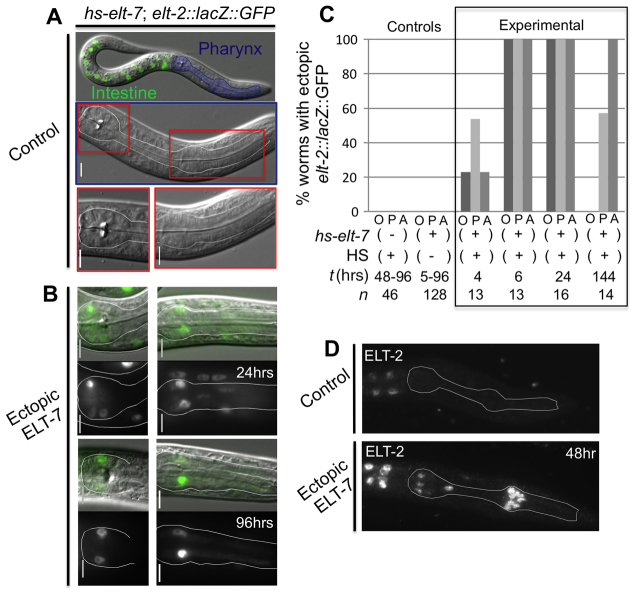
Fig. 2.
Maintenance of intestinal differentiation markers in pharyngeal cells. (A) The elt-2 reporter is normally expressed only in intestinal nuclei (green) in non-heat shocked hs-elt-7 larvae. Anterior (right) and posterior pharynx regions in middle panel are enlarged in lower panels. (B) 24 hours after ectopic ELT-7 expression, the elt-2 reporter is expressed in cells inside and outside the pharynx. At 96 hours, bright signal is maintained only in cells within the pharynx. Scale bars: 5 μm. (C) Percentage of worms expressing elt-2::lacZ::GFP in non-intestinal cells outside the pharynx (O), in the posterior pharynx (P) or in the anterior pharynx (A). n, number of worms observed; t, time since heat shock in hours. (D) Immunoreactive ELT-2 in non-heat-shocked hs-elt-7 control larvae and hs-elt-7 larvae 48 hours after heat shock.
Pharyngeal cells lose their original differentiated identity following activation of intestine differentiation
Several lines of evidence suggest that differentiated cells lose their normal identities following ectopic ELT-7 expression. Although animals exposed to brief END-1 expression grow and behave normally, those subjected to brief ectopic ELT-7 arrest abruptly in larval development (supplementary material Fig. S3, Table S2). Despite this arrest, animals remain alive for an extended period (for at least 8 days). Although active and motile, these non-developing larvae are defective in chemosensation (supplementary material Fig. S3) and molting (not shown), perhaps reflecting a switch in gene expression states in the epidermis and neurons that transiently express the elt-2 reporter after ectopic ELT-7 expression (supplementary material Fig. S4).
Pharyngeal cells that maintain long-term ELT-2 expression lose pharynx-specific gene expression (Fig. 3). Expression of a marker for the myo-2 gene, which encodes a pharynx muscle-specific myosin, is attenuated and undetectable only hours after forced ubiquitous ELT-7 in embryos (supplementary material Fig. S5, Movie 1). Further, expression of myo-2::GFP and ceh-22::GFP, an NK-2 family homeobox transcription factor required for normal pharynx muscle gene expression (Okkema and Fire, 1994) progressively diminishes over several days following a pulse of ectopic ELT-7 expression in larvae (Fig. 3A-D). Loss of pharynx muscle-specific gene expression is not attributable to developmental arrest, starvation, or general necrosis, as marker expression persists under such conditions (Fig. 3B,D; supplementary material Fig. S6).
