Abstract
Background
Sesame oil is a potent antioxidant dietary source for human health. Oxidative stress through generation of free radicals damages the myocardium in different experimental condition. The present research was designed to evaluate the antioxidant property of chronic oral administration of sesame oil against isoproterenol induced myocardial injury.
Methods and results
Male Wistar albino rats were randomly divided into five groups (n = 6) and treated as per treatment protocol with two different doses of sesame oil (5 and 10 ml/kg b.w.) orally for thirty days. At the end of the treatment all the rats (except control rats) were administered with isoproterenol (85 mg/kg) two consecutive days and subjected to biochemical and histopathological estimation. Isoproterenol (group ISO) induced the oxidative myocardial damage via alteration in the endogenous antioxidant enzymes and myocardial marker enzymes. Sesame oil in both the dose (group S1 and S2) shows protective mechanism via decreasing thiobarbituric acid reactive substance (TBARS) and enhancing the endogenous antioxidant enzymes (reduced glutathione (GSH), superoxide dismutase (SOD) and Catalase). Sesame oil also increased the lactate dehydrogenase (LDH), creatine kinase (CK) and aspartate transaminase (AST) as a myocardial marker enzyme in heart homogenate. As histologically evident isoproterenol induced myocardial injury was well preserved by the chronic administration of sesame oil. The protective role of sesame oil was compared with the reference standard α-tocopherol (group S3) also showing the similar effect.
Conclusion
From this finding it has been concluded that chronic administration of sesame oil offers cardio protective action via putative antioxidant property.
Keywords: Antioxidants, Ischemic reperfusion injury, Myocardial infarction, Neutraceutical
1. Introduction
Isoproterenol (ISO) is an adrenergic agonist and acute administration of ISO in experimental animals causes myocardial injury is well established animal model to find out the cardio protective activity of pharmacologically active agents.1 ISO damage the myocardium via calcium accumulation in cytosolic membrane, generation of reactive oxygen species (ROS) and procoagulant activity.2,3 ISO causes the patchy pathological changes in the myocardial tissue, which is almost clinically relevant to myocardial infarction of ischemic heart disease (IHD).4 Oxidative stress plays a major role to cause ISO induced myocardial injury. Acute administration of ISO causes depletion of myocardial endogenous antioxidant systems. Several studies have been made to protect the change in antioxidant enzyme via exogenous administration of antioxidants to scavenge the ROS. The approach was succeeded in some research and also failed in some research because of exogenous administration of antioxidants not able to reach the site where ROS is generated.5,6 Drugs to enhance the endogenous antioxidant enzymes to protect the heart from stress have been paid more interest. Natural antioxidants plays a major role to reduce the oxidative stress by scavenging the excess free radicals.7 Administration of antioxidants (exist in plant extracts, food supplements and even drugs) during ischemic reperfusion injury (IRI) ameliorates the severity of IRI through augmentation of endogenous antioxidants, which might be a promising loom to treat IHD.8,9 Nutraceuticals plays a major role to protect the human health from various life threatening diseases like cancer, cardiovascular disease, diabetes, arthritis etc., Worldwide variety of food products used as nutraceuticals, which is categorized as dietary fiber, probiotics, and natural antioxidants.10 Sesame oil is one of the major cooking oil used in human diet with known antioxidant constituents.11,12 Sesame oil, obtained from the seeds of Sesamum indicum L. belongs to the family Pedaliaceae. It contains different fatty acids and nonfat antioxidants, as well as tocopherol, sesamin, sesamolin, and sesamol.13 Ahmad et al7 reported the anti-oxidative property of sesame oil in 6-hydroxydopamine induced neurotoxicity. Karatzi et al14 reported that the beneficial effect of daily intake of sesame oil in endothelial dysfunction in hypertensive men. Recently several researchers reported the antioxidant role of sesame oil in experimental models and also protect the heart via eliminating the risk factor.15,16 However, there were no studies conducted in this direction. Recently we found that chronic administration of sesame oil enhances the endogenous antioxidants in ischemic myocardium.17 So, we hypothesized that the protective role of sesame oil against ISO induced myocardial injury may be via antioxidant system. Based on this hypothesis the present investigation is undertaken to find out the potency of antioxidant activity of chronic administration of sesame oil and to estimate the cardio protective action rendered by sesame oil.
