Abstract
Background
Previously published methods for determination of efavirenz (EFV) in human dried blood spots (DBS) employ costly and complex liquid chromatography/mass spectrometry. We describe the validation and evaluation of a simple and inexpensive high-performance liquid chromatography (HPLC) method for EFV quantification in human DBS and dried plasma spots (DPS), using ultraviolet (UV) detection appropriate for resource-limited settings.
Methods
100μl of heparinized whole blood or plasma were spotted onto blood collection cards, dried, punched, and eluted. Eluates are injected onto a C-18 reversed phase HPLC column. EFV is separated isocratically using a potassium phosphate and ACN mobile phase. UV detection is at 245nm. Quantitation is by use of external calibration standards. Following validation, the method was evaluated using whole blood and plasma from HIV-positive patients undergoing EFV therapy.
Results
Mean recovery of drug from dried blood spots is 91.5%. The method is linear over the validated concentration range of 0.3125 – 20.0μg/mL. A good correlation (Spearman r=0.96) between paired plasma and DBS EFV concentrations from the clinical samples was observed, and hematocrit level was not found to be a significant determinant of the EFV DBS level. The mean observed CDBS/Cplasma ratio was 0.68. A good correlation (Spearman r=0.96) between paired plasma and DPS EFV concentrations from the clinical samples was observed. The mean percent deviation of DPS samples from plasma samples is 1.68%.
Conclusions
Dried whole blood spot or dried plasma spot sampling is well suited for monitoring EFV therapy in resource limited settings, particularly when high sensitivity is not essential.
Keywords: Efavirenz, Dried Blood Spots, Dried Plasma Spots, HPLC
Introduction
Dried blood spots (DBS) sampled from whole blood spotted onto filter paper have been used for over 45 years in applications ranging from neonatal screening of inborn errors of metabolism, therapeutic drug monitoring, epidemiological screening, toxicokinetic monitoring of drug exposure in preclinical animal models, to assessment of the systemic exposure of a wide variety of biologically active compounds.1-4 The robustness of DBS sampling was illustrated when the first clinical study demonstrating DBS methodology to quantify drug levels and generate pharmacokinetic (PK) data for regulatory purposes was published in 2009.5 In recent years several articles have been published extending the knowledge, applicability and relevance of DBS sampling for clinical PK studies.1,6-7
The use of DBS has several advantages over traditional plasma sampling strategies. Since DBS techniques require a substantially smaller volume of blood than traditional plasma sampling techniques, as little at 5 μL when coupled to an HPLC-MS/MS assay,8 they allow for serial sampling in PK studies involving pediatric patients or small mammals which would be limited to highly variable composite profiles requiring larger patient populations by traditional methods.9-10 Additionally, DBS methodologies offer economic advantages over plasma sampling strategies making them ideal for use in international trials in resource-limited areas of the world.1 The DBS sampling procedure is less invasive and requires less training than traditional venipuncture methods as the sample can be obtained from a simple finger- or heel-prick. Unlike traditional plasma-based methodologies, collection of DBS samples does not require refrigerated centrifugation, aliquoting, or freezing. DBS samples have much lower costs of shipping and storage as they do not require shipment on dry ice or special packaging since they can be stable for long periods at room temperature and present a lower biohazard risk than traditional plasma samples. While use of dried plasma spots (DPS) still requires traditional plasma collection and processing methods, DPS sampling offers similar storage and shipping advantages as DBS, and represents an alternative strategy in resource-limited settings.