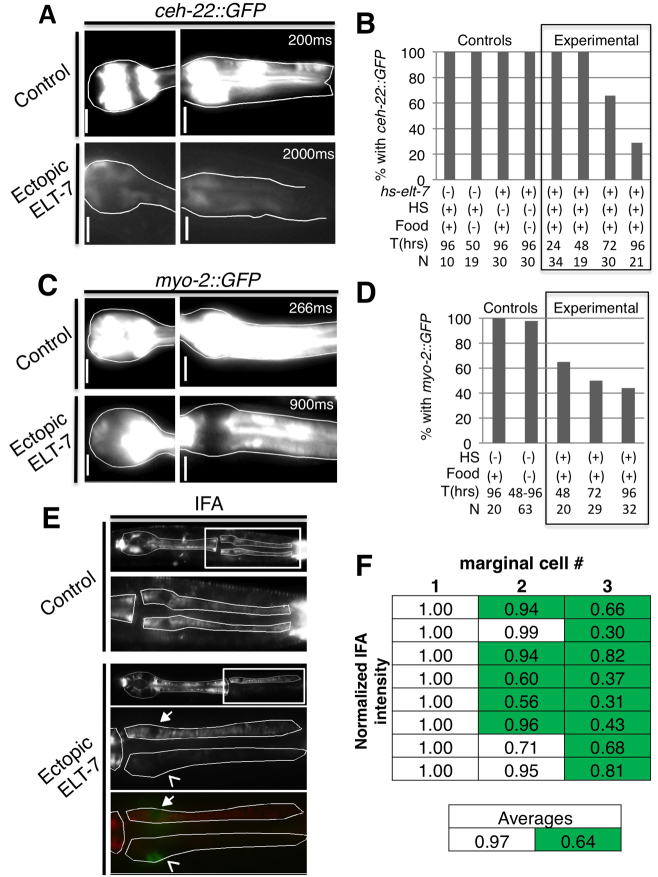
Fig. 3.
Loss of pharynx-specific markers following a pulse of ELT-7 expression. Expression of pharynx muscle-specific ceh-22::GFP (A,B) or myo-2::GFP (C,D) reporters diminishes by 3 days after ectopic ELT-7 expression compared with non-heat shocked hs-elt-7 control larvae. Scale bars: 5 μm. Exposure time is indicated in the upper right-hand corner. (E) Decreased IFA in cells expressing elt-2::lacZ::GFP compared with non-GFP-expressing marginal cells within the same section of the pharynx (boxed region shown in lower panels). Bottom panel, overlay of IFA (red) and elt-2::lacZ::GFP (green). Closed arrow indicates non-elt-2::lacZ::GFP-expressing marginal cell nucleus; open arrow indicates GFP-expressing nucleus. Faint green signal over the non-expressing nucleus is the out-of-plane signal from the positive nucleus of the third marginal cell. (F) IFA average pixel intensity minus background was normalized to the cell with the highest pixel intensity within each worm. Normalized signal in anterior marginal cells with strong elt-2 reporter expression (green highlighted) was lower than in cells with weak or no expression of elt-2 (white). Each row is an individual animal and each column indicates the individual marginal cells ordered based on increasing GFP signal. The average overall IFA signal is reduced in the elt-2::lacZ::GFP-positive marginal cells.
We found that structural components of fully differentiated marginal cells are lost in parallel with acquisition of intestinal markers following ectopic ELT-7 expression. We quantified the intermediate filament protein IFA (Francis and Waterston, 1991) in individual marginal cells of larvae based on average pixel intensities of immunoreactive protein and found that marginal cells with strong elt-2::lacZ::GFP-positive nuclei showed diminished IFA expression compared with marginal cells with low or undetectable expression in the same animal (Fig. 3E,F; eight worms analyzed, 24 total cells, 13 elt-2::lacZ::GFP-expressing cells). The disappearance of otherwise stable pharynx-specific proteins, coincident with maintenance of intestine-specific gene expression, is consistent with bona fide transdifferentiation of differentiated, post-mitotic pharyngeal marginal cells into intestinal cells.