2. Materials and method
2.1. Drugs and chemical
Sesame oil was obtained from VV & Sons edible oil Ltd., Virudhunagar, India as gift sample. All chemicals were of analytical grade purchased from sigma chemicals, USA.
2.2. Experimental animals
Male Wistar albino rats of body weight 180–200 g were obtained from the Institute Animal House. The animals were fed with a standard pellet diet (Sai Durga Feeds and Foods, Bangalore) and water ad libitum. The rats were accimelated in the department animal house and we followed the guidelines given by the Committee for the Purpose of Supervision and Control of Experiments on Animals (CPCSEA), India. The research protocol was approved by the Institute animal ethical committee (1220/a/08/CPCSEA/ANCP).
2.3. Experiment protocol to induce myocardial necrosis
30 healthy rats were divided into 5 different groups (n = 6) and fed with sesame oil by oral route once a day for thirty days. At the end of the treatment period rats from all groups except control group were administered isoproterenol (ISO) 85 mg/kg s.c., for two consecutive days to induce myocardial injury.18,19 After 48 h of the first dose of isoproterenol the rats were sacrificed under light ether anesthesia, hearts were isolated and subjected to biochemical and histopathological studies.
2.3.1. Treatment protocol
The groups were studied
Group C: Saline (10 ml/kg) treated rats
Group ISO: Saline treated rats received ISO (85 mg/kg)
Group S1: Sesame oil (5 ml/kg) treated rats received ISO (85 mg/kg)
Group S2: Sesame oil (10 ml/kg) treated rats received ISO (85 mg/kg)
Group S3: α-tocopherol (10 mg/kg) treated rats received ISO (85 mg/kg)
2.4. Estimation of biochemical parameters
The following biochemical parameters were studied in the heart homogenate.
2.4.1. Myocardial thiobarbituric acid reactive substances (TBARS)
TBARS activity in the myocardium was measured by a method of Okhawa et al20 Hearts were homogenized in 10% trichloro acetic acid in 4 °C. 0.2 ml homogenate was pipetted into a test tube, followed by the addition of 0.2 ml of 8.1% sodium dodecyl sulfate (SDS), 1.5 ml of 20% acetic acid (pH-3.5) and 1.5 ml of 0.8% TBA. Tubes were boiled for 60 min at 95 °C and then cooled in ice. Double distilled water (1.0 ml) and n-butanol:pyridine (15:1 v/v) mixture (5.0 ml) were added to the test tubes and centrifuge at 4000× g for 10 min. The absorbance of developed color in organic layer was measured at 532 nm. Data are expressed as nmole of TBARS/g wet.wt.
2.4.2. Myocardial reduced glutathione (GSH)
GSH was estimated by the method of Ellman et al21 The reaction mixture contained 0.1 ml of supernatant, 2.0 ml of 0.3 M phosphate buffer (pH-8.4), 0.4 ml of double distilled water and 0.5 ml of DTNB (5,5 dithiobis-2-nitrobenzoic acid). The reaction mixture was incubated for 10 min and the absorbance was measured at 412 nm. Data are expressed as mole/g wet.wt.
2.4.3. Superoxide dismutase (SOD)
SOD levels in the hearts were determined by the method of McCord & Firdovich modified by Kakkar et al22 A sample (0.6 ml) was added to sodium pyrophosphate buffer (pH-8.3) followed by the addition of 0.1 ml of 186 M phenazine methosulphate, 0.3 ml of 300 mM nitro blue tetrazolium and 0.2 ml of 780 M NADH. The reaction mixture was incubated for 90 s at 30 °C and stopped the reaction by adding 1 ml of acetic acid. n-Butanol (4 ml) was then added and centrifuged at 3000× g for 10 min. The absorbance of organic layer was measured at 560 nm. Data are expressed as units per mg protein.
