Abstract
Timely delivery of information is essential for proper function of the nervous system. Precise regulation of nerve conduction velocity is needed for correct exertion of motor skills, sensory integration and cognitive functions. In vertebrates, the rapid transmission of signals along nerve fibers is made possible by the myelination of axons and the resulting saltatory conduction in between nodes of Ranvier. Myelin is a specialization of glia cells and is provided by oligodendrocytes in the central nervous system. Myelination not only maximizes conduction velocity, but also provides a means to systematically regulate conduction times in the nervous system. Systematic regulation of conduction velocity along axons, and thus systematic regulation of conduction time in between neural areas, is a common occurrence in the nervous system. To date, little is understood about the mechanism that underlies systematic conduction velocity regulation and conduction time synchrony. Node assembly, internode distance (node spacing) and axon diameter - all parameters determining the speed of signal propagation along axons - are controlled by myelinating glia. Therefore, an interaction between glial cells and neurons has been suggested.
This review summarizes examples of neural systems in which conduction velocity is regulated by anatomical variations along axons. While functional implications in these systems are not always clear, recent studies in the auditory system of birds and mammals present examples of conduction velocity regulation in systems with high temporal precision and a defined biological function. Together these findings suggest an active process that shapes the interaction between axons and myelinating glia to control conduction velocity along axons. Future studies involving these systems may provide further insight into how specific conduction times in the brain are established and maintained in development.
Throughout the text, conduction velocity is used for the speed of signal propagation, i.e. the speed at which an action potential travels. Conduction time refers to the time it takes for a specific signal to travel from its origin to its target, i.e. neuronal cell body to axonal terminal.
Keywords: conduction velocity regulation, neuronal isochronicity, internode distance, auditory system, coincidence detection
Introduction
When signals are conveyed from one neuron to another, temporal accuracy is essential for proper information processing in the nervous system. Exertion of fine motor skills, sensory processing and higher integrative functions require precise regulation of nerve conduction velocity. If the speed of conduction in nerves is altered by illness, impairments of motor, sensory (Compston and Coles, 2002) and possibly cognitive skills follow (Nave, 2010). In the peripheral and central nervous system a specialization of glial cells, the myelin sheath enwrapping of axons, was the last major evolutionary invention for the nervous system of vertebrates (Bullock et al., 1984; Zalc et al., 2008). Myelination of axons, first described in 1854 (Virchow, 1854), increases the speed of conduction significantly by saltatory nerve conduction between nodes of Ranvier (Ranvier, 1871). Qualitative differences of myelination along axons, such as variations in internode distance and myelin sheath thickness, enable systematic regulation of conduction velocity.
Precise temporal signal transmission is of particular importance for the processing of auditory information. One of the important functions of the auditory system is spatial hearing, which enables us to localize sound and to extract acoustic information in a noisy environment (Sound segregation or “cocktail party effect”) (Blauert, 1997), a task that fails with aging. The two main acoustic cues used for these tasks are interaural time differences (ITDs) and interaural intensity differences (IIDs). Processing ITDs and IIDs requires binaural inputs that are temporally correlated. Abnormal timing and synchrony in the auditory brainstem haven been suggested to contribute to auditory neuropathy (Oertel, 2005; Zeng et al., 2005), a form of hearing impairment with normal cochlear conduction but disordered neural conduction (Starr et al., 1996; Starr et al., 2000). Likewise, patients with demyelinating diseases like MS display loss of hearing acuity and impaired temporal processing in the auditory brainstem (Noffsinger et al., 1972; Levine et al., 1993; Rappaport et al., 1994; Jones et al., 2002). At a cellular level, dysmyelination has been shown to cause increase in spike jitter and failures (Kim et al., 2013). Similarly, temporal and speech processing is diminished in elderly listeners who have normal hearing thresholds (Goŕdon-Salant and Fitzgibbons, 1993; Anderson et al., 2012).
Systematic timing of signal propagation is a common occurrence in the nervous system and required for proper neural function. A particularly precise timing of neuronal fibers is displayed by the circuits in the auditory brainstem, detecting time disparities in the microsecond range and providing binaural inputs in temporal register. The auditory brainstem of birds and mammals presents a unique opportunity to study the development and mechanisms of CV regulation. The sound localization circuits in the auditory brainstem have temporally precise axonal projections, and a well-defined function. Future experiments may utilize these systems to study the regulation of conduction time in the nervous system, both during development and at advanced age. The results will contribute to the understanding and treatment of myelin-related diseases – from hereditary leukodystrophies to multiple sclerosis and the degeneration of myelin with age.
Determinants of conduction velocity regulation
Measurements of conduction velocity in axons attracted attention very early in the history of neuroscience. Empirical (Zotterman, 1937; Hursh, 1939; Gasser and Grundfest, 1939; Hutchinson et al., 1970) and theoretical studies (Rushton, 1951) showed a linear relationship between diameter and conduction velocity in myelinated axons. The myelin sheath is provided by specialized glial cells: the oligodendrocytes in the central nervous system (CNS) and Schwann cells in the peripheral nervous system (PNS). The myelin sheath is interrupted in regular intervals by nodes of Ranvier, where sodium channels are present in high density, thereby enabling saltatory conduction (Tasaki, 1939), the basis of fast signal propagation along myelinated axons.
Conduction velocity is influenced by myelin sheath thickness and internode distance (i.e. the distance along the axon between the nodes of Ranvier) (Hursh, 1939), and both parameters are linearly related to axon diameter. Conduction velocity increases with increasing internode distance up to 2000 μm (Brill et al., 1977). Another factor influencing the speed of signal propagation is the composition and density of sodium channels at the nodes of Ranvier (Waxman, 1975) as they influence the onset of the action potential generated at the node. Thus, speed of conduction in myelinated axons is dependent on a number of parameters and variation of any of those parameters can regulate signal propagation speed. These parameters and their influence on conduction velocity have been described in a number of reviews (Waxman, 1975; Waxman and Swadlow, 1977; Waxman, 1980; Waxman, 1997).
Conduction velocity variations in axons
Evolutionary pressures have lead to a maximization of conduction velocity in some axons. However, optimizing nerve fiber function means more than maximizing conduction velocity: Certain neural functions require adjustment of conduction velocity to regulate the relative timing of inputs, which in some cases entails a slowing down or a delay of signal propagation along axons relative to other axonal inputs.
One of the earliest studies on differential conduction velocity described systematic variations in axon diameter of motor axons innervating the mantle of the squid (Pumphrey and Young, 1938). These variations reduced temporal discrepancies as the longer fibers conducted more quickly than the shorter ones. While these differences in diameter do not achieve isochronicity of inputs (the concurrent arrival of inputs at the target), they do ensure nearly simultaneous contraction of the mantle muscles, resulting in a faster reaction time.
Another well-studied example is found in the classical model of the electromotor system in electric fish. In this organ, so-called electrocytes on the body surface provide an electrical discharge used to incapacitate prey. In order to fully deploy its potential, electrocyte discharges need to be synchronized. The electrocytes are connected to a pacemaker nucleus controlling their deployment and due to their location along the body, the axons providing input from the pacemaker nucleus differ in length. Internode distance along those axons is adjusted so that conduction velocity compensates for different axon lengths and simultaneous firing is achieved (Bennett, 1970; Waxman, 1971; see Figure 1 in Waxman, 1997). In the electric fish Sternarchus, two types of nodes of Ranvier exist: a small, typical type that actively generates spikes, and a larger electrically passive node type that adds capacitance to delay the propagation of action potentials along the axon and modify the waveform of the voltage spike (Waxman et al., 1972). Hence, in addition to the number of nodes of Ranvier along an axon, the structure of nodes can influence conduction velocity.