While DBS has several advantages over traditional plasma sampling, DBS techniques also require additional assay validation steps. The DBS card matrix often contains proprietary chemicals that may cause matrix effects such as ion suppression in tandem mass spectrometry detection that must be investigated during assay validation.1 Additionaly, the use of whole blood as the liquid matrix requires considerations as to variability in sample hematocrit, and volume of blood spotted can lead to heterogenous spotting. Further, variability in fraction unbound (fu) and blood cell affinity (ρ) of an analyte can lead to blood partitioning (Cb/C) variability that needs to be characterized during assay validation.1, 6
International studies evaluating the epidemiology of infectious diseases and efficacy of anti-infectives are often conducted in resource-limited environments. Thus, it is not surprising that much of the published work on DBS methodologies has been focused on the measurement of drugs used to treat diseases such as malaria (quinine, chloroquine, and proguanil),11-12 tuberculosis (moxifloxacin),13 and HIV (amprenavir, atazanavir, darunavir, indinavir, lopinavir, nelfinavir, ritonavir, saquinavir, efavirenz, etravirine, nevirapine, and raltegravir).14-18 While the anti-malarial methodologies used rapid and simple ELISA and HPLC-UV detection methods, the anti-tubercular and anti-retroviral methods exclusively employ costly HPLC-MS/MS.
Efavirenz (EFV, Sustiva®) is a non-nucleoside reverse transcriptase inhibitor (NNRTI) that was FDA-approved in 1998 for the treatment of HIV as part of highly active antiretroviral therapy (HAART). EFV is currently used in combination with lamivudine and zidovudine or tenofovir and emtricitabine as the preferred NNRTI-based combination regimen for treatment-naïve HIV patients.19 Two DBS methods for determination of EFV in human whole blood have been published, and both have used HPLC-MS/MS.14-15 The first published DBS-based EFV determination method reported an 81% recovery, limit of detection of 0.05 μg/mL, and lower limit of quantitation of 0.102 μg/mL from 5 μL human whole blood spots, however the method was not validated to FDA regulatory quidelines.14 The second published DBS-based EFV quantification method was reported to be linear over a concentration range of 0.1 to 20 μg/mL, 102-104% recovery, and was validated according to FDA guidelines, but only reported stability testing out to 7 days.15
The aim of this study was to develop and validate in accordance with FDA guidelines a simple and inexpensive HPLC-based method for the determination of EFV in human DBS using ultraviolet detection for use in patients enrolled in IMPAACT clinical trials. After validation, the method was evaluated using clinical samples from HIV-positive adult patients treated with EFV as part of their HAART regimen.
Materials and Methods
Blood collection cards (Whatman Protein Saver 903) were purchased from Whatman Inc. EFV was provided by the NIH Research and Reference Reagent Program and Sequoia Research Products, United Kingdom. HPLC grade water and Acetonitrile (ACN), as well as reagent grade O-phosphoric acid (85%) were purchased from Fisher Scientific. Potassium hydroxide was purchased from RICCA Chemical Company. All other chemicals and solvents were of highest purity available from commercial sources and were used without further purification.
Preparation of Calibrators and Controls
DBSs for calibration, precision, accuracy, recovery, and stability were prepared from stock EFV standards. EFV 1mg/mL in methanol was diluted 1:50 in a total volume of 10mL heparinized whole blood to give a concentration of 20 μg/mL. The other calibration curve standards were made through serial 1:2 dilutions with heparinized whole blood to create calibration samples of 20, 10, 5, 2.5, 1.25, 0.625, and 0.3125 μg/mL. Controls were prepared using a similar method at concentrations of 18, 4.5, 1.5, 0.625, and 0.3125 μg/mL in heparinized whole blood. 100 μL of the calibration standards and controls were spotted onto blood collection cards, dried overnight at room temperature, and then stored in Ziploc bags with desiccant and a humidity indicator card at −20°C until ready to assay.
Clinical Samples
With approval from the University of California, San Diego Institutional Review Board, a total of 31 leftover whole blood samples were collected from the UCSD Antiviral Research Center (AVRC). These 31 samples had been collected via venipuncture from HIV-positive adult patients known to be taking oral EFV capsules (Sustiva®) during their regular Owen Clinic appointments for laboratory monitoring of their disease at the UCSD Medical Center. These samples were processed and analyzed within one month of collection. Plasma, dried blood spot (DBS), and dried plasma spot (DPS) EFV assay samples were prepared from each of the clinical samples by taking aliquots from the sample collection tubes when sufficient whole blood volume was present, and the hematocrit (HCT) for each clinical sample was collected retrospectively from the donors’ medical charts when available. DBS and DPS clinical assay samples were prepared using the same method as the standards following the spotting of 100 μL heparinized whole blood and plasma from each clinical sample respectively by pipette.
