Abstract
Although second-order motion may be detected by early and automatic mechanisms, some models suggest that perceiving second-order motion requires higher-order processes, such as feature or attentive tracking. These types of attentionally mediated mechanisms could explain the motion aftereffect (MAE) perceived in dynamic displays after adapting to second-order motion. Here we tested whether there is a second-order MAE in the absence of attention or awareness. If awareness of motion, mediated by high-level or top-down mechanisms, is necessary for the second-order MAE, then there should be no measurable MAE if the ability to detect directionality is impaired during adaptation. To eliminate the subject’s ability to detect directionality of the adapting stimulus, a second-order drifting Gabor was embedded in a dense array of additional crowding Gabors. We found that a significant MAE was perceived even after adaptation to second-order motion in crowded displays that prevented awareness. The results demonstrate that second-order motion can be passively coded in the absence of awareness and without top-down attentional control.
Keywords: Vision, Attention, Perception, Motion, Motion perception, Second-order motion, Crowding, Awareness, Consciousness, First-order motion, MAE, Contrast-defined
1. Introduction
Both first-order luminance-defined motion and second-order contrast-defined motion can produce the motion-aftereffect (MAE)1 (Mather, Verstraten, & Anstis, 1998a). However, the characteristics of the second-order MAE are distinct from those of the first-order MAE (for a review, see Mather, Verstraten, & Anstis, 1998b), leading many authors to suggest that second-order motion is processed independently from first-order motion (Ashida, Seiffert, & Osaka, 2001; Cavanagh & Mather, 1989; Derrington & Badcock, 1985; Ledgeway & Smith, 1994b; McCarthy, 1993; Nishida, Ashida, & Sato, 1994; Nishida & Sato, 1995; Seiffert & Cavanagh, 1998; c.f., Ledgeway & Smith, 1994a). Second-order motion could be processed by feature-tracking mechanisms (Cavanagh, 1992; Derrington, Allen, & Delicato, 2004), which may involve or require attention to operate (Ashida et al., 2001; Seiffert & Cavanagh, 1999). Indeed, dividing attention between different second-order moving stimuli reduces sensitivity to the motion (Allen & Ledgeway, 2003; Del Vecchio, von, & Faubert, 2001; Ho, 1998; Lu, Liu, & Dosher, 2000), and visual search for low contrast second-order motion is serial (Ashida et al., 2001). Together, these results suggest that attention plays at least a modulating role in the perception of low-contrast and low-speed second-order motion.
Common among all experiments on second-order motion, and the second-order MAE, is that the stimuli are always salient and visible. This is the case even for low-contrast and low-speed second-order motion displays, making it difficult to test the necessity or modulatory role of attention. As long as subjects are aware of the stimuli, attentionally modulated (Nishida & Ashida, 2000) or mediated (Ashida et al., 2001; Cavanagh, 1992) second-order motion mechanisms could operate; although several studies have varied attentional load, no study has successfully eliminated awareness of second-order motion and examined the consequence. Yet, this is the only way to rule out attention as a modulator or mediator of the second-order MAE. If attention acts to modulate the dynamic second-order MAE, then removing the subject’s ability to attend should eliminate the modulation. Likewise, if second-order motion perception is mediated by high-level mechanisms requiring attention (Cavanagh, 1992), then there should be no MAE without the awareness of directional motion.
In the experiments below, we test whether attention to or awareness of motion is necessary for the second-order MAE, using a crowding technique developed by He and co-workers (He, Cavanagh, & Intiligator, 1997; He, Cavanagh, & Intriligator, 1996). The results will show that adaptation to second-order motion, even when subjects cannot identify the direction of the motion, produces a direction-and location-specific MAE.
2. Experiment 1: Second-order MAE following crowded adaptation
The goal of the first experiment was to measure whether second-order motion adaptation occurs passively—even when subjects are not aware of the direction of motion adaptation. To test this, subjects adapted to a crowded display of drifting second-order patterns (Fig. 1). Following adaptation, the second-order motion aftereffect was measured.
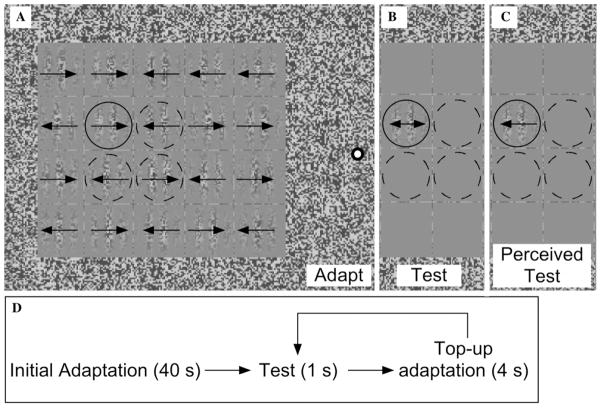
Fig. 1.