Reprogrammed pharyngeal cells exhibit morphological characteristics of intestinal cells
We found that reprogrammed marginal cells form organelles that resemble those in intestinal cells. Within 3-5 days of a pulse of ELT-7 expression, 11% of larvae (n=129) exhibit birefringent and autofluorescent lysosome-related rhabditin granules (Clokey and Jacobson, 1986) surrounding elt-2::LacZ::GFP-expressing nuclei (Fig. 4A-E′; supplementary material Fig. S9, Table S3). Rhabditin granules are normally present only in differentiated intestinal cells and are never observed in the pharynx of untreated larvae (n>1000), or in larvae ectopically expressing END-1 (n=36). Moreover, the nuclei of cells with ectopic rhabditin granules are strikingly reminiscent of typical differentiated intestinal nuclei (so-called ‘fried egg nuclei’), with a clear nucleoplasm and prominent dense nucleolus that are not typical of pharyngeal nuclei (Fig. 4C′-E′). Formation of ectopic gut granules does not appear to be stage dependent as we have observed them in the pharynx following ectopic ELT-7 expression at later L1 (n=4), L2 (n=6), L3 (n=5) and L4 stages (n=5) (n=144 worms observed).
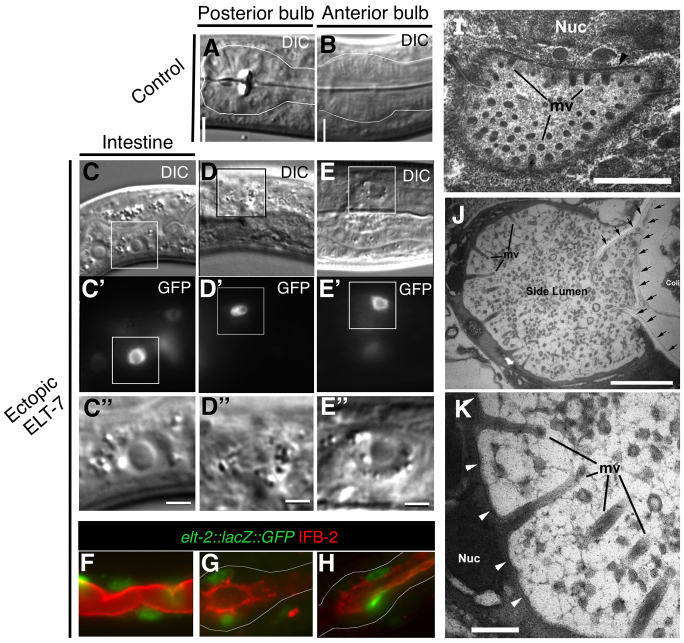
Fig. 4.
Reprogrammed pharyngeal cells have intestine-specific morphology. Morphology of the posterior (A) and anterior (B) bulbs of the pharynx in non-heat shocked hs-elt-7 control animals and morphology of the intestine (C), posterior bulb (D) and anterior bulb (E) after heat shock (D, 72 hours; E, 96 hours) are seen using DIC. Boxed region (C′-E′) outlines elt-2::lacZ::GFP-expressing nuclei in C′-E′. Scale bars: 2 μm. (F-H) Overlay of IFB-2 (red) and elt-2::lacZ::GFP (green) signal in the endogenous intestine (F), and posterior (G) and anterior (H) pharynx 5 days after ectopic ELT-7. (I-K) Morphology of the intestine and remodeled pharynx by TEM. (Extensive images of the ultrastructure of the wild-type pharynx for comparison can be viewed at wormatlas.org.) (I) Cross-section of normal L1 larva intestinal lumen. Microvilli (mv) on the top are seen at nearly full length, whereas those at the bottom are at oblique angles. Black arrow indicates the terminal web. Scale bar: 1 μm. (J) Accessory lumen in the anterior pharynx of hs-elt-7 L1 larva 6 days after brief ectopic ELT-7 expression. Arrows show remaining buccal cuticle. Several long microvilli (mv) are seen and the circular profiles in the middle of the side lumen are apparently additional microvilli extending at extreme angles from other edges of the side lumen. Some E. coli (Coli) lie within the buccal channel. Scale bar: 1 μm. (K) Higher magnification of ectopic microvilli (mv). Scale bar: 0.2 μm. Arrowheads indicate an electron-dense line, an apparent terminal web structure, running subjacent to the plasma membrane. The microvilli appear surrounded by diffuse glycocalyx. Nuc, nucleus.