Preparation of Assay Samples
The frozen blood collection cards were thawed at room temperature before two quarter-inch discs were punched and placed in capped microcentrifuge tubes with 400 μL of elution buffer (10mM KH2PO4 w/ 75% ACN). The microcentrifuge tubes were then vortexed for 15 seconds and allowed to elute for 2 hours at room temperature with gentle agitation using a rotary mixer at 100 rpm. All eluted standards, controls, and samples were then transferred to 400 μL HPLC inserts within 1.5mL HPLC auto-sampler injection vials.
HPLC Methodology
The HPLC system used was the Thermo Separation Products (TSP) Spectra System (Thermo Electron Corp) with a single pump (Spectra System P4000-040), an autosampler (Spectra System AS3000-021), a diode-array detector (Spectra Focus Forward Optical Scanner SF200-0000), a degasser (LC Access 920603001), and an integrator using the Chrom Quest software (version 4.0) as the system controller. The analytical column was a reverse-phase C-18 column (MAC-MOD Ace 5 C-18, 15cm × 4.6mm) with a compatible pre-column filter (MAC-MOD Analytical catolog #MMCS-210).
EFV standards, controls, and samples were autosampled at an injection volume of 100 μL.. Analytes were separated isocratically using a mobile phase of 51% buffer (10mM potassium phosphate buffer, pH 3.1-3.15) and 49% ACN (mobile phase A) at ambient temperature. The UV detector was set at 245 nm. The chromatogram was run for 25 minutes at a flow rate of 0.75 mL/min before the column was purged with a mobile phase of 80% ACN and 20% water (mobile phase B) for 3 minutes. The column was then re-equilibrated with mobile phase A for 7 minutes prior to injection of additional samples. The EFV retention time using this method was 21-22 minutes. Quantitation of EFV was by use of external calibration standards to generate a curve using a least-squares linear regression algorithm to plot the peak area versus concentration with 1/response weighting. Linearity was verified using estimates of the correlation coefficient (r), where r had to be >0.99 to meet the acceptance criteria of the calibration curve. Additionally, for the calibration curve to meet acceptance criteria the mean back-calculated values for the six standards had to be within 15% of the nominal values except for the lowest standard (0.3125 μg/mL) which had to be within 20% of the nominal value.
Limits of Quantitation
The limits of quantitation are the lowest and highest points on the calibration curve that could be accurately and reproducibly quantified. For this validation the lowest limit of quantitation (LLOQ) was 0.3125 μg/mL. The upper limit of quantitation (ULOQ) was 20 μg/mL. Sample chromatograms of the lowest and highest limits of quantitation are shown in Figure, Supplemental Digital Content 1, http://links.lww.com/TDM/A33.
Precision and Accuracy
Precision and accuracy of this method was validated by analysis of the human DBS control sample prepared at the LLOQ and at four additional concentrations spanning the calibration range. Precision was defined as the percent coefficient of variation (%CV) of each control sample after a series of replications using the equation:
Accuracy was defined as the percent deviation (%DEV) from the theoretical value of each control sample using the following equation:
The acceptance criteria for validation of the method require the means of the control samples to have a CV% and %DEV of <15%, except for the LLOQ which must be <20%.
Intra- and Inter-Assay Precision and Accuracy
To assess the within and between assay precision and accuracy, 6 aliquots of each control sample were evaluated on each assay day for 6 days.
Partial Volumes Precision and Accuracy
To assess the precision and accuracy of determining EFV concentrations above the calibration range by dilution following the elution step, DBS sample concentrations 4 times greater than the ULOQ were eluted and diluted with elution buffer using 3 dilution factors (1:4, 1:8, and 1:16) to generate measured concentrations that fell within the calibration curves’ range. The acceptance criteria for validation of the method require the means of the diluted samples to have a CV% and %DEV of <15%.