Stimulus from the first experiment. (A) An array of second-order, contrast-defined Gabors was presented so closely spaced that the direction of motion in the central Gabors could not be identified (due to crowding). The direction of motion in each of the Gabors was randomized across trials, except the four central Gabors (circled), whose motion was fixed (to build adaptation). (B) During the test period a dynamic Gabor (drift-balanced contrast-modulated sinusoids moving in opposite directions) was presented in one of the four adapted regions (circled). In this example, the test Gabor contains perfectly drift-balanced motion (no net physical motion) (C) Because of the prior motion adaptation, the dynamic test Gabor in (B) displayed an illusory motion aftereffect (MAE) opposite the direction of prior adaptation. (D) Trial sequence. Subjects initially adapted for 40 s to the array in (A), followed by interleaved test and top-up adaptation periods.
2.1. Methods
To examine whether second-order motion is processed in the absence of awareness, it is important to ensure that the second-order (contrast-defined) stimuli are free of luminance artifacts. For this reason, the methods in the first experiment involved 2 stages. First, to eliminate luminance artifacts we measured the equiluminance point of our second-order moving stimuli using a minimum motion technique. Second, we measured the MAE following adaptation to second-order drifting patterns. Two experienced subjects with normal or corrected-to-normal visual acuity participated in all experiments. Stimuli were presented on a high-resolution CRT monitor (Sony Multiscan G520, 1024 × 768 pixels, 100 Hz refresh) using an Apple G4 Power Macintosh with OS9. Subjects were seated in a dark room and immobilized with a chin rest placed 49 cm from the screen.
In the first stage, a minimum motion technique similar to that used by previous authors was administered to find each subject’s equiluminance value (Anstis & Cavanagh, 1983; Lu & Sperling, 2001a; Nishida, Edwards, & Sato, 1997; Seiffert & Cavanagh, 1998). The purpose of this was to establish psychophysical equiluminance of the second-order moving stimuli (to ensure that the second-order stimuli were, in fact, free of luminance artifacts). Subjects fixated on a point (1.2 deg diameter) 18.7 deg to the right of a circular aperture (center to center) that was 4.7 deg in diameter. Inside the aperture, a luminance-defined sine wave (0.71 cyc/deg) was flickered in counterphase at 5 Hz. A second, contrast-defined (i.e., second-order) grating was also presented in counterphase at 5 Hz. The contrast-defined grating consisted of a random-dot pattern (each dot was 0.2 by 0.2 deg), modulated by a contrast-defined sinusoid, also 0.71 cycles/deg. The luminance and contrast-defined gratings were interleaved in a four-frame sequence such that each sine wave was shifted by 90 deg (i.e., quadrature phase, luminance gratings presented in even frames, contrast-modulated gratings presented in odd frames). If only the luminance defined gratings were visible, or if the contrast-defined gratings were perfectly equiluminant, there would be no directional motion percept; however, if the contrast-defined grating visibly deviates from equiluminance, the subject perceives unidirectional motion. On each trial (0.5 s duration), the luminance midpoint of the contrast-modulated grating was randomly varied (one of 11 values centered on physical equiluminance, while keeping the minimum and maximum luminance values constant) and, using a method of constant stimuli task, subjects were asked to judge the direction of motion in the aperture (two alternative forced choice, leftward/rightward). Each subject participated in 220 trials (20 trials for each of the 11 luminance values). A logistic psychometric function was fit to the data, and the point of subjective equality (PSE) was measured. The PSE revealed the luminance midpoint (the relative luminance between the contrast-modulated segments of the second-order grating) that produced a percept of ambiguous motion; this is the point of equiluminance (Anstis & Cavanagh, 1983; Nishida et al., 1997; Seiffert & Cavanagh, 1998). The equiluminance point was measured for second-order patterns with three different contrast modulation depths (0.31, 0.58, and 0.9). For the highest contrast second-order pattern, the equiluminance point for subjects DB and DW was 34.0, and 34.4, respectively, while physical mean luminance was 34.8 cd/m2. These equiluminance settings reveal a very slight compressive non-linearity (Lu & Sperling, 2001a). The small size of the distortion (<1%) is consistent with previous studies (Scott-Samuel & Georgeson, 1999).