The switch from pharynx-specific to intestine-specific gene expression in fully differentiated cells is accompanied by progressive remodeling of cellular structure. The marginal cells accommodate the space between muscle cells along the length of the pharynx and have a rigidly fixed wedge-like morphology supported by radial bundles of intermediate filament proteins (Albertson and Thomson, 1976; Francis and Waterston, 1991). We found that immunoreactive IFB-2, an intestine-specific intermediate filament that localizes to the intestine terminal web (Bossinger et al., 2004), progressively localizes to the apical surface of marginal cells after ectopic ELT-7 expression in L1 larvae and adults (Fig. 4F-H; supplementary material Fig. S7). Expression and apical localization of IFB-2 suggests that the cells may form an intestinal terminal web structure following a brief pulse of ELT-7. Although apical surfaces are narrow in normal marginal cells, they expand in area following ELT-7 expression, consistent with remodeling to intestinal cell structure (supplementary material Fig. S8).
Using transmission electron microscopy, we observed that the fine-structure morphology of cells in the pharynx showed clear evidence for transdifferentiation into intestinal cells (Fig. 4I-K). Specifically, cells in the pharynx exhibit accessory lumens (Fig. 4J) that contain distinct microvilli with apparent glycocalyx on the apical surfaces. We also observed a subjacent intestine-like terminal web (Fig. 4K), as was indicated by immunofluorescence analysis (Fig. 4G,H). Taken together with the expression data, our observations suggest that several days after a 15-minute pulse of ELT-7 expression, terminally differentiated pharyngeal cells become transformed both at the gene expression and fine cellular structure levels into differentiated intestine-like cells.
Pharynx to intestine transdifferentiation takes place through mixed-identity post-mitotic cells
We found that a brief pulse of ectopic ELT-7 expression leads to transdifferentiation of pharyngeal marginal cells, as evidenced by maintained elt-2 reporter expression, stable ELT-2 protein, formation of rhabditin granules, cellular remodeling to form a terminal web and microvilli, as well as loss of normally stable marginal cell proteins. Activation of endogenous ELT-2 by ectopic ELT-7, and establishment of the intestine auto-regulatory circuitry, likely drives transdifferentiation of marginal cells into intestinal cells. Increased expression of intestine-specific proteins, and remodeling of the cell, occur prior to repression of pharyngeal cell-specific identity, suggesting that, unlike some examples of naturally occurring transdifferentiation (Jarriault et al., 2008), this in vivo reprogramming takes place through intermediate cellular states in which characteristics of both the original and final cell types are present. We found no evidence of cell division during the reprogramming process based on cell number and position, indicating that erasing original cell identity through cell division is not a necessary step in forced transdifferentiation. Although some morphological characteristics of pharynx tissue are present in the animals with transdifferentiated cells, these ultrastructural features are excluded in the regions that have undergone transdifferentiation and that exhibit an intestine-like morphology.
Transcription factor identity and cellular context influence susceptibility to forced transdifferentiation
Although transcription factors that regulate specification of each of the three germ layers can reprogram the fate of pre-gastrulation blastomeres in C. elegans, they are unable to reprogram cells in later stage embryos without prior removal of inhibitory factors (reviewed by Joshi et al., 2010). We found that ELT-7 can initiate intestine gene expression in many differentiated cell types in embryos, larvae, and adults without removal of inhibitory factors, demonstrating that not all transcription factors adhere to the MCT. A growing number of in vitro studies demonstrate that transcription factors can redirect differentiated cell identity without reversion to a pluripotent intermediate; however, the context and factors that result in efficient and stable transdifferentiation of any cell type remain relatively unclear (reviewed by Ladewig et al., 2013).
We found that only pharynx cells appear to become fully transdifferentiated and reprogrammed by ELT-7. We considered the possibility that this reflects tissue-specific expression of the hsp-16-41/2 promoter used in the study, which is expressed at somewhat higher levels in the pharynx (Stringham et al., 1992). However, we found that longer heat shocks (30 minutes and 60 minutes) also failed to result in cellular reprogramming outside the pharynx, although such a regimen results in higher heat-shock promoter activity in epidermal and muscle cells than in the pharynx under the brief (15 minutes) heat-shock conditions used throughout this study. Thus, the cell-type specificity does not appear to be attributable to differences in expression levels of the transgene.