Stability
Stability of the EFV DBS was evaluated under a number of conditions. The freeze/thaw stability of the DBS samples was determined following 3 freeze/thaw cycles (2 hours at room temperature/overnight at −20°C) for 3 consecutive days by analysis of 3 replicates of 3 control sample concentrations (18, 1.5, and 0.625 μg/mL). The elution buffer matrix stability was determined by re-injection of 3 control sample concentrations (18, 1.5, and 0.625 μg/mL) after storage in auto-sampler vials at room temperature for 10 days. Thermal stabilities were also determined at 5 different temperatures (45°C, 37°C, room temperature, 4°C, and −70°C) by analysis of 3 replicates of 3 control sample concentrations (18, 1.5, and 0.625 μg/mL) after storage for one month. Additionally, the long-term storage stability of EFV DBS samples was determined at −20°C by analysis of 6 replicates of 3 control sample concentrations (18, 1.5, and 0.625 μg/mL) after storage for one week, one month, three months, six months, and one year.
Matrix Recovery
Recovery was determined in triplicate at two concentration levels (20 and 0.8 μg/mL) by comparing the mean area found in eluted DBS with that found in un-spotted sample as measured in elution buffer. Recovery samples were prepared by serial dilution of the stock 1.0 mg/mL EFV solution (1:50, then 1:25) in elution buffer and in heparinized whole blood to produce the un-spotted and spotted sample solutions, respectively. 10 μL of the spiked whole blood was spotted onto filter paper in duplicate, dried overnight, and EFV from 2 quarter-inch discs punched from the DBS were eluted with 400 μL of elution buffer to produce the spotted sample. 20 μL of EFV spiked elution buffer was added to 380 μL of elution buffer to create the un-spotted sample. For the validation of the method the acceptance criteria for recovery was consistency, precision, and reproducibility with a CV% <15%.
Specificity
The specificity of the method was determined by examining the susceptibility of the assay to interference by biogenic constituents in blank DBSs, as well as interference from concomitant medications. Interference from biogenic matrix effects was evaluated by determining EFV concentration in human DBS both before and after spiking the heparinized whole blood from six different sources with 6 μg/ml of EFV. The blank and spiked heparinized whole blood samples were then spotted, dried, eluted and assayed. Potential interferences from concomitant medications was evaluated by defining the retention time of potentially co-eluting compounds injected at concentrations within the 10-20 μg/mL range.
Results
Intra- and Inter-Assay Precision and Accuracy
The intra- and inter-assay precision and accuracy results are shown in Tables, S1 and S2, Supplemental Digital Content 2, http://links.lww.com/TDM/A34. At the LLOQ (0.3125μg/mL) the within day precision ranged from 5.7 – 12.1% CV over six days and accuracy ranged from −1.7 – 9.1 %DEV. The within day precision (%CV) at the extra low, low, middle and high validation samples ranged from: 2.8 −10.4, 4.1 −8.5, 3.5 −11.2, 3.8 −14.5 %CV respectively. The within day accuracy (%DEV) at the extra low, low, middle, and high validation samples ranged from: −5.9 – 4.4, −6.4 −10.5, −3.5 – 13.6, −4.3 – 5.6 %DEV respectively.
For all validation samples (n = 36) the between assay precision and accuracy ranged from 6.0 – 8.9%CV, and 1.0 – 5.1 %DEV, respectively.
Partial Volumes Precision and Accuracy
The detailed results of the partial volumes precision and accuracy test are shown in Table S3, Supplemental Digital Content 2, http://links.lww.com/TDM/A34.. The mean %DEV for diluted DBS samples with a dilution factors of 4, 8 and 16 were 6.1, 8.9, and 11.5 respectively. Mean %CV were 2.9, 3.1, and 4.0 respectively.
Stability
The results of the freeze/thaw stability, elution buffer stability, and thermal stability tests are summarized in Table S4, Supplemental Digital Content 2, http://links.lww.com/TDM/A34All stability tests produced acceptable accuracy and precision values with a maximum observed %CV of 13.9% and a maximum observed %DEV of −14.5%, fulfilling acceptance criteria of the methodology. The results of the long-term storage stability test at −20 °C are summarized in Table S5, Supplemental Digital Content 2, http://links.lww.com/TDM/A34.When stored for 6 months at −20 °C the high quality control sample (18 μg/mL) had on observed %DEV outside the acceptable range of 15% (17.6%), however, when stored for 1 year both the %CV and %DEV were within acceptance criteria at 2.8 and 2.6% respectively.