2.4.4. Estimation of catalase
Catalase was estimated by the method described by Aebi & Bergmeyer.23 Sample was added to a 3 ml cuvette that contained 1.95 ml of 50 mM phosphate buffer (pH-7.0). Then 1 ml of 30 mM hydrogen peroxide was added and changes in absorbance were followed for 30 s at 240 nm at an interval of 15 s. Data are expressed as units per mg protein.
2.4.5. Estimation of protein
Protein estimation for the tissue sample of SOD and catalase were done by the method of Bradford.24 Sample was added up to 20 μL with double distilled water, 50 μL in NaOH and 1 ml of Bradford reagent and kept aside for 10 min after vortexing. The absorbance was measured at 595 nm.
LDH levels in the myocardium were determined by the method described by King.25 Creatine kinase (CK) & Aspartate transaminase (AST) levels in the myocardium were determined by the method described by Saxenaet al.26 by using respective kits.
2.5. Histological examination
The rat hearts were removed, washed immediately with saline and then fixed in 10% buffered formalin. The hearts were embedded in paraffin sections cut at 5 μ stained with hematoxylin and eosin. These sections were then examined under the light microscope for histological changes.
2.6. Statistical analysis
All values are expressed as mean ± SEM for 6 animals in each group. Data for various biochemical parameters were analyzed using One-way analysis of variance followed by Tukey's multiple comparison test (GraphPad Version 3.06, La Jolla, CA, USA). Significance is set at p < 0.05.
3. Results and discussion
Oxidative stress plays a major role in the development of IRI through generation of free radicals and depletion of endogenous antioxidant enzymes. Enhancement of antioxidant via administration of drugs may be one of the effective therapeutic approaches to treat IHD. The antioxidant property of drugs has been proved in various types of stressful conditions, like ischemia, ROS, endotoxins and protects the myocardium from consequent exposure to injuries of similar or more severe in nature.27–29
Cardiotoxicity mediated by ISO due to stimulation of β1 – adrenergic receptor and this stimulation not only rapidly generate ROS, but also depresses the myocardial endogenous antioxidant system leading to cause myocardial IRI.30,31 Depletion in the level of myocardial endogenous antioxidants develops a divergence of mitochondrial function. It has already reported that the decrease in the antioxidant level resulted in a diminished hunt of free radical in the ISO–induced myocardial damage in rats.32 Generation of reactive oxygen species during ISO administration initiate lipid peroxidation leads to loss of membrane structural and functional integrity.32,33
There was no mortality in the Sesame oil ingested rats and the Sesame oil ingested rats subjected to ISO administration. The results obtained in the different groups subjected to in-vivo (ISO induced) myocardial injury were presented below. (Table 1).
Table 1.
Effect of chronic administration of Sesame oil on TBARS, GSH, SOD, Catalase, LDH, CK and AST in rat heart homogenate.