A particularly elegant example of systematic regulation of conduction velocity in mammals is found in the fibers from the inferior olive (IO) to cerebellar purkinje cells in rats. Purkinje cells in the cerebellar cortex display a high level of spiking synchronicity, supported by electrical coupling of IO cells through gap junctions. In rats, the olivocerebellar path length varies considerably between the different parts of the cerebellar cortex (Sugihara et al., 1993), however their conduction times are fairly equal (Sugihara et al., 1993; Lang and Rosenbluth, 2003). Variations in axon diameter as well as differential myelination contribute to these isochronic inputs (Sugihara et al., 1993; Lang and Rosenbluth, 2003). It should be noted that these findings are not without controversy; these results have not been observed in the cat, suggesting that the isochronicity of the olivocerebellar pathway is due to a restricted brain size in smaller animals (Aggelopoulos et al., 1995; Baker and Edgley, 2006).
Another representation of intra-axonal variation of conduction velocity was illustrated in the thalamocortical pathway of mice by Salami and colleagues (Salami et al., 2003). Neurons in the ventrobasal nucleus of the thalamus project to different areas in the cortex and their projections differ widely in length. The axon partitions in the intracortical region, however, cover a similar distance, due to the architecture of the cortical areas. Despite the length difference of the projections, action potentials elicited in the thalamus arrive around the same time at their target. Within cortical areas, conduction velocity slows down about 10-fold in thalamocortical axons, most likely the result of decreased myelination. Thus, the longer, more variable axon segments propagate action potentials quickly, while the shorter, uniform segments contribute most to overall conduction time.
In the retina, variations in conduction velocity minimize conduction time differences among retinal ganglion cell axons (Stanford, 1987). This presumably ensures spatiotemporal representation of the retinal image so that differences between conduction times to the lateral geniculate nucleus are minimized. Another such example is found in the projection from the lateral amygdala to distributed perirhinal sites. Conduction times are similar, despite the fact that the signals must travel different distances (Pelletier and Paré, 2002). The authors hypothesize that these synchronized inputs facilitate Hebbian associations (Hebb, 1949) between coincident, but spatially distributed, activity patterns in the perirhinal cortex.
Cortical areas in both hemispheres of the brain are connected via projections through the corpus callosum. Recent findings show that the axons forming these projections differ in length and possess different fiber caliber (Tomasi et al., 2012). Moreover, axon diameter depends on both the origin and the target of the projection (Innocenti et al., 2013). Although the exact functional significance is unknown, Innocenti and colleagues speculate that specific conduction times of projections from and within the cerebral cortex contribute to information encoding and processing in the brain through a complex system of lines of communication with specific time computing properties.
Cortical layer V neurons project to a number of different areas, both ipsilaterally and contralaterally, via the corpus callosum. Chomiak et al. showed that conduction velocity in the minor axon branches, connecting the ipsilateral targets, is related to the axonal length from the origin neuron (Chomiak et al., 2008). Conduction velocity is decreased in shorter axons, and vice versa, to allow isochronic spiking at the target nuclei. The much longer, contralateral axon branch is excluded from this strategy: conduction time to the other hemisphere is more than twice as long as the time needed for a signal to reach ipsilateral targets. It thus seems unlikely that this fiber branch undergoes the same temporal adjustment.
Intra-axonal differences, i.e. differences within a single axon, in signal propagation velocity have been suggested in various pathways. In the CNS, direct measurements of response times of antidromically elicited spikes showed that conduction velocity changes between the optic nerve and the optic tract (Baker and Stryker, 1990). This conduction velocity variation takes place presumably takes place in different parts of the same axons, but this was not explicitly determined. In the PNS, differences in internodal length, myelin sheath thickness and axon caliber were found for ventral motor neuron axons (Fraher, 1976; Fraher, 1978a; Fraher, 1978b). Similarly Traub and Mendell (Traub and Mendell, 1988) observed a sequential reduction in conduction velocity for receptor cell axons as they enter the dorsal root and then the spinal cord.
Conduction velocity regulation of axons and thus the specific control of conduction time seem unlikely to be a random occurrence. The exact functional implications of precisely timed inputs in the above-described examples, however, remain undefined.
The auditory system as a model system to study conduction velocity regulation
Auditory brainstem circuitry and function have been especially well described for mammals and birds, and both systems have been used for decades as research models (Friauf and Lohmann, 1999; Rubel and Fritzsch, 2002; Grothe, 2003; Kandler et al., 2009; Grothe et al., 2010; Burger et al., 2011; Ashida and Carr, 2011). Precise temporal signal transmission is of particular importance for the processing of auditory information.
One of the cues used for sound localization and segregation (suppression of unwanted noise, the “cocktail party effect”, which allows us to focus on a single conversation in a noisy room) are interaural time differences (ITD), or the difference in a sound’s arrival time at each ear. ITDs are defined by the speed of sound and the inter ear distance and as a result occur in the microsecond range. The primary circuitry underlying this ability involves ipsilateral and contralateral excitatory collaterals of single axons from monaurally innervated neurons in the cochlear nucleus to binaurally activated ITD-detecting neurons.
In the avian brainstem, the circuit responsible for encoding sub-millisecond ITDs consists of axonal delay lines innervating an array of coincidence detector neurons. Information from the ears is transferred to neurons in nucleus magnocellularis (NM), a part of the avian cochlear nucleus, on each side of the brainstem. Individual NM axons bifurcate and project to both the ipsilateral nucleus laminaris (NL) and the contralateral NL. Thus, NL coincidence detector neurons receive inputs from both ears. The circuit comprised by NM and NL embodies a modified Jeffress model (Jeffress, 1948; Overholt et al., 1992; Köppl and Carr, 2008; Seidl et al., 2010), in which external arrival time differences are matched by an internal delay line. NL neurons form a bilateral map of sound source locations (Figure; Carr and Konishi, 1990; Overholt et al., 1992; Köppl and Carr, 2008; for review see Palmer, 2004; Burger et al., 2011). Equivalent interaural delays occur when a sound stimulus originates from straight ahead, arriving at both ears simultaneously and leading to an ITD of 0 μs. Action potential (AP) travel times have to be regulated precisely to ensure coincident arrival of information within several microseconds at individual detector neurons in NL. NM neurons send out a circuitous, but short axon branch to the ipsilateral NL and a much longer axon branch to the contralateral NL (Figure; Seidl et al., 2010). The length difference between these axon branches exceeds 1600 μm, making coincident arrival of inputs at 0 ITD a challenge. In fact, coincident binaural inputs would be impossible at any naturally occurring ITD. Anatomical data describes variations in axon diameter and distance between nodes of Ranvier between ipsilateral and contralateral inputs to NL, suggesting that axon length differences are counterbalanced by differences in conduction velocity (Seidl et al., 2010). Direct measurements of conduction velocity confirm this hypothesis and show that signal propagation is twice as fast in the longer, contralateral axon (Seidl et al., 2013). Thus, within one axon, conduction velocities differ systematically between two branches enabling coincidence detection in the microsecond range.
These findings in the chicken auditory brainstem were foreshadowed by a study in the barn owl (Carr and Konishi, 1990). Here, differences between the ipsilateral and contralateral NM axon branches were found but were not as dramatic as in the chicken. Carr and Konishi also reported variations in axon diameter and internode distances and speculated that these anatomical variations might provide a means to regulate conduction times (Carr and Konishi, 1990).