Matrix Recovery
The mean percent recovery of EFV from DBS when spotted at 20 and 0.8 μg/mL was 90.2% and 92.8% respectively. Overall, a mean percent recovery of 91.5% and a precision (CV%) of 3.8% was observed for the elution methodology.
Specificity
The specificity of the method was determined by examining the susceptibility to the assay to interference by biogenic constituents in blank DBSs, as well as interference from concomitant medications. There were no observed endogenous peaks that interfered with the quantitation of EFV from each lot of six blank DBS. The mean measured concentration for EFV spikes was 5.865 μg/mL, which equates to a mean %DEV of −2.3% from the 6 μg/mL theoretical value. Potential interferences from 37 potential concomitant medications (21 antiretrovirals) was evaluated by defining the retention time of potentially co-eluting compounds injected at concentrations within the 10-20 μg/mL range. As can be seen in Table S6, Supplemental Digital Content 2, http://links.lww.com/TDM/A34none of the 37 tested compounds co-elutes with EFV at 21 minutes, the closest being lopinavir which has a mean retention time of 18.1 minutes.
Clinical Samples
A total of 31 distinct human heparinized whole blood samples were collected to evaluate this method following validation. Of the 31 collected samples 28 had detectable EFV levels. Four samples had insufficient volume for DPS analysis. Two samples had no associated HCT level, while 4 other samples only had HCT levels from previous site visits (< 60 days prior). All together, there were 19 samples for which there was collection of plasma, DBS, DPS, and HCT all drawn on the same day. For plasma, DBS, and DPS the observed EFV concentration range was 1.092-4.131, 0.60-4.380, and 1.092-4.131 μg/mL respectively. The observed hematocrit range was 0.348-0.480.
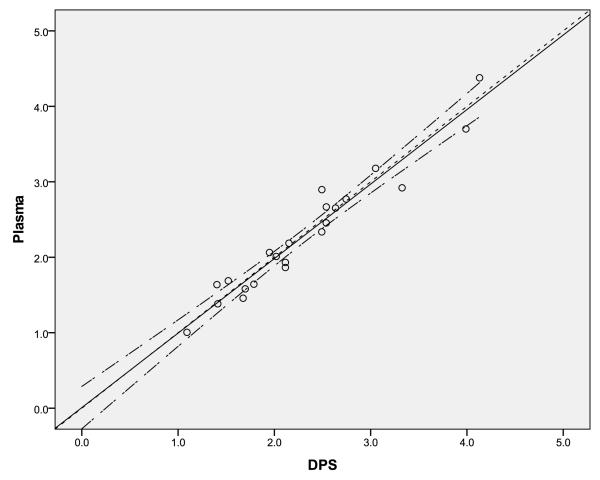
As can be seen in Figure 1, the 22 paired plasma and DPS samples showed good correlation. The Spearman Correlation coefficient was 0.96, and the line of identity was entirely within the 95% confidence interval of the regression line of the observed data. The mean %DEV of DPS samples from plasma samples is 1.68%.
Figure 1.
Correlation of EFV concentration (μg/mL) from matching plasma and dried plasma spot samples (DPS). The solid line is the line of regression, the long-dashed line is the 95% confidence interval of the regression, and the short-dashed line is the line of identity.