The main experiment (second stage) utilized the subject’s equiluminance value to test the MAE following adaptation to second-order motion in an array of crowded Gabors (Fig. 1). Each trial of the experiment consisted of two phases, the adaptation and test phases. In the adaptation phase, subjects fixated on a point (8.7 deg from center to horizontal midpoint of the nearest Gabor) while adapting to a rectangular array of 20 Gabors (5 horizontally by 4 vertically), each with either rightward or leftward motion, chosen randomly on each trial (except for 4 central adapting Gabors, whose motion direction remained constant). Fig. 1 shows an example of the adaptation stimulus. Each Gabor consisted of a dynamic random-dot pattern (each dot was 0.2 by 0.2 deg, refreshed every 10 ms) modulated by a contrast-defined sinusoid, which was 0.71 cycles/deg and drifted 2.65 deg/s. The second-order motion in the Gabors was produced by drifting the sinusoidal contrast modulation. A Gaussian contrast-modulated envelope blurred the edges of the Gabor (2.82 deg full-width at half-maximum amplitude). This Gaussian contrast envelope was static at all times. Hereafter, these Gabors will be termed “second-order Gabors” to distinguish them from the more familiar first-order variant (Fig. 2 shows an example second-order Gabor). Three contrast modulation depths (the maximum Michelson contrast between elements that form the second-order Gabors) were tested (0.31, 0.58, and 0.90), centered on the subject’s equiluminance value.
Fig. 2.
Example second-order Gabor used in the first experiment. (A). The Gabor consisted of a dynamic random dot pattern, modulated by a contrast-defined sinusoid (dashed line in B) that drifted either leftward or rightward, and a Gaussian contrast-modulated envelope (to blur the edges; solid line in B). The sinusoidal contrast modulation (visible here as random dots alternating with gray bars) was the only moving component. The Gaussian contrast-modulated envelope was always static. The dynamic random dot background was updated every frame and produced a broadband noise (e.g., T.V. snow). The pictured pattern has a sinusoidal modulation with exaggerated contrast depth to reproduce in print; the actual Gabor had much lower contrast. Formally, the Gabor is described as: , where L(x,y,t) is the luminance at any point at time t; E is physical equiluminance (mean luminance); V is subject’s equiluminance value (see Methods); R(x,y,t) is a random-dot array in time; D is the depth of the contrast modulation (the incremental contrast above and below E); SF is the spatial frequency of the sine wave contrast modulation (pixels/cycle); TF is the temporal frequency of the sine wave (cyc/frame); r is distance of (x,y) from the center of the Gabor; σ is the standard deviation of the static Gaussian contrast envelope; and M is the maximum radius of the Gaussian envelope. Because the monitor’s refresh was 100 Hz, t is defined in 10 ms increments. (B) The luminance contrast profile of the second-order Gabor. The sinusoidal contrast modulation (dashed line) varies from low to high contrast, and is the only drifting component. The static Gaussian contrast-modulated envelope is indicated by the solid line.
The second-order Gabors were separated from each other horizontally and vertically by 4.72 deg from center to center, and each Gabor was presented on a square gray patch (4.67 by 4.67 deg). The background was a static random-dot pattern (each dot was 0.2 by 0.2 deg) with a contrast depth of 0.31, 0.58, and 0.90 (to match the contrast modulation depth of the second-order Gabors). A thin strip (0.05 deg) of the background was visible between each patch, giving the array an appearance of a grid. The purpose of the grid and the random-dot background was to provide a continuously visible static reference that would not interfere with the task or adaptation, but would help subjects maintain fixation and make fine judgments of relative motion. Neither second-order motion adaptation nor the MAE influence the perception of the grid, because second-order motion adaptation does not produce a static remote MAE (von Grünau & Dube, 1992).