In the mammalian auditory brainstem variations in axon diameter and internode distance have been found as well. While the exact mechanism of ITD processing in mammals is still being resolved (for review see Joris and Yin, 2007; Grothe et al., 2010; Portfors and von Gersdorff, 2013), it appears to involve a strong, temporally precise inhibitory input in addition to binaural excitatory coincidence detection onto neurons in the medial superior olive (MSO) (Brand et al., 2002; Pecka et al., 2008). This inhibitory projection from the ipsilateral medial nucleus of the trapezoid body (MNTB) is driven by an excitatory input from the contralateral cochlear nucleus. Recent findings have revealed caliber and internode variations that would selectively speed up the excitatory inputs to MNTB compared to those to MSO (Ford et al., 2012). These findings support the hypothesis that inhibitory inputs to MSO are precisely timed in relation to the contralateral excitatory inputs targeting the same nucleus, suggesting a role for inhibition in shaping ITD tuning of MSO neurons. These findings are not without precedence in the mammalian auditory brainstem: in the cat, binaural axonal inputs to the lateral superior olive, (LSO), involved in the processing of interaural level differences (ILD, occurring when sound is louder at one ear over the other) have been shown to differ in diameter as well (Warr, 1966; Brownell, 1975; Spangler et al., 1985; Spirou et al., 1990; Smith et al., 1991; Smith et al., 1993; reviewed in Joris and Yin, 1998).
Conduction velocity properties of axons providing inputs to primary binaural centers are regulated systematically. Plasticity in ITD and ILD processing can be induced during development and in adulthood by deprivation of meaningful acoustic cues (Seidl and Grothe, 2005; Siveke et al., 2012). This plasticity in response patterns correlates with qualitative changes of inhibitory axonal inputs that depend on normal auditory experience during development (Kapfer et al., 2002; Werthat et al., 2008). Precise timing of excitatory axonal inputs is important for both ITD and ILD coding (ITD: Rose et al., 1966; Goldberg and Brown, 1969; ILD: Joris and Yin, 1995; Park et al., 1996; Park et al., 1997; Tollin, 2003). Deprivation of acoustic cues may also cause a change in the development of myelination and contribute to alteration of timing properties of afferent inputs to MSO and LSO. Therefore, myelin plasticity may play a role in shaping ITD and ILD processing as well. Recent data suggest that myelination can be a substrate for adult plasticity (Liu et al., 2012). It is, thus, possible that changes in myelination can be induced by unnatural acoustic environments during adulthood and result in altered responses of the auditory system (Siveke et al., 2012).
Regulation of conduction time in the auditory system is different from other systems for two reasons. First, adjustment of conduction time serves a known biological function, the encoding of ITDs. The circuitry comprised by NM and NL embodies a Jeffress model of sound localization for which the timing of inputs is crucial (Jeffress, 1948). Second, conduction time regulation needs to be more precise, as ITDs occur in the sub-millisecond range. For the chicken (Calford and Piddington, 1988), and likewise for small mammals like the gerbil (Maki and Furukawa, 2005), the maximum ITD occurring between the two ears is less than 200 μs. If the timing of inputs were off by more than that, no binaural coincidence detection would occur at NL. Hence, precise adjustment of conduction times is a prerequisite. The differential regulation of conduction time in the avian auditory brainstem is remarkable because it takes place in two major branches of a single axon. This sets it apart from differential regulation in the mammalian auditory brainstem (projections with different origin and target) and other systems, where differential regulation occurs along the same axon or in terminal axon branches that make out only a small part of total axon length.
Because of the aforementioned characteristics, the auditory system in birds and mammals presents an interesting model system to study mechanisms and development of internode distance regulation.
Mechanisms of internode distance regulation
Axon diameter, internode distance, and myelin sheath thickness all influence the speed of action potential propagation. Moreover, these factors are to a certain degree correlated with each other. As node assembly, node spacing, and axon diameter are controlled by myelinating glia (de Waegh et al., 1992; Garcia et al., 2003; Court et al., 2004; Susuki and Rasband, 2008), the key to understanding conduction velocity regulation lies most likely with glial cells and their interaction with axons.
Traditionally, growth during development and the ensuing stretching of axons is thought to be responsible for determining the distance between nodes of Ranvier (Hiscoe, 1947; Schlaepfer and Myers, 1973; Friede et al., 1981; Friede et al., 1985; Hildebrand et al., 1989; Hildebrand et al., 1996). Consequently, the determining factor in internode elongation should be the developmental time period, during which myelination occurs coincident with axonal growth. Such a passive mechanism has major implications on the ability to repair damage after demyelination caused by neurological disease and trauma. Remyelination would not have access to the same processes to control internode distance in adult organisms after growth, and thus the elongation of axons, has stopped. A recent study by Horner and colleagues, however, provides evidence for a dynamic remyelination that is independent of axonal growth (Powers et al., 2013). Here, myelin that is restored by oligodendrocytes after spinal cord injury in mice does not differ from myelin in control animals with respect to internode distance and myelin sheath thickness. Myelin sheath length increased over the course of 6 months to achieve control values. This suggests that establishing internode length may involve a process that does not require lengthening of axons.
Much knowledge about internode distance and its relation to axon diameter has been gained from studies in the PNS, where Schwann cells provide myelination. Myelin formed by Schwann cells is similar to myelin formed by oligodendrocytes in certain aspects, such as composition of Na+ channels at the node, anchoring proteins at the node and paranode, as well as K+ channels at the juxtaparanode (Peles and Salzer, 2000). There are, however, fundamental characteristics that separate oligodendrocyte-mediated myelin from that formed by Schwann cells: Schwann cells can only provide one internode per axon, whereas oligodendrocytes extend several processes, each of which myelinates distinct internodes, often on different axons (Baumann and Pham-Dinh, 2001). A local regulation between axon segments and oligodendrocyte processes has been suggested to affect myelin sheath thickness: In rats, myelin sheaths of different thickness are provided by the same oligodendrocyte. The existence of polyribosomes and/or rough endoplasmic reticulum in oligodendrocyte processes near the myelinated axons suggests a local mechanism in myelin formation (Waxman and Sims, 1984). Nerve growth factor (NGF) stimulates myelination by Schwann cells but inhibits oligodendrocyte-mediated myelination (Chan et al., 2004). After birth, the brain is less susceptible to growth than the rest of the body (Thompson, 1917; Moore, 1983). Hence the degree of change needed for internodes may differ between the PNS and the CNS.
How is the position of nodes of Ranvier along axons determined? An early event in the establishment of nodes of Ranvier is the formation of a junction at the paranode between the axon and the glial sheet and sodium channel clustering at the node (Poliak and Peles, 2003; Sherman and Brophy, 2005; Salzer et al., 2008; Susuki and Rasband, 2008). The formation of a paranodal junction and sodium channel clustering at the node appear linked, however the exact mechanism is unclear. At least for the CNS, myelination does not seem to be a prerequisite for node formation, as contact between oligodendrocytes and the neurites of retinal ganglion cells is not necessary for the induction of sodium channel clustering (Kaplan et al., 1997). Similarly, axon diameter can be controlled by oligodendrocytes in the absence of myelination (Sánchez et al., 1996). Conversely, individual axons have been shown to profoundly regulate the myelinating potential of single oligodendrocytes (Almeida et al., 2011).