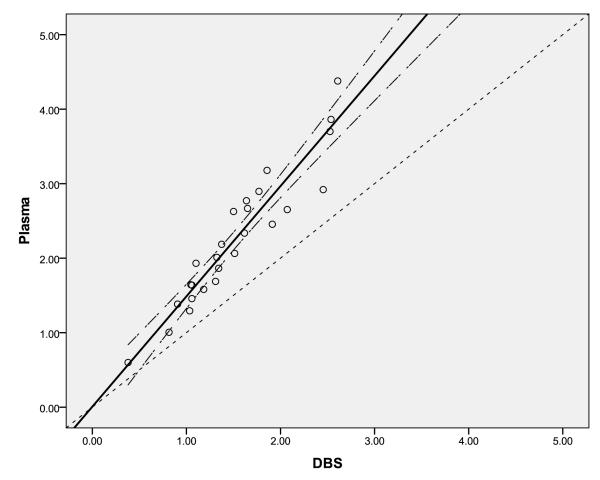
The correlation between the 26 paired plasma and DBS samples can be seen in Figure 2. The line of regression has the equation:
where the constant is not statistically significant. The Spearman correlation coefficient for this relationship is 0.96. Multivariable linear regression methods were attempted to assess the significance of hematocrit values as a covariate for the correlation between observed plasma and DBS EFV concentrations using the 19 samples containing all three parameters. As can be seen in the scatterplot of residuals (from the equation above) verses hematocrit in Figure 3, hematocrit was not found to be a significant covariate. The mean observed CDBS/Cplasma ratio was 0.68 with a variation (CV%) of 11.8%.
Figure 2.
Correlation of EFV concentrations (μg/mL) from matching plasma and dried blood spot (DBS) samples. The solid line is the line of regression, the long-dashed line is the 95% confidence interval of the regression, and the short-dashed line is the line of identity.
Figure 3.
Scatterplot of Residuals from the linear regression of observed EFV concentrations in Plasma (μg/mL) and DBS plotted against the associated hematocrit (HCT) values. The solid line is the line of regression, the long-dashed line is the 95% confidence interval of the regression, and the short-dashed line is the residual equals zero line.
Discussion
A validated method for the determination of EFV in human DBS is needed to measure concentrations in DBS from patients enrolled in IMPAACT clinical trials, particularly for those conducted in resource limited environments wherein plasma sampling methodologies are impractical. Assay design was focused on development of a rapid and simple method for establishing therapeutic adherence, facilitated by ease of collection, shipping and storage. An internal standard was not used to maximumize the simplicity of sample preparation and because excellent accuracy and precision within the specified dynamic range of EFV concentrations were obtained without it. Thus, it was especially critical to demonstrate excellent and consistent recovery of drug from dried blood spots. Stability characteristics of EFV in human dried blood spots under various storage and processing conditions were also characterized, to evaluate the robustness of specimen shipment options. Freeze/thaw stability was important to demonstrate since long-term storage of the EFV DBS was intended to be −20°C.
Despite theoretical limitations of using a UV-based detection method (sensitivity and selectivity), the results of this validation compare favorably to published LC-MS/MS EFV DBS methods. Owing to reduced resolution limitations, LC-MS/MS enables reduced elution times (6 verses 21 minutes) and thus HPLC run times.14-15 LC-MS/MS methodologies exhibit a more sensitive lower limit of detection (0.05 μg/mL),14 but this HPLC-UV assay was fully validated down to similar lower limit of quantitation as was validated for the LC-MS/MS (0.325 vs 0.1 μg/mL).15 However, since therapeutic levels of EFV are >1 μg/mL,20 the present HPLC-UV method provides a well characterized methodology for establishing therapeutic adherence without the extra expense of LC/MS/MS, making this HPLC-UV assay ideal in resource-limited settings where HIV is prevalent. The reported steady-state EFV Cmin is 1.8 μg/mL (in adults receiving 600 mg daily) and it has a long half-life (40-55 hours).22 Given the assay’s LLOQ of 0.325 μg/mL, the present HPLC-UV methodology can detect EFV for several days after the last administered EFVdose.
Hematocrit and volume of blood spotted have been reported as influential variables affecting determination of drug levels from DBS sampling techniques.9 As HCT is a determinant of blood viscosity, high HCT values can reduce blood spreading across the surface of the filter paper leading to reduced blood spot sizes and heterogenous DBS. ter Heine et al reported that volume of blood spotted (ranging from 20-60 μL) had no influence on the amount of EFV present in the punched out disc.15 We now report that HCT (ranging from 0.35-0.48) appears to have little influence on the amount of EFV present in the punched out disc.