| Groups | Biochemical parameters |
||||||
|---|---|---|---|---|---|---|---|
| TBARS nmol/g wet wt | GSH μg/g wet wt | SOD I.U/mg protein | Catalase I.U/mg protein | LDH U/l | CK U/l | AST U/l | |
| Group C | 4.1 ± 0.98 | 327.6 ± 22.86 | 1.5 ± 0.20 | 138.3 ± 8.54 | 163 ± 1.01 | 8.6 ± 1.08 | 192 ± 1.01 |
| Group ISO | 31.1 ± 5.71# | 128.1 ± 13.86# | 0.85 ± 0.15ns | 42.4 ± 5.17# | 66 ± 2.03# | 4.8 ± 2.19# | 80.7 ± 1.17# |
| Group S1 (Sesame oil 5 ml/kg) | 12.6 ± 1.86** | 268.3 ± 44.40* | 2.5 ± 0.37*** | 83.2 ± 2.36* | 121 ± 0.98*** | 7.3 ± 0.87*** | 105.5 ± 1.94*** |
| Group S2 (Sesame oil 10 ml/kg) | 9.1 ± 1.01*** | 293.8 ± 29.68** | 2.2 ± 0.23** | 115.6 ± 7.19*** | 146 ± 4.23*** | 6.9 ± 0.02*** | 157.5 ± 1.56*** |
| Group S3 (α-tocoferol 10 mg/kg) | 13.5 ± 3.22** | 373.4 ± 41.68*** | 1.9 ± 0.08** | 109.2 ± 14.96*** | 162 ± 1.03*** | 7.2 ± 2.03*** | 166 ± 0.33*** |
All values are expressed as Mean ± SEM [n = 6] #p < 0.0001 vs. group C; *p < 0.01 vs. group ISO; **p < 0.001 vs. group ISO; ***p < 0.0001 vs. group ISO; ns = Non significant. (TBARS-Thiobarbituric acid reactive substance, GSH-Reduced glutathione, SOD-Superoxide dismutase, LDH-Lactate dehydrogenase, CK-Creatinine kinase, AST-Aspartate transaminase).
Myocardial TBARS in ISO group was significantly (p < 0.0001) higher than that in control C group. In sesame oil treated groups (S1 and S2) and α-tocopherol treated (S3) there was a significantly (S1-p < 0.001, S2-p < 0.0001, S3-p < 0.001) lower TBARS in comparison to ISO group. Myocardial endogenous antioxidants (GSH and Catalase) in ISO group were significantly (p < 0.0001 and p < 0.0001 respectively) lower than that in the control group. There was no significant change in the level of SOD in ISO group. In sesame oil treated groups (S1 and S2) and α-tocopherol treated (S3) there was a significantly higher GSH (S1-p < 0.01, S2-p < 0.001, S3-p < 0.0001), SOD (S1-p < 0.0001, S2-p < 0.001, S3-p < 0.001), Catalase (S1-p < 0.01, S2-p < 0.0001, S3-p < 0.0001) in comparison to ISO group. Myocardial markers (LDH, CK and AST) in ISO group were significantly (p < 0.0001) lower than that in control group C. In sesame oil treated groups (S1 and S2) and α-tocopherol treated (S3) there was a significantly higher LDH, CK and AST (S1-p < 0.0001, S2-p < 0.0001, S3-p < 0.0001) in comparison to ISO group. Light microscopy of the heart in group C showed the normal myofibrillar structure with striations (Fig. 1a). Group ISO showed focal and the collection of neutrophils with edema and more necrosis (Fig. 1b). Heart of group S1 (Sesame oil 5 ml/kg) showed preserved myofibrillar structure with minimal inflammation (Fig. 1c). The tissue sections of group S2 (Sesame oil 10 ml/kg) showed preserved myofibrillar structure with mild focal changes and minimal inflammation without necrosis (Fig. 1d). Heart of group S3 (α-tocopherol 10 mg/kg) showed normal myocardium (Fig. 1e).
Fig. 1.