Some insight about node formation might come from an axonal neighbor, the axon initial segment (AIS). The AIS and the nodes of Ranvier share the molecular composition of ion channels, anchoring proteins and other classes of proteins (Ogawa and Rasband, 2008; Rasband, 2010). Na+ and K+ channel clustering at the AIS further suggests that nodes of Ranvier are evolutionary derivatives of the AIS (Hill et al., 2008). A series of excellent studies recently demonstrated plasticity at the AIS: in the avian auditory brainstem, the position of the AIS is tuned for optimal function in a sound localization circuit (Kuba et al., 2006). The same neurons respond with change in AIS length and position after sensory deprivation (Kuba et al., 2010). Similar results have been reported in dissociated neurons of mammals, suggesting that plasticity at the AIS may be a universal mechanism to regulate neuronal excitability (Grubb and Burrone, 2010; Evans et al., 2013).
Information processing in the auditory brainstem of mammals and birds, together with the synchronized current discharge in electrical fish, present the highest level of temporal precision in any neural system. Considering the temporal resolution needed to differentiate and encode ITDs that differ by only a few microseconds, it is intriguing to speculate about an active process that adjusts conduction time along axons conveying information from the ears. In the avian auditory brainstem, conduction velocity is regulated differentially within two branches of the same axon to overcome a dramatic fiber length difference and achieve isochronic inputs (Seidl et al., 2010; Seidl et al., 2013). Myelination of the delay line circuit of the barn owl occurs at a time in development when auditory responses are present (Kubke and Carr, 2000) and coincides with the period of head growth and attainment of stable ITD cues (Cheng and Carr, 2007). Therefore, myelination in this circuit may be affected by the electrical activity of NL neurons and/or signals of axons from NM.
We can only speculate how a systematic arrangement of conduction velocities is achieved during development. Sensory inputs appear to play a role in myelination. In the visual system, deprivation or enhancement of sensory inputs cause a change in myelination (Gyllensten and Malmfors, 1963; Tauber et al., 1980). Similar to barn owls, acoustically evoked activity is present in chickens (Jackson and Rubel, 1978) when myelination occurs (Hartman et al., 1979; Macklin and Weill, 1985; Korn and Cramer, 2008). Therefore the contribution of sensory activity to systematic regulation of internode distance seems possible.
If sensory activity influences myelination, how is oligodendrocyte myelination around axons controlled? Electrical activity of CNS axons has been shown to affect proliferation and differentiation of myelinating glia (Demerens et al., 1996; Stevens et al., 2002; Ishibashi et al., 2006); likely by the release of glutamate from synaptic vesicles along axons that promotes myelin induction (Wake et al., 2011). Moreover, oligodendrocytes are electrically active as well (Káradóttir et al., 2008) and their membrane potential can influence conduction velocity (Yamazaki et al., 2007). Recently, Teneurin-4 has been identified as a regulator of oligodendrocyte differentiation and myelination (Suzuki et al., 2012) and, as it is expressed in the auditory brainstem, it might play a role in functional myelination (Kenzelmann-Broz et al., 2010).
Conclusion
In the past few decades, enormous progress has been made in the myelin field. It will be exciting to find out more about interactions between axon and glial cells and how these interactions shape myelin plasticity. Recent studies suggest that an activity-dependent process may shape the organization of myelin along an axon, but it is currently unknown how internode distances are regulated. Future experiments are needed to establish how specific internode distances are arranged during development, what is needed for the maintenance of specific conduction times. Another interesting question will be if conduction times of networks change with age. Understanding these mechanisms has implications for the formation and regulation of myelinated axons throughout life and for mechanisms that may need to be considered for their repair. Demyelinating diseases like multiple sclerosis lead to are responsible for the breakdown of precise conduction velocity regulation, thereby compromising motor skills and sensory processing (Compston and Coles, 2002). Cognitive functions may be susceptible to oligodendrocyte defects also (Nave, 2010), as myelination shows plasticity in adulthood triggered by social interactions (Liu et al., 2012) and transmission along transcallosal projections seems to be timed precisely (Tomasi et al., 2012; Innocenti et al., 2013). Knowledge about the development and mechanisms of conduction regulation in axons may lead to new therapies for neurological and neuropsychiatric diseases.
Figure 1.
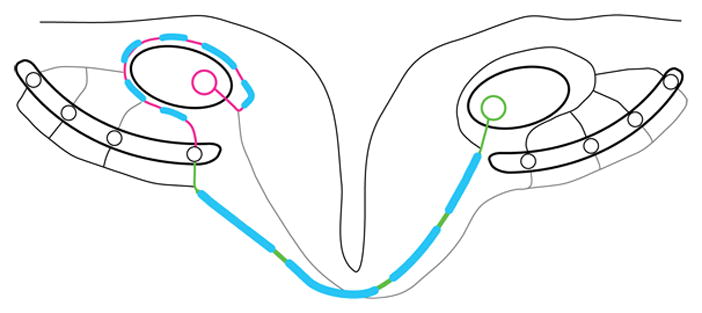
Figure Differential regulation of conduction velocity in two major branches of single axon
Nucleus magnocellularis (NM) and nucleus laminaris (NL) embody a modified Jeffress model (Jeffress, 1948) where contralateral NM axons form a delay line on the ventral side of a NL cell line. This delay line sequentially slows down the contralaterally evoked sound signal and enables coincidence with the ipsilateral inputs that creates no delay between terminals. Depending on the ITD presented, coincident inputs occur at a different location in NL, rendering the NL cell line a map or sound source location along the azimuth (Carr and Konishi, 1990; Köppl and Carr, 2008). The ipsilateral and contralateral axon branches of the NM axon display a length difference of more than 1600 μm. Conduction velocities in the ipsilateral and contralateral branches are adjusted utilizing variations in internode distance and axon diameter so that conduction times achieve isochronicity in the microsecond range. In the shorter axon branch, internode distance and axon diameter are shorter and thinner, respectively, than in the longer axon branch. The differential regulation of conduction time in the sub-millisecond range between two major collaterals of a single axon to fulfill a well-described biological function is unprecedented (Seidl et al., 2010). Note that both axon segments are part of a single NM axon. Magenta, ipsilateral axon branch; green, contralateral axon branch; blue, myelin sheath.
Highlights.
This review presents neural systems where axon conduction times are systematically regulated.
Recent studies in the auditory system are highlighted where high temporal precision is achieved.
Mechanisms of neuron-glia interaction may achieve activity-dependent plasticity of myelin.
Acknowledgments
I am grateful to Melissa L. Caras, Stephanie A. Furrer and Claire J. Creutzfeldt for comments on the manuscript. I also thank the anonymous reviewers for their helpful comments and constructive criticism.