Analysis of the clinical samples demonstrated a strong correlation between EFV concentrations measured from DBS and from plasma, with a mean CDBS/Cplasma ratio of 0.68 (standard deviation 0.08). Thus, while EFV concentrations obtained from DPS (mean CDPS/Cplasma ratio of 1.02 with a standard deviation of 0.08) can be used directly to monitor EFV therapy, concentrations derived from DBS methodologies cannot be used interchangeably with plasma reference levels and require conversion using the blood partitioning ratio (Cb/C). EFV is very highly bound in the plasma, mostly to albumin, and a clinical study evaluating EFV fraction unbound and intracellular accumulation reported a median EFV fu of 0.63% with an observed range of 0.4-1.5%.21 Since EFV is highly bound to plasma proteins, the low observed CDBS/Cplasma ratio in this study suggests much lower binding to RBC components.
The DBS HPLC-UV method reported herein is a simple, economical, and accurate method for measurement of efavirenz within the concentration range of 0.3125 and 20 μg/mL.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge support from the National Institute of Mental Health (Center award P30 MH62512 to the HIV Neurobehavioral Research Center), and National Institute of Allergy and Infectious Diseases (Award U01 AI 068632 IMPAACT Network Pharmacology Specialty Laboratory).
Glossary
- EFV
Efavirenz
- DBS
Dried blood spot
- HPLC
high-performance liquid chromatography
- UV
ultra-violet
- PK
Pharmacokinetic
- NNRTI
non-nucleoside reverse transcriptase inhibitor
- HAART
highly-active antiretroviral therapy
- ACN
acetonitrile
- DPS
dried plasma spot
- HCT
hematocrit
- LLOQ
lowest limit of quantitation
- ULOQ
upper limit of quantitation
- CV%
coefficient of variation
- %DEV
percent deviation
- fu
fraction unbound
Footnotes
Potential Conflicts of Interest Dr Scott Letendre is receiving research funding from and is currently on the advisory board for Merck and Co, INC. For the remaining authors no potential conflicts of interest were declared.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Majumdar TK, Howard DR. The use of dried blood spots for concentration assessment in pharmacokinetic evaluations. In: Bonate PL, Howard DR, editors. Pharmacokinetics in Drug Development. Springer Science; New York: 2011. pp. 91–114. [Google Scholar]
- 2.Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketoneuria in large populations of newborn infants. Pediatrics. 1963;32:338–343. [PubMed] [Google Scholar]
- 3.Edelbroek PM, van der Heijden J, Stolk LML. Dried blood spot methods in therapeutic drug monitoring: methods, assays, and pitfalls. Ther Drug Monit. 2009;31:327–336. doi: 10.1097/FTD.0b013e31819e91ce. [DOI] [PubMed] [Google Scholar]
- 4.Mei JV, Alexander JR, Adam BW, et al. Use of filter paper for the collection and analysis of human whole blood specimens. J Nutr. 2001;131:1631S–1636S. doi: 10.1093/jn/131.5.1631S. [DOI] [PubMed] [Google Scholar]
- 5.Spooner N, Lad R, Barfield M. Dried blood spots as a sample collection technique for the determination of pharmacokinetics in clinical studies: considerations for the validation of a quantitative bioanalytical method. Anal Chem. 2009;81:1557–1563. doi: 10.1021/ac8022839. [DOI] [PubMed] [Google Scholar]
- 6.Rowland M, Emmons GT. Use of dried blood spots in drug development: pharmacokinetic considerations. AAPS J. 2010;12:290–293. doi: 10.1208/s12248-010-9188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emmons G, Rowland M. Pharmacokinetic considerations as to when to use dried blood spot sampling. Bioanalysis. 2010;2:1791–1796. doi: 10.4155/bio.10.159. [DOI] [PubMed] [Google Scholar]