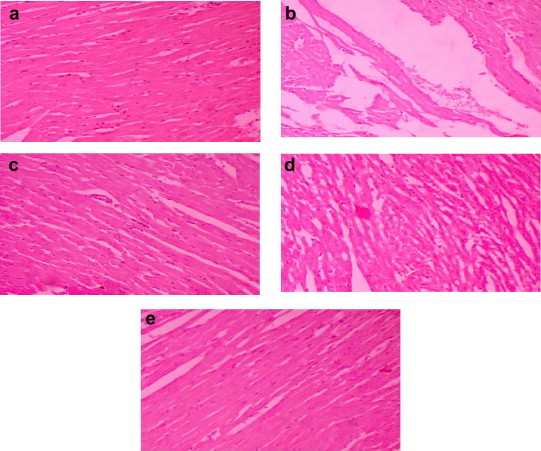
Histopathological report. a) Group C: saline treated rats shows normal myocardium. b) Group ISO: ISO treated rats shows necrotic myocardium. c) Group S1: Sesame oil 5 ml/kg treated rats shows well preserved myocardium. d) Group S2: Sesame oil 10 ml/kg treated rats shows well preserved myocardium. e) Group S3: α-tocopherol 10 mg/kg treated rats show well preserved myocardium.
In the present research we have observed that the myocardial damage via administration of ISO was associated with oxidative stress, as evidenced by increase in myocardial TBARS and depletion of myocardial endogenous antioxidant status. Same findings were reported by other studies also.18,19 Oxidative stress was evidenced by the generation of free radical which might be scavenged by augmentation of endogenous antioxidants in the myocardium after pharmacological intrusion.34 Chronic oral administration of sesame oil alleviates the oxidative stress and the morphological changes associated with IRI. The protective role of sesame oil may be due to myocardial adaptation and enhancement of myocardial endogenous antioxidants.35
Ischemic necrosis during ISO administration liberates the cardiac marker enzyme like LDH, CK, and AST from the heart due to destruction of myocardium.36–39 Cardiac marker enzymes like AST, CK and LDH give information about severity of infarction in the heart. Estimation of these enzymes in heart and serum is required to assess the size of infarction.40,41 Decreased levels of AST, CK and LDH in the myocardium indicated that ISO caused necrotic damage in the heart. The loss of marker enzymes in the myocardium reflects the consequences of cellular injury due to lipid peroxides.42 Sesame oil caused significant changes in the cardiac markers (LDH, CK and AST) in the 5 and 10 ml/kg treated groups. It may be due to its membrane stabilization action. In S1 and S2 groups, the alterations in the level of AST, CK and LDH were found to be near normal when compared with those in ISO. The same effect also observed with the reference standard α-tocopherol.
Finally, in the histopathological observation of heart exhibited near normal pattern, which further supported the protective role of sesame oil against ischemic injury. Significant rise in the levels of endogenous antioxidant (GSH, SOD and catalase) were observed in all the treatment groups, and healthier protection observed with 10 ml/kg treated rats. The limitation of this present research is ISO does not produce any significant changes in the level of SOD. It has been reported that the equilibrium between SOD and catalase is important to scavenge the free radical before generating the hydroxyl radical.43–45 There were no significant changes in the level of SOD in group ISO and significant rise in the level of SOD and catalase in sesame oil treated groups were conforming the protection via antioxidant mechanism. In our previous study it was observed that the sesame oil shows protective role via enhancing the myocardial endogenous antioxidant system in the ischemic heart.17 The same effect also observed in the present study could be an evidence for antioxidant mechanism of sesame oil as cardio protective agent.
4. Conclusion
Sesame oil offered significant protection against isoproterenol induced oxidative myocardial injury through augmentation of antioxidant enzyme, which was evidenced by the result of enhancement of cardiac marker. Finally, we concluded that the protective role of sesame oil against myocardial injury in the treated rats is attributed to reduced oxidative stress.
Conflicts of interest
All authors have none to declare.
Acknowledgment
The first author thanks to Management, Annamacharya College of Pharmacy to provide the facilities to carry out the research work.
