Abstract
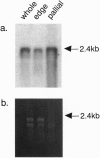
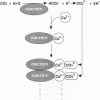
It is believed that the polymorphism observed in calcium carbonate crystals, such as aragonite and calcite in mollusk shells, is controlled by organic matrix proteins secreted from the mantle epithelia. However, the fine structures of these proteins are still unknown, and to understand the molecular mechanisms of mineralization process, detailed structural analyses of the organic matrix proteins are essential. For this, we have carried out purification, characterization, and cDNA cloning of nacrein, which is a soluble organic matrix protein in the nacreous layer of oyster pearls. Northern blot analysis showed that the nacrein transcript was specifically expressed in mantle pallial. Analysis of the deduced amino acid sequence revealed that the protein contained two functional domains: one was a carbonic anhydrase and another was a Gly-Xaa-Asn (Xaa = Asp, Asn, or Glu) repeat domain; however, the carbonic anhydrase domain was split into two subdomains with insertion of the Gly-Xaa-Asn repeat domain between them. Our findings suggest that nacrein actually functions as a matrix protein whose repeated Gly-Xaa-Asn domain possibly binds calcium and as a carbonic anhydrase that catalyzes the HCO3- formation, thus participating in calcium carbonate crystal formation of the nacreous layer.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addadi L., Berman A., Oldak J. M., Weiner S. Structural and stereochemical relations between acidic macromolecules of organic matrices and crystals. Connect Tissue Res. 1989;21(1-4):127–135. doi: 10.3109/03008208909050003. [DOI] [PubMed] [Google Scholar]
- Addadi L., Weiner S. Interactions between acidic proteins and crystals: stereochemical requirements in biomineralization. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4110–4114. doi: 10.1073/pnas.82.12.4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskey A. L. Mineral-matrix interactions in bone and cartilage. Clin Orthop Relat Res. 1992 Aug;(281):244–274. [PubMed] [Google Scholar]
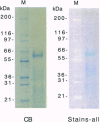
- Campbell K. P., MacLennan D. H., Jorgensen A. O. Staining of the Ca2+-binding proteins, calsequestrin, calmodulin, troponin C, and S-100, with the cationic carbocyanine dye "Stains-all". J Biol Chem. 1983 Sep 25;258(18):11267–11273. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Davis J. G., Oberholtzer J. C., Burns F. R., Greene M. I. Molecular cloning and characterization of an inner ear-specific structural protein. Science. 1995 Feb 17;267(5200):1031–1034. doi: 10.1126/science.7863331. [DOI] [PubMed] [Google Scholar]
- Gorski J. P. Acidic phosphoproteins from bone matrix: a structural rationalization of their role in biomineralization. Calcif Tissue Int. 1992 May;50(5):391–396. doi: 10.1007/BF00296767. [DOI] [PubMed] [Google Scholar]
- Hauschka P. V., Carr S. A. Calcium-dependent alpha-helical structure in osteocalcin. Biochemistry. 1982 May 11;21(10):2538–2547. doi: 10.1021/bi00539a038. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Montgomery J. C., Venta P. J., Tashian R. E., Hewett-Emmett D. Nucleotide sequence of human liver carbonic anhydrase II cDNA. Nucleic Acids Res. 1987 Jun 11;15(11):4687–4687. doi: 10.1093/nar/15.11.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami H., Marelich G. P., Grubb J. H., Kyle J. W., Sly W. S. Cloning, expression, and sequence homologies of cDNA for human carbonic anhydrase II. Genomics. 1987 Oct;1(2):159–166. doi: 10.1016/0888-7543(87)90008-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashian R. E. The carbonic anhydrases: widening perspectives on their evolution, expression and function. Bioessays. 1989 Jun;10(6):186–192. doi: 10.1002/bies.950100603. [DOI] [PubMed] [Google Scholar]
- Vera J. C. Measurement of microgram quantities of protein by a generally applicable turbidimetric procedure. Anal Biochem. 1988 Oct;174(1):187–196. doi: 10.1016/0003-2697(88)90534-9. [DOI] [PubMed] [Google Scholar]
- Weiner S. Aspartic acid-rich proteins: major components of the soluble organic matrix of mollusk shells. Calcif Tissue Int. 1979 Nov 26;29(2):163–167. doi: 10.1007/BF02408072. [DOI] [PubMed] [Google Scholar]
- Weiner S., Hood L. Soluble protein of the organic matrix of mollusk shells: a potential template for shell formation. Science. 1975 Dec 5;190(4218):987–989. doi: 10.1126/science.1188379. [DOI] [PubMed] [Google Scholar]
- Wheeler A. P., George J. W., Evans C. A. Control of calcium carbonate nucleation and crystal growth by soluble matrx of oyster shell. Science. 1981 Jun 19;212(4501):1397–1398. doi: 10.1126/science.212.4501.1397. [DOI] [PubMed] [Google Scholar]