In each session, there was an initial adaptation period of 40 s, with 8 s top-up adaptation periods between each trial (Fig. 1). Following each adaptation period, in the test phase of each trial, a dynamic test pattern was presented for 1 s at one of the four central positions (chosen randomly each trial, Fig. 1). The test pattern consisted of dynamic random noise (identical to the dynamic random noise in the adaptation Gabors above) modulated by two interleaved contrast-defined sine waves that drifted in opposite directions. The temporal and spatial frequencies of the sine wave modulations were equivalent to those of the adaptation second-order Gabors (above). In each trial, the contrast modulation depth of the two oppositely drifting sine waves was manipulated in opposite directions to make one direction more salient than the other (the average contrast of the two sine waves always remained fixed at 0.31). For example, the contrast of the rightward drifting sine wave could be increased while the contrast of the leftward drifting sine wave was decreased (Fig. 3A). When the contrast depth of the two oppositely drifting sine waves was equated, there was no net directional motion (Fig. 3B). Importantly, although the contrast depth was manipulated, no first-order luminance defined motion was present—the motion in the test stimuli (whether balanced or not) was always second-order. This type of drift-balanced second-order motion is known to successfully display a second-order MAE (Ledgeway & Smith, 1994a). The difference in contrast depth between the two sine waves (the net direction of motion in the test Gabor) could be one of six different values, while the mean contrast of the two sine waves was constant (Michelson contrast modulation depth; positive values indicate a contrast imbalance providing a stronger motion signal in the direction of motion adaptation).
Fig. 3.
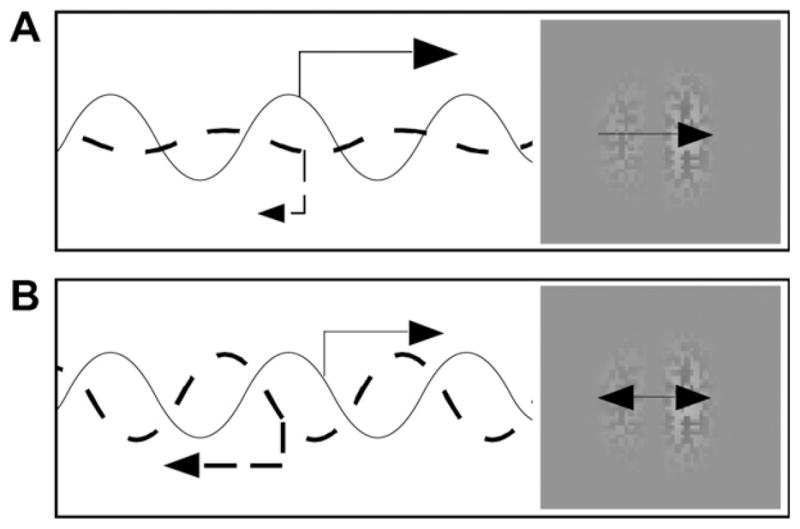
Nulling method used in the first experiment. (A) To measure the MAE, two contrast-modulated sine waves were drifted in opposite directions. The perceived motion in the pattern is dictated by the relative contrast of the two sine waves. If the contrast of the rightward sinusoid is higher, the perceived motion in the pattern follows a rightward direction. (B) If the contrast of the two sine waves is equal, then they are perfectly drift balanced and there is no perceived motion (i.e., they just flicker).
Using a yes/no task in a method of constant stimuli design, subjects judged which direction the test Gabor drifted (leftward or rightward). There were 96 trials for each of the six tested Gabor motion conditions; data were collected in separate 48 trial sessions to reduce fatigue. Three contrast modulation depths were tested, for a total of 1728 trials per subject. Data were averaged across sessions and separate PSEs were estimated for each of the 3 Gabor types (each of the adaptation Gabor contrast modulation depths) from the logistic function f(x) = [1/(1 + exp[a(x + b)])], where (b) estimates the relative contrast required to null the MAE (the point of subjective equality, PSE; Finney, 1971; McKee, Klein, & Teller, 1985) and (a) indicates the slope of the function. If the contrast depths of the two sine waves were equal and there was no MAE, subjects should not report a motion bias in either direction. However, if subjects report seeing movement in a direction opposite to that of the adapting stimuli when the contrast depth of the test stimulus is balanced, this would indicate an MAE. The relative Michelson contrast depth needed to cancel the MAE (the PSE) measures the strength of the effect. Statistical significance of the PSE was established using the maximum likelihood ratio test.
In a separate session, the experiment above was repeated with one modification: subjects made two judgments in each trial. During each top-up adaptation period, subjects first judged the direction of motion in the adaptation Gabor. Subjects were allowed to make their judgment at any time during the trial, but were encouraged to be as accurate as possible. The adaptation and test locations were fixed in every trial in the upper dashed circle in Fig. 1; subjects therefore had no uncertainty about the location of the Gabor to be judged. During the test period, subjects made a second judgment of motion direction in the test Gabor, using the same procedure and stimuli described above to measure the MAE. Each subject participated in 64 trials for each of six Gabor test conditions, for a total of 384 trials. The purpose of this additional test was to confirm that crowding was effective.