References
- 1.Karthikeyan K., Bai B.R.S., Devaraj S.N. Grape seed proanthocyanidins ameliorates isoproterenol-induced myocardial injury in rats by stabilizing mitochondrial and lysosomal enzymes: an in vivo study. Life Sci. 2007;81:1615–1621. doi: 10.1016/j.lfs.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 2.Chagoya S.V., Hernández-Muñoz R., López-Barrera F., Yañez L., Vidrio S., Suárez J. Sequential changes of energy metabolism and mitochondrial function myocardial infarction induced by isoproterenol in rats: a long-term and integrative study. Can J Physiol Pharmacol. 1997;75:1300–1311. [PubMed] [Google Scholar]
- 3.Pinelli A., Trivulzio S., Tomasoni L., Brenna S., Bonacina E., Accinni R. Isoproterenol-induced myocardial infarction in rabbits. Protection by propranolol or labetalol: a proposed non-invasive procedure. Eur J Pharm Sci. 2004;23:277–285. doi: 10.1016/j.ejps.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Senthil S., Sridevi M., Pugalendi K.V. Cardioprotective effect of oleanolic acid on isoproterenol-induced myocardial ischemia in rats. Toxicol Pathol. 2007;35:418–423. doi: 10.1080/01926230701230312. [DOI] [PubMed] [Google Scholar]
- 5.Das D.K., Engelman R.M., Kimura Y. Molecular adaptation of cellular defences following preconditioning of the heart by repeated ischaemia. Cardiovasc Res. 1993;27:578–584. doi: 10.1093/cvr/27.4.578. [DOI] [PubMed] [Google Scholar]
- 6.Mohanty I.R., Maheshwari U., Joseph D., Deshmukh Y. Tribulusterrestris protects rat myocardium against isoproterenol-induced ischemic injury: role of HSP 70 and cardiac endogenous antioxidants. Alt Med Studies. 2011;1:35–39. e9. [Google Scholar]
- 7.Ahmad S., Khan M.B., Hoda M.N. Neuroprotective effect of sesame seed oil in 6-hydroxydopamine induced neurotoxicity in mice model: cellular, biochemical and neurochemical evidence. Neurochem Res. 2012 Mar;37:516–526. doi: 10.1007/s11064-011-0638-4. [DOI] [PubMed] [Google Scholar]
- 8.Visweswaran P., Massin E.K., Dubose T.D. Mannitol induced acute renal failure. J Am Soc Nephrol. 1997;8:1028–1033. doi: 10.1681/ASN.V861028. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee S.K., Dinda A.K., Manchanda S.C., Maulik S.K. Chronic garlic administration protects rat heart against oxidative stress induced by ischemic reperfusion injury. BMC Pharmacol. 2002;2:16. doi: 10.1186/1471-2210-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das L., Bhaumik E., Raychaudhuri U., Chakraborty R. Role of nutraceuticals in human health. J Food Sci Technol. 2012;49:173–183. doi: 10.1007/s13197-011-0269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gauthaman K., Mohamed Saleem T.S. Nutraceutical value of sesame oil. Phcog Rev. 2009;3:264–269. [Google Scholar]
- 12.Saleem T.S.M., Chetty M., Kavimani S. Nutraceutical value of sesame oil – an update. Int J Res Pharm Sci. 2012;3:650–656. [Google Scholar]
- 13.Fukuda Y. Food chemical studies on the antioxidants in sesame seed. Nippon Shokuhin Kogyo Gakkaishi. 1990;37:484–492. [Google Scholar]
- 14.Karatzi K., Kimon S., Maritta L. Sesame oil consumption exerts a beneficial effect on endothelial function in hypertensive men. Euro J Preventive Cardiol. 2013 Apr;20:202–208. doi: 10.1177/2047487312437625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arumugam P., Ramesh S. Protective effects of sesame oil on 4-NQO-induced oxidative DNA damage and lipid peroxidation in rats. Drug Chem Toxicol. 2011;34:116–119. doi: 10.3109/01480541003782310. [DOI] [PubMed] [Google Scholar]