- 8.Thomas A, Deglon J, Steimer T, et al. Online desorption of dried blood spots coupled to hydrophilic interaction/reversed-phase LC/MS/MS system for the simultaneous analysis of drugs and their polar metabolites. J Sep Sci. 2010;33:873–879. doi: 10.1002/jssc.200900593. [DOI] [PubMed] [Google Scholar]
- 9.Patel P, Mulla H, Tanna S, et al. Facilitating pharmacokinetic studies in children: a new use of dried blood spots. Arch Dis Child. 2010;95:484–7. doi: 10.1136/adc.2009.177592. [DOI] [PubMed] [Google Scholar]
- 10.Oandya HC, Spooner N, Mulla H. Dried blood spots, pharmacokinetic studies and better medicines for children. Bioanalysis. 2011;3:779–786. doi: 10.4155/bio.11.19. [DOI] [PubMed] [Google Scholar]
- 11.Rowell V, Rowell FJ. Rapid enzyme-linked immunosorbet assay (ELISA) with a visual end-point for detecting quinine in urine, serum, and dried blood spots. Analyst. 1987;112:1437–1439. doi: 10.1039/an9871201437. [DOI] [PubMed] [Google Scholar]
- 12.Lejeune D, Souletie I, Houze S, et al. Simultaneous determination of monodesethylchloroquine, chloroquine, cycloguanil and proguanil on dried blood spots by reverse-phase liquid chromatography. J Pharm Biomed Anal. 2007;43:1106–1115. doi: 10.1016/j.jpba.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 13.Vu DH, Koster RA, Alffenaar JWC, et al. Determination of moxifloxacin in dried blood spots using LC-MS/MS and the impact of the hematocrit and blood volume. J Chromatogr B. 2011;879:1063–1070. doi: 10.1016/j.jchromb.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Koal T, Burhenne H, Romling R, et al. Quantification of antiretroviral drugs in dried blood spot samples by means of liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrum. 2005;19:2995–3001. doi: 10.1002/rcm.2158. [DOI] [PubMed] [Google Scholar]
- 15.ter Heine R, Rosing H, van Gorp ECM, et al. Quantification of protease inhibitors and non-nucleoside reverse transcriptase inhibitors in dried blood spots by liquid chromatography-triple quadruple mass spectrometry. J Chromatgr B. 2008;867:205–212. doi: 10.1016/j.jchromb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 16.ter Heine R, Rosing H, van Gorp ECM, et al. Quantification of etravirine (TMC125) in plasma, dried blood spots and peripheral blood mononuclear cell lysate by liquid chromatography tandem mass spectrometry. J Pharm Biomed Anal. 2009;49:393–400. doi: 10.1016/j.jpba.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 17.ter Heine R, Hillebrand MJX, Rosing H, et al. Quantification of the HIV-integrase inhibitor raltegravir and detection of its main metabolite in human plasma, dried blood spots and peripheral blood mononuclear cell lysate by means of high-performance liquid chromatography tandem mass spectrometry. J Pharm Biomed Anal. 2009;49:451–458. doi: 10.1016/j.jpba.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 18.ter Heine R, Mulder JW, van Gorp ECM, et al. Clinical evaluation of the determination of plasma concentrations of darunavir, etravirine, raltegravir, and ritonavir in dried blood spot samples. Bioanalysis. 2011;3:1093–1097. doi: 10.4155/bio.11.72. [DOI] [PubMed] [Google Scholar]
- 19.Boyd SD. A Review of recommendations and treatment options regarding the management of HIV infection. Am J Health Syst Pharm. 2011;68:991–1001. doi: 10.2146/ajhp100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marzolini C, Telenti A, Decosterd LA, et al. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. Aids. 2001;15:71–75. doi: 10.1097/00002030-200101050-00011. [DOI] [PubMed] [Google Scholar]
- 21.Almond LM, Hoggard PG, Edirisinghe D, et al. Intracellular and plasma pharmacokinetics of efavirenz in HIV-infected individuals. J Antimicrobial Chemother. 2005;56:738–744. doi: 10.1093/jac/dki308. [DOI] [PubMed] [Google Scholar]
- 22.Sustiva® [package insert] Bristol-Meyers Squibb Company; Princeton, NJ: 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.