2.2. Results
After adaptation to an array of second-order Gabors (Figs. 1 and 2), subjects perceived a significant MAE in a direction opposite that of the adaptation direction (Figs. 3 and 4). Fig. 4A shows a psychometric function for one subject in the condition in which the contrast modulation depth of the adaptation Gabor was 0.58. The PSE was 0.09 (the smallest measured effect), indicating that for the test Gabor to display no perceived motion, the contrast of the sine wave drifting in the same direction as the adaptation direction had to be increased by ~9% (χ2(1) = 7.80, P < 0.01).
Fig. 4.
Results of the first experiment.(A) A psychometric function for one subject in one condition following adaptation to second-order motion in the crowded array from Fig. 1. The abscissa shows the relative contrast modulation depth of the two sine wave contrast modulations (i.e., as in Fig. 3). Negative values indicate that the test Gabor contained net motion opposite the direction of prior adaptation (in the direction of an MAE); positive values indicate that the net motion in the test Gabor was in the same direction as adaptation. The ordinate shows the proportion of subject responses that were opposite the direction of adaptation (in the direction of the MAE). The PSE (the 50% point on the curve) was ~0.10 (the weakest effect of any measured), indicating that the test Gabor needed to contain net motion (higher contrast) in the direction of adaptation to null the perceived MAE (a significant MAE; χ2 = 7.8, P < 0.01). (B) Results for two subjects, showing a significant MAE perceived on each of the tested Gabor contrasts. The open symbols show the perceived second-order MAE in experimental sessions in which the subjects were required to judge the direction of motion in the adaptation Gabor as well as the test pattern. When guessing the direction of motion in the adaptation Gabor, subjects DB and DW were at 50.8% and 53% accuracy, respectively, which reveals that crowding was effective at preventing awareness of motion direction. Error bars, ±SEM.
Fig. 4B shows the results for both subjects across several adaptation contrasts. Each condition yielded a significant MAE; the least significant MAE was observed by subject DB in the 0.90 contrast depth condition (χ2(1) = 5.42, P < 0.05, all other effects P < 0.01). It is clear that there is a substantial second-order MAE at each tested contrast, suggesting that the MAE here is not a product of a first-order motion contaminant (or one would expect a larger MAE with increasing contrast). The lowest tested Michelson contrast modulation depth was 0.31, which was the contrast required for 80% direction discrimination. Such a high threshold indicates that judging the direction of motion in the second-order Gabors, even without crowding, was extremely difficult. The open symbols in Fig. 4B show the perceived second-order MAE in trials in which subjects were required to guess the direction of motion in the adaptation region (a dual-judgment task). Although subjects were at chance when guessing the direction of adaptation (DB accuracy = 50.8%, χ2(1) = 0.16, P = 0.69; DW, accuracy = 53%, χ2(1) = 1.2, P = 0.27), the MAE in these trials remained significant (for subject DW, χ2(1) = 8.5, P < 0.01; DB, χ2(1) = 9.7, P < 0.01). This experiment demonstrated that the MAE did not hinge on awareness of the direction of motion adaptation.
The results suggest that there is adaptation to second-order motion even without the ability to scrutinize or attend to any individual Gabor. However, before we can conclude that second-order motion mechanisms operate in the absence of attention or awareness, several control experiments are necessary.
3. Experiment 2: Crossover MAEs for first-and second-order motion
The first experiment demonstrated that second-order motion adaptation occurs even when subjects are not aware of the direction of motion adaptation. Previous studies have found that first-order motion adaptation in crowded displays also produces a motion aftereffect (Aghdaee, 2005; Aghdaee & Zandvakili, 2005; Blake, Tadin, Sobel, Raissian, & Chong, 2006; Whitney, 2005, 2006). Is it possible that the MAE in Experiment 1 was generated by a first-order luminance artifact? The minimum motion technique of establishing equiluminant contrast-defined motion helped reduce this possibility. However, to more definitively rule out the possibility of luminance artifacts in Experiment 1, we conducted a second experiment. The goal of this experiment was to test whether there was a luminance artifact in our second-order stimuli and to test if this could generate a second-order MAE. To test this, we measured crossover adaptation effects for low-contrast first-and second-order motion. If the second-order MAE in Experiment 1 were due to a first-order contaminant, then this should be measurable on a first-order test pattern.