- 16.Alipoor B., Haghighian M.K., Sadat B.E., Asghari M. Effect of sesame seed on lipid profile and redox status in hyperlipidemic patients. Int J Food Sci Nutr. 2012 Sep;63:674–678. doi: 10.3109/09637486.2011.652077. [DOI] [PubMed] [Google Scholar]
- 17.Saleem T.S.M., Chetty M., Kavimani S. Sesame oil enhances endogenous antioxidants in ischemic myocardium of rat. Rev Bras Farmacogn. 2012;22:669–675. [Google Scholar]
- 18.Gauthaman K., Mohamed Saleem T.S., Jayaprakash S., Peter T., Karthikeyan K., Niranjali D. Cardioprotective effect of the Hibiscus rosasinensis flowers in an oxidative stress model of myocardial ischemic reperfusion injury in rat. BMC Complement Altern Med. 2006;6:32. doi: 10.1186/1472-6882-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohamed Saleem T.S., Lokanath N., Prasanthi A., Madhavi M., Mallika G., Vishnu M.N. Aqueous extract of Saussurealappa root ameliorate oxidative myocardial injury induced by isoproterenol in rats. J Adv Pharm Tech Res. 2013;4:94–100. doi: 10.4103/2231-4040.111525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okhawa H., Oohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Ann Bichem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 21.Ellman G.L. Tissue sulfhydryl groups. Arch Biochem Biophy. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 22.Kakkar P., Das B., Viswanathan P.N. A modified spectrophotometric assay of superoxide dismutase. Ind J Biochem Biophy. 1984;21:130–132. [PubMed] [Google Scholar]
- 23.Aebi H., Bergmeyer H.U. 2nd ed. vol. 2. Chemic Academic Press Inc., Verlag; 1974. pp. 673–685. (Methods of Enzymatic Analysis). [Google Scholar]
- 24.Bradford M.M. A rabid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;772:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 25.King J. A routine method for the estimation of lactic dehydrogenase activity. J Med Lab Tech. 1959;16:265–272. [PubMed] [Google Scholar]
- 26.Saxena K.K., Gupta B., Srivastava V.K., Saxena R.S., Singh R.C., Prasad D.N. Creatine kinase and aspartate transaminase in experimental model to predict the size of cardiac infarct. Ind J Exp Biol. 1988;26:235–236. [PubMed] [Google Scholar]
- 27.Engelman D.T., Watanbe M., Engelman R.M. Hypoxic preconditioning preserves anti- oxidant reserve in the working rat heart. Cardiovasc Res. 1995;29:133–140. [PubMed] [Google Scholar]
- 28.Schaefer J., Nierhaus K.H., Lohff B., Peters T., Schaefer T., Vos R. Mechanisms of autoprotection and the role of stress-proteins in natural defenses, autoprotection, and salutogenesis. Med Hypothesis. 1998;51:153–163. doi: 10.1016/s0306-9877(98)90110-4. [DOI] [PubMed] [Google Scholar]
- 29.Maulik N., Watanabe M., Engelman D.T. Myocardial adaptation to ischemia by oxidative stress induced by endotoxin. Am J Physiol. 1995;38:C907–C916. doi: 10.1152/ajpcell.1995.269.4.C907. [DOI] [PubMed] [Google Scholar]
- 30.Rathore N., John S., Kale M., Bhatnagar D. Lipid peroxidation and antioxidant enzymes in isoproterenol induced oxidative stress in rat tissues. Pharmacol Res. 1998;38:297–303. doi: 10.1006/phrs.1998.0365. [DOI] [PubMed] [Google Scholar]