Footnotes
Disclosures: The author declares no conflicts of interest, financial or otherwise.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggelopoulos NC, Duke C, Edgley SA. Non-uniform conduction time in the olivocerebellar pathway in the anaesthetized cat. J Physiol. 1995;486 (Pt 3):763–768. doi: 10.1113/jphysiol.1995.sp020851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida RG, Czopka T, ffrench-Constant C, Lyons DA. Individual axons regulate the myelinating potential of single oligodendrocytes in vivo. Development. 2011;138:4443–4450. doi: 10.1242/dev.071001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Parbery-Clark A, White-Schwoch T, Kraus N. Aging affects neural precision of speech encoding. J Neurosci. 2012;32:14156–14164. doi: 10.1523/JNEUROSCI.2176-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashida G, Carr CE. Sound localization: Jeffress and beyond. Curr Opin Neurobiol. 2011;21:745–751. doi: 10.1016/j.conb.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker GE, Stryker MP. Retinofugal fibres change conduction velocity and diameter between the optic nerve and tract in ferrets. Nature. 1990;344:342–345. doi: 10.1038/344342a0. [DOI] [PubMed] [Google Scholar]
- Baker MR, Edgley SA. Non-uniform olivocerebellar conduction time in the vermis of the rat cerebellum. J Physiol. 2006;570:501–506. doi: 10.1113/jphysiol.2005.099176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- Bennett MV. Comparative physiology: electric organs. Annu Rev Physiol. 1970;32:471–528. doi: 10.1146/annurev.ph.32.030170.002351. [DOI] [PubMed] [Google Scholar]
- Blauert J. Spatial Hearing: The Psychophysics of Human Sound Localization. Cambridge, MA: MIT Press; 1997. Spatial hearing with multiple sound sources and in enclosed spaces; pp. 201–287. [Google Scholar]
- Brand A, Behrend O, Marquardt T, McAlpine D, Grothe B. Precise inhibition is essential for microsecond interaural time difference coding. Nature. 2002;417:543–547. doi: 10.1038/417543a. [DOI] [PubMed] [Google Scholar]
- Brill MH, Waxman SG, Moore JW, Joyner RW. Conduction velocity and spike configuration in myelinated fibres: computed dependence on internode distance. J Neurol Neurosurg Psychiatry. 1977;40:769–774. doi: 10.1136/jnnp.40.8.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell WE. Organization of the cat trapezoid body and the discharge characteristics of its fibers. Brain Res. 1975;94:413–433. doi: 10.1016/0006-8993(75)90226-7. [DOI] [PubMed] [Google Scholar]
- Bullock TH, Moore JK, Fields RD. Evolution of myelin sheaths: both lamprey and hagfish lack myelin. Neurosci Lett. 1984;48:145–148. doi: 10.1016/0304-3940(84)90010-7. [DOI] [PubMed] [Google Scholar]
- Burger RM, Fukui I, Ohmori H, Rubel EW. Inhibition in the balance: binaurally coupled inhibitory feedback in sound localization circuitry. J Neurophysiol. 2011;106:4–14. doi: 10.1152/jn.00205.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calford M, Piddington R. Avian interaural canal enhances interaural delay. J Comp Physiol. 1988;162:503–510. [Google Scholar]
- Carr CE, Konishi M. A circuit for detection of interaural time differences in the brain stem of the barn owl. J Neurosci. 1990;10:3227–3246. doi: 10.1523/JNEUROSCI.10-10-03227.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JR, Watkins TA, Cosgaya JM, Zhang C, Chen L, Reichardt LF, Shooter EM, Barres BA. NGF Controls Axonal Receptivity to Myelination by Schwann Cells or Oligodendrocytes. Neuron. 2004;43:183–191. doi: 10.1016/j.neuron.2004.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SM, Carr CE. Functional delay of myelination of auditory delay lines in the nucleus laminaris of the barn owl. Dev Neurobiol. 2007;67:1957–1974. doi: 10.1002/dneu.20541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomiak T, Peters S, Hu B. Functional architecture and spike timing properties of corticofugal projections from rat ventral temporal cortex. J Neurophysiol. 2008;100:327–335. doi: 10.1152/jn.90392.2008. [DOI] [PubMed] [Google Scholar]
- Compston A, Coles A. Multiple sclerosis. The Lancet. 2002;359:1221–1231. doi: 10.1016/S0140-6736(02)08220-X. [DOI] [PubMed] [Google Scholar]
- Court FA, Sherman DL, Pratt T, Garry EM, Ribchester RR, Cottrell DF, Fleetwood-Walker SM, Brophy PJ. Restricted growth of Schwann cells lacking Cajal bands slows conduction in myelinated nerves. Nature. 2004;431:191–195. doi: 10.1038/nature02841. [DOI] [PubMed] [Google Scholar]
- Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci U S A. 1996;93:9887–9892. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MD, Sammons RP, Lebron S, Dumitrescu AS, Watkins TBK, Uebele VN, Renger JJ, Grubb MS. Calcineurin Signaling Mediates Activity-Dependent Relocation of the Axon Initial Segment. The Journal of Neuroscience. 2013;33:6950–6963. doi: 10.1523/JNEUROSCI.0277-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford M, Alexandrova O, Cossell L, Attwell D, Grothe B. Fine tuning of action potential conduction velocity in myelinated CNS axons. Society for Neuroscience. 2012;2012 [Google Scholar]
- Fraher JP. The growth and myelination of central and peripheral segments of ventral motoneurone axons. A quantitative ultrastructural study. Brain Res. 1976;105:193–211. doi: 10.1016/0006-8993(76)90421-2. [DOI] [PubMed] [Google Scholar]
- Fraher JP. Quantitative studies on the maturation of central and peripheral parts of individual ventral motoneuron axons. I. Myelin sheath and axon calibre. J Anat. 1978a;126:509–533. [PMC free article] [PubMed] [Google Scholar]
- Fraher JP. Quantitative studies on the maturation of central and peripheral parts of individual ventral motoneuron axons. II. Internodal length. J Anat. 1978b;127:1–15. [PMC free article] [PubMed] [Google Scholar]
- Friauf E, Lohmann C. Development of auditory brainstem circuitry. Activity-dependent and activity-independent processes. Cell Tissue Res. 1999;297:187–195. doi: 10.1007/s004410051346. [DOI] [PubMed] [Google Scholar]
- Friede RL, Brzoska J, Hartmann U. Changes in myelin sheath thickness and internode geometry in the rabbit phrenic nerve during growth. J Anat. 1985;143:103–113. [PMC free article] [PubMed] [Google Scholar]
- Friede RL, Meier T, Diem M. How is the exact length of an internode determined. J Neurol Sci. 1981;50:217–228. doi: 10.1016/0022-510x(81)90168-4. [DOI] [PubMed] [Google Scholar]
- Garcia ML, Lobsiger CS, Shah SB, Deerinck TJ, Crum J, Young D, Ward CM, Crawford TO, Gotow T, Uchiyama Y, Ellisman MH, Calcutt NA, Cleveland DW. NF-M is an essential target for the myelin-directed “outside-in” signaling cascade that mediates radial axonal growth. J Cell Biol. 2003;163:1011–1020. doi: 10.1083/jcb.200308159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser HS, Grundfest H. Axon diameters in relation to the spike dimensions and the conduction velocity in mammalian A fibres. Brain Res. 1939;127:393–414. [Google Scholar]
- Goldberg JM, Brown PB. Response of binaural neurons of dog superior olivary complex to dichotic tonal stimuli: some physiological mechanisms of sound localization. J Neurophysiol. 1969;32:613–636. doi: 10.1152/jn.1969.32.4.613. [DOI] [PubMed] [Google Scholar]