3.1. Methods
Experiment 2 had two stages: initially we tested first-and second-order MAEs following adaptation to first-order motion. Using those same test patterns, we then tested first-and second-order MAEs following adaptation to second-order motion. Together, these two stages measure the degree of crossover adaptation. If there is no crossover adaptation, then we can be confident that our second-order moving Gabors did not contain a first-order artifact.
In the first part of the experiment, during adaptation, we presented a crowded array of low contrast luminance-defined (first-order) drifting Gabors (0.0042 Michelson contrast; Fig. 5). The parameters of the crowded array, the Gabors, and the procedure were identical to that in the first experiment. In the test period, we presented either a dynamic first-order or second-order test Gabor, determined randomly on each trial, in one of the adapted locations (as in Fig. 1). The first-order test Gabor was similar to the second-order pattern, except that there was no random noise in the background: two luminance-defined sine waves (each 0.04 Michelson contrast) drifted in opposite directions. The average contrast of the two sine waves was pegged, and the contrast of each was manipulated in opposite directions to bias the perceived direction of movement (as in Fig. 3). The second-order test Gabor (0.73 contrast) was identical to that in the first experiment.
Fig. 5.

Stimulus used in the first stage of Experiment 2. (A) Subjects adapted to an array of low contrast first-order Gabors (the contrast is greatly exaggerated here for visibility). During the test period, second-order (B) or first-order Gabors (C) could be presented.
In the second part of the experiment, we repeated the first experiment (a crowded array of second-order adaptation Gabors was presented, each 0.67 contrast). Either first-or second-order test patterns were presented (identical to test patterns described above and in Fig. 5). Importantly, the first-and second-order test Gabors were identical in both the first and second stage of Experiment 2; the only difference was whether luminance or contrast-defined motion was adapted.
3.2. Results
In the first part of the experiment, we measured the dynamic first-and second-order MAE following adaptation to an array of drifting low-contrast first-order (luminance defined) Gabors (Fig. 5; see Methods). Fig. 6A shows that there was a significant MAE observed on the first-order test Gabor (DW, χ2(1) = 5.6, P < 0.02; DB, χ2(1) = 4.32, P < 0.05) whereas there was not a significant MAE observed on the second-order test Gabor (DW, χ2(1) = 0.64, P > 0.05; DB, χ2(1) = 1.12, P > 0.05). The drift-balanced first-order test pattern was more sensitive than the second-order test pattern to first-order motion adaptation. Using these same test Gabors, we measured the MAE following adaptation to the crowded array of second-order Gabors (as in Experiment 1; Fig. 1). If the second-order adaptation Gabors contained a luminance artifact, then the first-order test Gabors should have displayed a stronger MAE (as found in Fig. 6A). However, Fig. 6B shows that the second-order test displayed a stronger MAE than the first-order test Gabor following second-order motion adaptation (for the second-order test Gabor, the least significant effect: DW, χ2(1) = 4.9, P < 0.05; for the first-order test Gabor, the most significant effect: DW, χ2(1) = 0.87, P > 0.05). These results are consistent with Nishida et al. (1997), who found extremely weak or non-existent crossover adaptation. These results further confirm that our second-order Gabors were free of luminance artifacts.
Fig. 6.
Results of the second experiment. The MAE is plotted for first-and second-order Gabors as a function of first-or second-order adaptation. Each graph’s ordinate has two scales: on the right is the raw MAE magnitude (as in Fig. 4). This scale refers to the circular data points connected with dashed lines. On the left is the MAE expressed as a multiple of threshold discrimination (referring to the bars in the graph); to more directly compare first and second-order MAEs, we divided the PSE (contrast required to null the MAE) by the threshold contrast discrimination (estimated as half the distance between the 25% and 75% response proportions on the psychometric function, as in Fig. 4). This accounted for the fact that discriminating first-order test patterns is much easier than second-order test patterns. The PSEs (average of two subjects, as in Fig. 4) are shown by the circles connected with a dashed line. Both estimates of the MAE yield identical results. (A) Following first-order adaptation (Fig. 5), the MAE was significant for luminance-defined test Gabors, but was insignificant for contrast-defined test Gabors (asterisks indicate significance at the 0.01 level; χ2 test). A dynamic first-order test pattern was therefore a more sensitive measure of luminance-defined motion adaptation. (B) Following second-order adaptation (e.g., Fig. 1), there was a significant MAE only for the contrast-defined test Gabors (P < 0.01). If the second-order adaptation Gabors had contained a luminance artifact, the first-order test Gabors should have displayed a stronger MAE. The fact that there was very little, if any, crossover adaptation (from second-order motion adaptation to first-order test patterns) shows that luminance artifacts are not responsible for the results in the first experiment.