- 31.Srivastava S., Chandrasekar B., Gu Y. Down regulation of Cu, Zn-superoxide dismutase contributes to beta-adrenergic receptor-mediated oxidative stress in the heart. Cardiovasc Res. 2007;74:445–455. doi: 10.1016/j.cardiores.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 32.Sangeetha T., Darlin S.Q. Preventive effects of S-allyl cysteine sulphoxide (Alliin) on mitochondrial dysfunction in normal and isoproterenol induced cardiotoxicity in male Wistar rats: a histopathological study. Mol Cell Biochem. 2009;32:1–8. doi: 10.1007/s11010-009-0066-9. [DOI] [PubMed] [Google Scholar]
- 33.Ansari M.N., Bhandari U., Pillai K.K. Protective role of curcumin in myocardial oxidative damage induced by isoproterenol in rats. Hum Exp Toxicol. 2007;26:933–938. doi: 10.1177/0960327107085835. [DOI] [PubMed] [Google Scholar]
- 34.Siveski-Iliskovic N., Hill M., Chow D.A., Singal P.K. Probucol protects against adriamycin cardiomyopathy without interfering with its antitumour effect. Circulation. 1995;91:10–15. doi: 10.1161/01.cir.91.1.10. [DOI] [PubMed] [Google Scholar]
- 35.Das D.K., Maulik N., Moraru Gene expression in acute myocardial stress: induction by hypoxia, ischemia, reperfusion, hypethermia and oxidative stress. J Mol Cell Cardiol. 1995;27:181–193. doi: 10.1016/s0022-2828(08)80017-x. [DOI] [PubMed] [Google Scholar]
- 36.Hagar H. Folic acid & vitamin B12 supplementation attenuates isoprenaline-induced myocardial infarction in experimental hyperhomocysteinemia rats. Pharmacol Res. 2002;46:213–219. doi: 10.1016/s1043-6618(02)00095-6. [DOI] [PubMed] [Google Scholar]
- 37.SabeenaFarvin K.H., Anandan R., Kumar S.H., Shiny K.S., Sankar T.V., Thankappan T.K. Effect of squalene on tissue defense system in isoproterenol-induced myocardial infarction in rats. Pharmacol Res. 2004;50:231–236. doi: 10.1016/j.phrs.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Gurgun C., Ildizli M., Yavuzgil O. The effects of short term statin treatment on left ventricular function and inflammatory markers in patients with chronic heart failure. Int J Cardiol. 2008;123:102–107. doi: 10.1016/j.ijcard.2006.11.152. [DOI] [PubMed] [Google Scholar]
- 39.Thippeswamy B.S., Patil U., Thakker S.P. Cardioprotective effect of Cucumistrigonus Roxb on isoproterenol-induced myocardial infarction in rat. Am J Pharmacol Toxicol. 2009;4:29–37. [Google Scholar]
- 40.Sasikumar C.S., Devi C.S. Protective effect of Abana, a polyherbal formulation, on isoproterenol induced myocardial infarction in rats. Ind J Pharmacol. 2008;32:198–201. [Google Scholar]
- 41.Ithayarasi P.A., Devi C.S. Effect of α-tocopherol on isoproterenol induced changes in lipid and lipoprotein profile in rats. Ind J Pharmacol. 1997;29:399–404. [Google Scholar]
- 42.Ganguly P.K., Srivastava M.L., Bora P.S., Gupta M.P. Lactate dehydrogenase isozymes. Effect of isoproterenol induced myocardial necrosis. Ind J Exp Biol. 1980;18:1443–1445. [PubMed] [Google Scholar]
- 43.Yim M.B., Chock P.B., Stadtman E.R. Copper, zinc super oxide dismutase catalyzes hydroxyl radical production from hydrogen peroxide. Natl Acad Sci USA. 1990;8713:5006–5010. doi: 10.1073/pnas.87.13.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones S.A., McArdle F., Jack C.I., Jackson M.J. Effect of antioxidant supplementation on the adaptive response of human skin fibroblasts to UV-induced oxidative stress. Redox Rep. 1999;46:291–299. doi: 10.1179/135100099101535133. [DOI] [PubMed] [Google Scholar]
- 45.Mohanty I.R., Arya D.S., Gupta S.K. Dietary Curcuma longa protects myocardium against isoproterenol induced hemodynamic, biochemical and histopathological alternations in rats. Int J Appl Res Nat Prod. 2009;1:19–28. [Google Scholar]