Transdifferentiation of only pharynx marginal cells implies that cellular context dictates susceptibility to reprogramming. With the exception of two cells in the posterior pharynx, the marginal cells are separated in cell lineage from intestinal cells at the first cell division (Sulston et al., 1983); therefore, relatedness in cell lineage does not appear to explain their particular susceptibility to reprogramming by ELT-7. The intestine and pharynx are both epithelial tubes that are a part of the digestive tract. It is conceivable that epithelial tube or digestive tract identity has a role in establishing the context for direct reprogramming into intestine per se; however, such an effect would not extend to rectal epithelial cells, as we did not observe evidence for transdifferentiation in that region. Although it is possible that marginal cells might be uniquely developmentally plastic, the results of Gilleard and McGhee (Gilleard and McGhee, 2001), Fukushige and Krause (Fukushige and Krause, 2005), and Turson and Patel (Turson and Patel, 2011) imply that differentiated marginal cells are not susceptible to transdifferentiation into epidermis, muscle and neurons, respectively. Rather, these cells may express a factor or set of factors that allow for transdifferentiation into intestine. Our current observations establish an in vivo model to investigate the role of cell context in stable transdifferentiation of somatic cells and reveal remarkable plasticity in cellular differentiation in an organism with a rigid pattern of cell division and identity.
MATERIALS AND METHODS
C. elegans strains, maintenance, synchronization and heat shock
Transgenic strains used were as follows: JR3410, wIs47 [hsp::end-1];rrIs1 [elt-2::lacZ::GFP] (Fukushige et al., 1998; Zhu et al., 1998); JR3373, wIs125[hsp::elt-7] (Sommermann et al., 2010); rrIs1; JR3457, wIs125; CuIs2 V [ceh-22::gfp]; and JR3471, unc-119(ed3); ruIsIII[unc-119(+), myo-2::GFP; wIs125. Larvae were synchronized as described previously (Stiernagle, 2006) and heat shocked at the desired stage in M9 buffer at 33°C for 15 minutes using a thermal cycler.
Immunofluorescence
Antibodies MH33 (anti-IFB-2) (Bossinger et al., 2004) and anti-ELT-2 were gifts from J. McGhee (University of Calgary, Canada). Anti-IFA (Francis and Waterston, 1991) was a gift from G. Gunderson (Columbia University, NY, USA). Cy3 goat anti-rabbit and Cy3 goat anti-mouse were obtained from Sigma. Fixation and permeabilization were carried out as described previously (Sommermann et al., 2010). Animals were viewed with a Zeiss Axioskop 2, Olympus BX60 or Nikon Eclipse Ti inverted microscope and imaged with a MicroFire camera or Hamamatsu flash Orca 2.8. Brightness and contrast of some images were modified using NIS Elements software or PowerPoint to reveal relevant details more clearly in the figures.
IFA intensity measurement
Using NIS Elements software, individual marginal cells in the anterior pharynx were outlined to create regions of interest (ROIs). Average intensity of the IFA signal was quantified within each of the three anterior marginal cells in individual worms that contained one or two GFP-positive nuclei, bright staining, and non-elongated or abnormal nuclei. We compared only cells within individual worms, in order to control for differences in staining efficiency between worms. Background was determined by measuring average pixel intensity in region outside the worm.