4. Experiment 3: Second-order MAE with versus without crowding
The first experiment demonstrated an MAE following adaptation to second-order motion that was crowded out of awareness. The second experiment showed that the second-order MAE is specific to contrast-defined motion adaptation and is not due to a luminance artifact. A remaining question is whether the magnitude of the second-order MAE is influenced by the presence of the crowding stimuli. For example, von Grünau and Dube (1992) presented an impressive case of a remote, dynamic MAE, suggesting that exposure to motion in one region can influence percepts of motion in remote areas of the visual field. Further studies have built on this finding, showing a number of different types of remote aftereffects of motion (Anstis & Reinhardt-Rutland, 1976; Ashida, Susami, & Osaka, 1996; Culham, Verstraten, Ashida, & Cavanagh, 2000; Snowden & Milne, 1997; Whitney & Cavanagh, 2003). The crowding display in Experiment 1 (Fig. 1) contained Gabors with sine wave contrast modulations drifting in opposite directions; in fact, the net motion in the display is, on average, zero. If the dynamic MAE is a global (Culham et al., 2000) or at least remote (von Grünau & Dube, 1992) effect, then the crowding display should reduce the magnitude of the MAE compared to the case in which a single adaptation Gabor is presented. In the third experiment we compared the second-order MAE with and without crowding (Fig. 7).
Fig. 7.
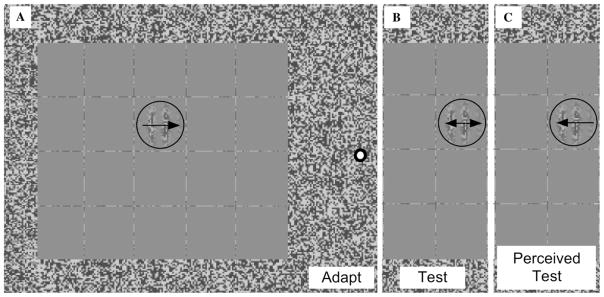
Stimulus used in Experiment 3. (A) Rather than adapting to an array of second-order Gabors, subjects adapted to a single Gabor. (B and C) The same test Gabors were used from Experiment 1, which display an MAE following adaptation.
4.1. Methods
Experiment 3 was identical to the dual-judgment session in Experiment 1 (stimulus in Fig. 1), except there were no crowding (distracting) Gabors surrounding the target adaptation Gabor and only one contrast modulation depth (0.31) was tested (Fig. 7). Subjects adapted to a single Gabor (at the same position and eccentricity as that tested in Experiment 1) and then were presented with a dynamic test (identical to Experiment 1). In each trial, subjects made two judgments (identical to the dual-judgment task in Experiment 1): the direction of motion in the adaptation Gabor and the direction of motion in the test Gabor. The test stimulus, task, and procedure were identical to those in the first experiment; the only difference was the presence of crowding Gabors.
4.2. Results
Fig. 8 shows that the magnitude of the MAE was slightly reduced with the addition of the surrounding crowding Gabors (Fig. 8; the most significant difference was for subject DB, χ2(1) = 1.83, P > 0.05). This suggests that the form of second-order motion adaptation that we measured here (and in Experiment 1) is mediated by a predominantly local mechanism. In contrast to many studies of motion adaptation and dynamic MAEs, the form of second-order adaptation here may be mediated by units with small receptive fields, without the necessary horizontal connections or feedback from higher motion processing areas that have been posited to explain previous remote effects of motion (Durant & Johnston, 2004; Nishida & Johnston, 1999; Whitney & Cavanagh, 2000; Zeki, 1990).
Fig. 8.