- Goŕdon-Salant S, Fitzgibbons PJ. Temporal factors and speech recognition performance in young and elderly listeners. J Speech Hear Res. 1993;36:1276–1285. doi: 10.1044/jshr.3606.1276. [DOI] [PubMed] [Google Scholar]
- Grothe B. New roles for synaptic inhibition in sound localization. Nature reviews Neuroscience. 2003;4:540–550. doi: 10.1038/nrn1136. [DOI] [PubMed] [Google Scholar]
- Grothe B, Pecka M, McAlpine D. Mechanisms of sound localization in mammals. Physiol Rev. 2010;90:983–1012. doi: 10.1152/physrev.00026.2009. [DOI] [PubMed] [Google Scholar]
- Grubb MS, Burrone J. Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature. 2010;465:1070–1074. doi: 10.1038/nature09160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllensten L, Malmfors T. Myelinization of the optic nerve and its dependence on visual function--a quantitative investigation in mice. J Embryol Exp Morphol. 1963;11:255–266. [PubMed] [Google Scholar]
- Hartman BK, Agrawal HC, Kalmbach S, Shearer WT. A comparative study of the immunohistochemical localization of basic protein to myelin and oligodendrocytes in rat and chicken brain. J Comp Neurol. 1979;188:273–290. doi: 10.1002/cne.901880206. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior. New York: Wiley & Sons; 1949. [Google Scholar]
- Hildebrand C, Loeliger S, Bjartmar C, Karlsson M. Sheath lengths of large motor axons in the ventral root L5 of neonatal and adult rats. Neurosci Lett. 1996;202:173–176. doi: 10.1016/0304-3940(95)12242-7. [DOI] [PubMed] [Google Scholar]
- Hildebrand C, Westerberg M, Mustafa GY. Influence of an experimental hindlimb maldevelopment on axon number and nodal spacing in the rat sciatic nerve. Brain Res Dev Brain Res. 1989;50:169–175. doi: 10.1016/0165-3806(89)90192-2. [DOI] [PubMed] [Google Scholar]
- Hill AS, Nishino A, Nakajo K, Zhang G, Fineman JR, Selzer ME, Okamura Y, Cooper EC. Ion channel clustering at the axon initial segment and node of Ranvier evolved sequentially in early chordates. PLoS Genet. 2008;4:e1000317. doi: 10.1371/journal.pgen.1000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscoe HB. Distribution of nodes and incisures in normal and regenerated nerve fibers. Anat Rec. 1947;99:447–475. doi: 10.1002/ar.1090990404. [DOI] [PubMed] [Google Scholar]
- Hursh JB. Conduction velocity and diameter of nerve fibres. American Journal of Physiology -- Legacy Content. 1939;127:131–139. [Google Scholar]
- Hutchinson NA, Koles ZJ, Smith RS. Conduction velocity in myelinated nerve fibres of Xenopus laevis. J Physiol. 1970;208:279–289. doi: 10.1113/jphysiol.1970.sp009119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti GM, Vercelli A, Caminiti R. The Diameter of Cortical Axons Depends Both on the Area of Origin and Target. Cereb Cortex. 2013 doi: 10.1093/cercor/bht070. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, Fields RD. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49:823–832. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson H, Rubel EW. Ontogeny of behavioral responsiveness to sound in the chick embryo as indicated by electrical recordings of motility. J Comp Physiol Psychol. 1978;92:682–696. doi: 10.1037/h0077496. [DOI] [PubMed] [Google Scholar]
- Jeffress LA. A place theory of sound localization. J Comp Physiol Psychol. 1948;41:35–39. doi: 10.1037/h0061495. [DOI] [PubMed] [Google Scholar]
- Jones SJ, Sprague L, Vaz Pato M. Electrophysiological evidence for a defect in the processing of temporal sound patterns in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2002;73:561–567. doi: 10.1136/jnnp.73.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris P, Yin TC. A matter of time: internal delays in binaural processing. Trends Neurosci. 2007;30:70–78. doi: 10.1016/j.tins.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Joris PX, Yin TC. Envelope coding in the lateral superior olive. I. Sensitivity to interaural time differences. J Neurophysiol. 1995;73:1043–1062. doi: 10.1152/jn.1995.73.3.1043. [DOI] [PubMed] [Google Scholar]
- Joris PX, Yin TC. Envelope coding in the lateral superior olive. III. Comparison with afferent pathways. J Neurophysiol. 1998;79:253–269. doi: 10.1152/jn.1998.79.1.253. [DOI] [PubMed] [Google Scholar]
- Kandler K, Clause A, Noh J. Tonotopic reorganization of developing auditory brainstem circuits. Nat Neurosci. 2009;12:711–717. doi: 10.1038/nn.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapfer C, Seidl AH, Schweizer H, Grothe B. Experience-dependent refinement of inhibitory inputs to auditory coincidence-detector neurons. Nat Neurosci. 2002;5:247–253. doi: 10.1038/nn810. [DOI] [PubMed] [Google Scholar]
- Kaplan MR, Meyer-Franke A, Lambert S, Bennett V, Duncan ID, Levinson SR, Barres BA. Induction of sodium channel clustering by oligodendrocytes. Nature. 1997;386:724–728. doi: 10.1038/386724a0. [DOI] [PubMed] [Google Scholar]
- Káradóttir R, Hamilton NB, Bakiri Y, Attwell D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci. 2008;11:450–456. doi: 10.1038/nn2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenzelmann-Broz D, Tucker RP, Leachman NT, Chiquet-Ehrismann R. The expression of teneurin-4 in the avian embryo: potential roles in patterning of the limb and nervous system. Int J Dev Biol. 2010;54:1509–1516. doi: 10.1387/ijdb.103139dk. [DOI] [PubMed] [Google Scholar]
- Kim JH, Renden R, von Gersdorff H. Dysmyelination of Auditory Afferent Axons Increases the Jitter of Action Potential Timing during High-Frequency Firing. The Journal of Neuroscience. 2013;33:9402–9407. doi: 10.1523/JNEUROSCI.3389-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn MJ, Cramer KS. Distribution of glial-associated proteins in the developing chick auditory brainstem. Dev Neurobiol. 2008;68:1093–1106. doi: 10.1002/dneu.20645. [DOI] [PubMed] [Google Scholar]
- Köppl C, Carr CE. Maps of interaural time difference in the chicken’s brainstem nucleus laminaris. Biol Cybern. 2008;98:541–559. doi: 10.1007/s00422-008-0220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba H, Ishii TM, Ohmori H. Axonal site of spike initiation enhances auditory coincidence detection. Nature. 2006;444:1069–1072. doi: 10.1038/nature05347. [DOI] [PubMed] [Google Scholar]
- Kuba H, Oichi Y, Ohmori H. Presynaptic activity regulates Na(+) channel distribution at the axon initial segment. Nature. 2010;465:1075–1078. doi: 10.1038/nature09087. [DOI] [PubMed] [Google Scholar]
- Kubke MF, Carr CE. Development of the auditory brainstem of birds: comparison between barn owls and chickens. Hear Res. 2000;147:1–20. doi: 10.1016/s0378-5955(00)00116-7. [DOI] [PubMed] [Google Scholar]
- Lang EJ, Rosenbluth J. Role of myelination in the development of a uniform olivocerebellar conduction time. J Neurophysiol. 2003;89:2259–2270. doi: 10.1152/jn.00922.2002. [DOI] [PubMed] [Google Scholar]
- Levine RA, Gardner JC, Fullerton BC, Stufflebeam SM, Carlisle EW, Furst M, Rosen BR, Kiang NY. Effects of multiple sclerosis brainstem lesions on sound lateralization and brainstem auditory evoked potentials. Hear Res. 1993;68:73–88. doi: 10.1016/0378-5955(93)90066-a. [DOI] [PubMed] [Google Scholar]
- Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, Vialou V, Lobo MK, Dietz DM, Nestler EJ, Dupree J, Casaccia P. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci. 2012;15:1621–1623. doi: 10.1038/nn.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklin WB, Weill CL. Appearance of myelin proteins during development in the chick central nervous system. Dev Neurosci. 1985;7:170–178. doi: 10.1159/000112285. [DOI] [PubMed] [Google Scholar]
- Maki K, Furukawa S. Acoustical cues for sound localization by the Mongolian gerbil, Meriones unguiculatus. J Acoust Soc Am. 2005;118:872–886. doi: 10.1121/1.1944647. [DOI] [PubMed] [Google Scholar]
- Moore K. The Developing Human. Philadelphia, PA: W. B. Saunders; 1983. [Google Scholar]
- Nave KA. Myelination and support of axonal integrity by glia. Nature. 2010;468:244–252. doi: 10.1038/nature09614. [DOI] [PubMed] [Google Scholar]
- Noffsinger D, Olsen WO, Carhart R, Hart CW, Sahgal V. Auditory and vestibular aberrations in multiple sclerosis. Acta Otolaryngol Suppl. 1972;303:1–63. [PubMed] [Google Scholar]
- Oertel D. Importance of timing for understanding speech. Focus on “perceptual consequences of disrupted auditory nerve activity”. J Neurophysiol. 2005;93:3044–3045. doi: 10.1152/jn.00020.2005. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Rasband MN. The functional organization and assembly of the axon initial segment. Curr Opin Neurobiol. 2008;18:307–313. doi: 10.1016/j.conb.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Overholt EM, Rubel EW, Hyson RL. A circuit for coding interaural time differences in the chick brainstem. J Neurosci. 1992;12:1698–1708. doi: 10.1523/JNEUROSCI.12-05-01698.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AR. Reassessing mechanisms of low-frequency sound localisation. Curr Opin Neurobiol. 2004;14:457–460. doi: 10.1016/j.conb.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Park TJ, Grothe B, Pollak GD, Schuller G, Koch U. Neural delays shape selectivity to interaural intensity differences in the lateral superior olive. J Neurosci. 1996;16:6554–6566. doi: 10.1523/JNEUROSCI.16-20-06554.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TJ, Monsivais P, Pollak GD. Processing of interaural intensity differences in the LSO: role of interaural threshold differences. J Neurophysiol. 1997;77:2863–2878. doi: 10.1152/jn.1997.77.6.2863. [DOI] [PubMed] [Google Scholar]
- Pecka M, Brand A, Behrend O, Grothe B. Interaural time difference processing in the mammalian medial superior olive: the role of glycinergic inhibition. J Neurosci. 2008;28:6914–6925. doi: 10.1523/JNEUROSCI.1660-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peles E, Salzer JL. Molecular domains of myelinated axons. Curr Opin Neurobiol. 2000;10:558–565. doi: 10.1016/s0959-4388(00)00122-7. [DOI] [PubMed] [Google Scholar]
- Pelletier JG, Paré D. Uniform range of conduction times from the lateral amygdala to distributed perirhinal sites. J Neurophysiol. 2002;87:1213–1221. doi: 10.1152/jn.00623.2001. [DOI] [PubMed] [Google Scholar]
- Poliak S, Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nature reviews Neuroscience. 2003;4:968–980. doi: 10.1038/nrn1253. [DOI] [PubMed] [Google Scholar]
- Portfors C, von Gersdorff H. Macrocircuits for Sound Localization Use Leaky Coincidence Detectors and Specialized Synapses. Neuron. 2013;78:755–757. doi: 10.1016/j.neuron.2013.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers BE, Sellers DL, Lovelett EA, Cheung W, Aalami SP, Zapertov N, Maris DO, Horner PJ. Remyelination reporter reveals prolonged refinement of spontaneously regenerated myelin. Proc Natl Acad Sci U S A. 2013;110:4075–4080. doi: 10.1073/pnas.1210293110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumphrey RJ, Young JZ. The Rates Of Conduction Of Nerve Fibres Of Various Diameters In Cephalopods. Journal of Experimental Biology. 1938;15:453–466. [Google Scholar]
- Ranvier L. Contributions à l’histologie et à la physiologie des nerfs périphériques. Comptes Rendus de l’Académie des Sciences. 1871;73:1168–1171. [Google Scholar]
- Rappaport JM, Gulliver JM, Phillips DP, Van Dorpe RA, Maxner CE, Bhan V. Auditory temporal resolution in multiple sclerosis. J Otolaryngol. 1994;23:307–324. [PubMed] [Google Scholar]
- Rasband MN. The axon initial segment and the maintenance of neuronal polarity. Nat Rev Neurosci. 2010;11:552–562. doi: 10.1038/nrn2852. [DOI] [PubMed] [Google Scholar]
- Rose JE, Gross NB, Geisler CD, Hind JE. Some neural mechanisms in the inferior colliculus of the cat which may be relevant to localization of a sound source. J Neurophysiol. 1966;29:288–314. doi: 10.1152/jn.1966.29.2.288. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Rushton WA. A theory of the effects of fibre size in medullated nerve. J Physiol. 1951;115:101–122. doi: 10.1113/jphysiol.1951.sp004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salami M, Itami C, Tsumoto T, Kimura F. Change of conduction velocity by regional myelination yields constant latency irrespective of distance between thalamus and cortex. Proc Natl Acad Sci U S A. 2003;100:6174–6179. doi: 10.1073/pnas.0937380100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer JL, Brophy PJ, Peles E. Molecular domains of myelinated axons in the peripheral nervous system. Glia. 2008;56:1532–1540. doi: 10.1002/glia.20750. [DOI] [PubMed] [Google Scholar]
- Sánchez I, Hassinger L, Paskevich PA, Shine HD, Nixon RA. Oligodendroglia regulate the regional expansion of axon caliber and local accumulation of neurofilaments during development independently of myelin formation. J Neurosci. 1996;16:5095–5105. doi: 10.1523/JNEUROSCI.16-16-05095.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer WW, Myers FK. Relationship of myelin internode elongation and growth in the rat sural nerve. J Comp Neurol. 1973;147:255–266. doi: 10.1002/cne.901470207. [DOI] [PubMed] [Google Scholar]
- Seidl AH, Grothe B. Development of sound localization mechanisms in the mongolian gerbil is shaped by early acoustic experience. J Neurophysiol. 2005;94:1028–1036. doi: 10.1152/jn.01143.2004. [DOI] [PubMed] [Google Scholar]
- Seidl AH, Rubel EW, Harris DM. Mechanisms for adjusting interaural time differences to achieve binaural coincidence detection. J Neurosci. 2010;30:70–80. doi: 10.1523/JNEUROSCI.3464-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl AH, Sanchez JT, Schecterson L, Tabor KM, Wang Y, Kashima DT, Poynter G, Huss D, Fraser SE, Lansford R, Rubel EW. Transgenic quail as a model for research in the avian nervous system: a comparative study of the auditory brainstem. J Comp Neurol. 2013;521:5–23. doi: 10.1002/cne.23187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman DL, Brophy PJ. Mechanisms of axon ensheathment and myelin growth. Nature reviews Neuroscience. 2005;6:683–690. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- Siveke I, Leibold C, Schiller E, Grothe B. Adaptation of binaural processing in the adult brainstem induced by ambient noise. J Neurosci. 2012;32:462–473. doi: 10.1523/JNEUROSCI.2094-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PH, Joris PX, Carney LH, Yin TC. Projections of physiologically characterized globular bushy cell axons from the cochlear nucleus of the cat. J Comp Neurol. 1991;304:387–407. doi: 10.1002/cne.903040305. [DOI] [PubMed] [Google Scholar]