Transmission electron microscopy (TEM)
L1 larvae of strain JR3373 were heat shocked and inspected by fluorescence microscopy after 6 days. Worms with bright elt-2::lacZ::GFP-expressing nuclei and intestine-like morphology in the anterior pharynx were placed on ice, shipped to the Hall lab, and subsequently transferred into a small planchette for the Bal-Tec HPM-010 High Pressure Freezing Machine using E. coli as the surrounding matrix. Fixation and embedding procedures generally followed Hall et al. (Hall et al., 2012). One or two animals were fast frozen per planchette, after which frozen samples were placed into an RMC FS-7500 Freeze Substitution unit, in a 1% osmium tetroxide solution in 98% acetone, 2% dH2O. Samples were held at -90°C for 4 days, then slowly warmed to 0°C, held for 3 days at 0°C, then rinsed in cold acetone and gradually infiltrated with EmBed 812 resin. Samples were flat embedded between Aclar sheets, then cured at 60°C for 2 days. Single worms were viewed under the dissecting microscope, cut out of the Aclar sandwich before re-embedding in fresh plastic resin and placed in a mold in precise orientation before curing again at 60°C. The embedded sample was serial thin-sectioned on an RMC PowerTome XL using a diamond knife, mounted on slot grids, post-stained with uranyl acetate and viewed with a Philips CM10 electron microscope. Digital images were collected using an SIS camera system and viewed using iTEM or Photoshop software platforms to analyze data and select images for illustrations.
Supplementary Material
Acknowledgments
We thank Andrew Sumner for assistance with chemosensation experiments, and Leslie Gunther-Cummins and Geoff Perumal for help with the Bal-Tec HPF device. We thank G. Gunderson and J. McGhee for reagents. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (p40 O8010440).
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
M.R.R. designed and performed experiments, and wrote the manuscript draft. A.W. performed experiments. K.C.Q.N. performed transmission electron microscopy experiments. D.H.H. interpreted the electron micrographs. J.H.R. conceived the project, and revised and approved the manuscript.
Funding
This work was supported by a training grant from the California Institute of Regenerative Medicine, and grants from the National Institutes of Health [OD 010943 to D.H.H. and HD062922 to J.H.R.]. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.103010/-/DC1
References
- Albertson D. G., Thomson J. N. (1976). The pharynx of Caenorhabditis elegans. Philos. Trans. R. Soc. B 275, 299–325 [DOI] [PubMed] [Google Scholar]
- Bossinger O., Fukushige T., Claeys M., Borgonie G., McGhee J. D. (2004). The apical disposition of the Caenorhabditis elegans intestinal terminal web is maintained by LET-413. Dev. Biol. 268, 448–456 [DOI] [PubMed] [Google Scholar]
- Ciosk R., DePalma M., Priess J. R. (2006). Translational regulators maintain totipotency in the Caenorhabditis elegans germline. Science 311, 851–853 [DOI] [PubMed] [Google Scholar]
- Clokey G. V., Jacobson L. A. (1986). The autofluorescent “lipofuscin granules” in the intestinal cells of Caenorhabditis elegans are secondary lysosomes. Mech. Ageing Dev. 35, 79–94 [DOI] [PubMed] [Google Scholar]
- Djabrayan N. J., Dudley N. R., Sommermann E. M., Rothman J. H. (2012). Essential role for Notch signaling in restricting developmental plasticity. Genes Dev. 26, 2386–2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis R., Waterston R. H. (1991). Muscle cell attachment in Caenorhabditis elegans. J. Cell Biol. 114, 465–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushige T., Hawkins M. G., McGhee J. D. (1998). The GATA-factor elt-2 is essential for formation of the Caenorhabditis elegans intestine. Dev. Biol. 198, 286–302 [PubMed] [Google Scholar]
- Fukushige T., Hendzel M. J., Bazett-Jones D. P., McGhee J. D. (1999). Direct visualization of the elt-2 gut-specific GATA factor binding to a target promoter inside the living Caenorhabditis elegans embryo. Proc. Natl. Acad. Sci. USA 96, 11883–11888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushige T., Krause M. (2005). The myogenic potency of HLH-1 reveals wide-spread developmental plasticity in early C. elegans embryos. Development 132, 1795–1805 [DOI] [PubMed] [Google Scholar]