- Smith PH, Joris PX, Yin TC. Projections of physiologically characterized spherical bushy cell axons from the cochlear nucleus of the cat: evidence for delay lines to the medial superior olive. J Comp Neurol. 1993;331:245–260. doi: 10.1002/cne.903310208. [DOI] [PubMed] [Google Scholar]
- Spangler KM, Warr WB, Henkel CK. The projections of principal cells of the medial nucleus of the trapezoid body in the cat. J Comp Neurol. 1985;238:249–262. doi: 10.1002/cne.902380302. [DOI] [PubMed] [Google Scholar]
- Spirou GA, Brownell WE, Zidanic M. Recordings from cat trapezoid body and HRP labeling of globular bushy cell axons. J Neurophysiol. 1990;63:1169–1190. doi: 10.1152/jn.1990.63.5.1169. [DOI] [PubMed] [Google Scholar]
- Stanford LR. X-cells in the cat retina: relationships between the morphology and physiology of a class of cat retinal ganglion cells. J Neurophysiol. 1987;58:940–964. doi: 10.1152/jn.1987.58.5.940. [DOI] [PubMed] [Google Scholar]
- Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI. Auditory neuropathy. Brain. 1996;119 (Pt 3):741–753. doi: 10.1093/brain/119.3.741. [DOI] [PubMed] [Google Scholar]
- Starr A, Sininger YS, Pratt H. The varieties of auditory neuropathy. J Basic Clin Physiol Pharmacol. 2000;11:215–230. doi: 10.1515/jbcpp.2000.11.3.215. [DOI] [PubMed] [Google Scholar]
- Stevens B, Porta S, Haak LL, Gallo V, Fields RD. Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron. 2002;36:855–868. doi: 10.1016/s0896-6273(02)01067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara I, Lang EJ, Llinás R. Uniform olivocerebellar conduction time underlies Purkinje cell complex spike synchronicity in the rat cerebellum. J Physiol. 1993;470:243–271. doi: 10.1113/jphysiol.1993.sp019857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susuki K, Rasband MN. Molecular mechanisms of node of Ranvier formation. Curr Opin Cell Biol. 2008;20:616–623. doi: 10.1016/j.ceb.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Fukushi M, Kosaki K, Doyle AD, de Vega S, Yoshizaki K, Akazawa C, Arikawa-Hirasawa E, Yamada Y. Teneurin-4 is a novel regulator of oligodendrocyte differentiation and myelination of small-diameter axons in the CNS. J Neurosci. 2012;32:11586–11599. doi: 10.1523/JNEUROSCI.2045-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki I. The electro-saltatory transmission of the nerve impulse and the effect of narcosis upon the nerve fiber. American Journal of Physiology -- Legacy Content. 1939;127:211–227. [Google Scholar]
- Tauber H, Waehneldt TV, Neuhoff V. Myelination in rabbit optic nerves is accelerated by artificial eye opening. Neurosci Lett. 1980;16:235–238. doi: 10.1016/0304-3940(80)90003-8. [DOI] [PubMed] [Google Scholar]
- Thompson D. On Growth and Form. Cambridge, UK: Cambridge University Press; 1917. [Google Scholar]
- Tollin DJ. The Lateral Superior Olive: A Functional Role in Sound Source Localization. The Neuroscientist. 2003;9:127–143. doi: 10.1177/1073858403252228. [DOI] [PubMed] [Google Scholar]
- Tomasi S, Caminiti R, Innocenti GM. Areal differences in diameter and length of corticofugal projections. Cereb Cortex. 2012;22:1463–1472. doi: 10.1093/cercor/bhs011. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Mendell LM. The spinal projection of individual identified A-delta- and C-fibers. J Neurophysiol. 1988;59:41–55. doi: 10.1152/jn.1988.59.1.41. [DOI] [PubMed] [Google Scholar]
- Virchow R. Über das ausgebreitete Vorkommen einer dem Nervenmark analogen Substanz in den tierischen Geweben. Virchows Arch Pathol Anat. 1854;6:562–572. [Google Scholar]
- de Waegh SM, Lee VM, Brady ST. Local modulation of neurofilament phosphorylation, axonal caliber, and slow axonal transport by myelinating Schwann cells. Cell. 1992;68:451–463. doi: 10.1016/0092-8674(92)90183-d. [DOI] [PubMed] [Google Scholar]
- Wake H, Lee PR, Fields RD. Control of local protein synthesis and initial events in myelination by action potentials. Science. 2011;333:1647–1651. doi: 10.1126/science.1206998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr WB. Fiber degeneration following lesions in the anterior ventral cochlear nucleus of the cat. Exp Neurol. 1966;14:453–474. doi: 10.1016/0014-4886(66)90130-0. [DOI] [PubMed] [Google Scholar]
- Waxman SG. An ultrastructural study of the pattern of myelination of preterminal fibers in teleost oculomotor nuclei, electromotor nuclei, and spinal cord. Brain Res. 1971;27:189–201. doi: 10.1016/0006-8993(71)90248-4. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Integrative properties and design principles of axons. International review of neurobiology. 1975;18:1–40. doi: 10.1016/s0074-7742(08)60032-x. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Determinants of conduction velocity in myelinated nerve fibers. Muscle Nerve. 1980;3:141–150. doi: 10.1002/mus.880030207. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Axon-glia interactions: building a smart nerve fiber. Current biology : CB. 1997;7:R406–R410. doi: 10.1016/s0960-9822(06)00203-x. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Pappas GD, Bennett MV. Morphological correlates of functional differentiation of nodes of Ranvier along single fibers in the neurogenic electric organ of the knife fish Stern archus. J Cell Biol. 1972;53:210–224. doi: 10.1083/jcb.53.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG, Sims TJ. Specificity in central myelination: evidence for local regulation of myelin thickness. Brain Res. 1984;292:179–185. doi: 10.1016/0006-8993(84)90905-3. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Swadlow HA. The conduction properties of axons in central white matter. Prog Neurobiol. 1977;8:297–324. doi: 10.1016/0301-0082(77)90009-0. [DOI] [PubMed] [Google Scholar]
- Werthat F, Alexandrova O, Grothe B, Koch U. Experience-dependent refinement of the inhibitory axons projecting to the medial superior olive. Dev Neurobiol. 2008;68:1454–1462. doi: 10.1002/dneu.20660. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Hozumi Y, Kaneko K, Sugihara T, Fujii S, Goto K, Kato H. Modulatory effects of oligodendrocytes on the conduction velocity of action potentials along axons in the alveus of the rat hippocampal CA1 region. Neuron Glia Biol. 2007;3:325–334. doi: 10.1017/S1740925X08000070. [DOI] [PubMed] [Google Scholar]
- Zalc B, Goujet D, Colman D. The origin of the myelination program in vertebrates. Current biology : CB. 2008;18:R511–R512. doi: 10.1016/j.cub.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Kong YY, Michalewski HJ, Starr A. Perceptual consequences of disrupted auditory nerve activity. J Neurophysiol. 2005;93:3050–3063. doi: 10.1152/jn.00985.2004. [DOI] [PubMed] [Google Scholar]
- Zotterman Y. A note on the relation between conduction rate and fibre size in mammalian nerves1. Skandinavisches Archiv Für Physiologie. 1937;77:123–128. [Google Scholar]