- Gilleard J. S., McGhee J. D. (2001). Activation of hypodermal differentiation in the Caenorhabditis elegans embryo by GATA transcription factors ELT-1 and ELT-3. Mol. Cell. Biol. 21, 2533–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. H., Hartwieg E., Nguyen K. C. (2012). Modern electron microscopy methods for C. elegans. Methods Cell Biol. 107, 93–149 [DOI] [PubMed] [Google Scholar]
- Horner M. A., Quintin S., Domeier M. E., Kimble J., Labouesse M., Mango S. E. (1998). pha-4, an HNF-3 homolog, specifies pharyngeal organ identity in Caenorhabditis elegans. Genes Dev. 12, 1947–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarriault S., Schwab Y., Greenwald I. (2008). A Caenorhabditis elegans model for epithelial-neuronal transdifferentiation. Proc. Natl. Acad. Sci. USA 105, 3790–3795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi P. M., Riddle M. R., Djabrayan N. J., Rothman J. H. (2010). Caenorhabditis elegans as a model for stem cell biology. Dev. Dyn. 239, 1539–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladewig J., Koch P., Brustle O. (2013). Leveling Waddington: the emergence of direct programming and the loss of cell fate hierarchies. Nat. Rev. Mol. Cell Biol. 14, 225–236 [DOI] [PubMed] [Google Scholar]
- Maduro M. F., Rothman J. H. (2002). Making worm guts: the gene regulatory network of the Caenorhabditis elegans endoderm. Dev. Biol. 246, 68–85 [DOI] [PubMed] [Google Scholar]
- Mango S. E. (2007). The C. elegans pharynx: a model for organogenesis (ed. The C. elegans Research Community). WormBook, doi/10.1895/wormbook.1.129.1 [DOI] [PMC free article] [PubMed]
- Okkema P. G., Fire A. (1994). The Caenorhabditis elegans NK-2 class homeoprotein CEH-22 is involved in combinatorial activation of gene expression in pharyngeal muscle. Development 120, 2175–2186 [DOI] [PubMed] [Google Scholar]
- Patel T., Tursun B., Rahe D. P., Hobert O. (2012). Removal of Polycomb repressive complex 2 makes C. elegans germ cells susceptible to direct conversion into specific somatic cell types. Cell Reports 2, 1178–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintin S., Michaux G., McMahon L., Gansmuller A., Labouesse M. (2001). The Caenorhabditis elegans gene lin-26 can trigger epithelial differentiation without conferring tissue specificity. Dev. Biol. 235, 410–421 [DOI] [PubMed] [Google Scholar]
- Sommermann E. M., Strohmaier K. R., Maduro M. F., Rothman J. H. (2010). Endoderm development in Caenorhabditis elegans: the synergistic action of ELT-2 and -7 mediates the specification→differentiation transition. Dev. Biol. 347, 154–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiernagle T. (2006). Maintenance of C. elegans (ed. The C. elegans Research Community). WormBook, doi/10.1895/wormbook.1.101.1 [DOI] [PMC free article] [PubMed]
- Stringham E. G., Dixon D. K., Jones D., Candido E. P. (1992). Temporal and spatial expression patterns of the small heat shock (hsp16) genes in transgenic Caenorhabditis elegans. Mol. Biol. Cell 3, 221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston J. E., Schierenberg E., White J. G., Thomson J. N. (1983). The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100, 64–119 [DOI] [PubMed] [Google Scholar]
- Tursun B., Patel T., Kratsios P., Hobert O. (2011). Direct conversion of C. elegans germ cells into specific neuron types. Science 331, 304–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzyuk T., Fakhouri T. H., Kiefer J., Mango S. E. (2009). The polycomb complex protein mes-2/E(z) promotes the transition from developmental plasticity to differentiation in C. elegans embryos. Dev. Cell 16, 699–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Fukushige T., McGhee J. D., Rothman J. H. (1998). Reprogramming of early embryonic blastomeres into endodermal progenitors by a Caenorhabditis elegans GATA factor. Genes Dev. 12, 3809–3814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Hill R. J., Heid P. J., Fukuyama M., Sugimoto A., Priess J. R., Rothman J. H. (1997). end-1 encodes an apparent GATA factor that specifies the endoderm precursor in Caenorhabditis elegans embryos. Genes Dev. 11, 2883–2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.