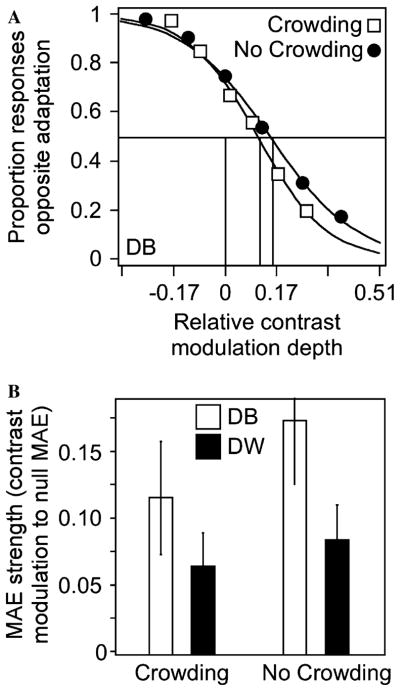
Results of Experiment 3. (A) Psychometric functions showing the MAE with and without crowding for subject DB. (B) There was a slight reduction in the MAE under crowding conditions for both subjects, but this difference was not significant (the most significant difference was for subject DB; χ2(1) = 1.80, P > 0.05). The fact that there is only a modest decrement in the MAE under crowding conditions suggests that adaptation was at a predominantly local spatial scale, and that global characteristics, pattern or ensemble statistics, and spatially distributed effects do not contribute (or contribute very little) to the passive second-order motion coding found in the first experiment. Error bars, ±SEM.
We might have expected a stronger modulatory role of attention or awareness on the magnitude of the MAE (Aghdaee & Zandvakili, 2005; Chaudhuri, 1990; Culham et al., 2000; Georgiades & Harris, 2000; Lankheet & Verstraten, 1995; Mukai & Watanabe, 2001; Nishida & Ashida, 2000; Rezec, Krekelberg, & Dobkins, 2004; Seiffert, Somers, Dale, & Tootell, 2003; Shulman, 1993; von Grunau, Bertone, & Pakneshan, 1998). For example, attentive tracking can generate motion capture and MAEs, even without any physical movement (Culham & Cavanagh, 1994; Culham et al., 2000), and attention is thought to influence motion processing even at early stages in the visual hierarchy (e.g., V1; Muckli, Kohler, Kriegeskorte, & Singer, 2005; Somers, Dale, Seiffert, & Tootell, 1999; Watanabe et al., 1998; Watanabe et al., 1998). Evidently, however, passive detectors are sufficient to code second-order motion in the absence of attentional or top-down mechanisms.
5. General discussion
The goal of the experiments here was to test whether second-order motion can be processed in the absence of attentional scrutiny and awareness. To test this we measured the MAE following adaptation to second-order drifting Gabors embedded in dense arrays of additional Gabors. These crowding displays prevented awareness of motion direction, and yet there was still a significant MAE produced following adaptation to second-order motion. The results indicate that there is a mechanism that codes second-order motion prior to the stage at which individual objects are selected for awareness—awareness of features or motion direction is not necessary for the visual system to code second-order motion.
Several models explicitly incorporate, or at least allow for the possibility that second-order motion detection is a passive process. These include the models of Lu and Sperling (1995b, 2001b) and the models of Johnston, Benton, and colleagues (Benton, Johnston, & McOwan, 2000; Benton, Johnston, McOwan, & Victor, 2001; Johnston, Benton, & McOwan, 1999). Our results are also consistent with psychophysical observations of (arguably) passive or subthreshold second-order motion detection (Lu & Sperling, 1999; Nishida, 1993; Patterson et al., 1997; Smith, 1994). The advantage of our experiments is that the crowding technique prevents awareness of motion direction with exactitude and therefore entirely bypasses arguments of top-down attentional control that might otherwise be invoked.
An interesting property of the second-order MAE revealed here (in addition to it not requiring awareness of motion adaptation) is that it was a predominantly local phenomenon (i.e., the mechanism responsible for the adaptation did not average motion signals over the array of Gabors). The traditional second-order MAE is selectively visible on dynamic test patterns (McCarthy, 1993; Nishida et al., 1994; Smith & Ledgeway, 1997), which generally produce global or spatially broad MAEs (Anstis & Reinhardt-Rutland, 1976; Ashida et al., 1996; Culham et al., 2000; Snowden & Milne, 1997; von Grünau & Dube, 1992; Whitney & Cavanagh, 2003). The data here support the existence of local, low-level, passive second-order motion detectors that precede explicit (conscious) shape and form computations. It is conceivable that the mechanism responsible simultaneously codes object form (second-order texture boundaries) as well as velocity information (Del Viva & Morrone, 1998). Several variants of this sort of detector have been proposed before for first-order motion (Burr, Ross, & Morrone, 1986a, 1986b; Nishida, 2004; Reisbeck & Gegenfurtner, 1999). Importantly, however, if this sort of mechanism were responsible, it must operate at an early level, prior to the stage at which objects/forms are selected for awareness. Likewise, because of the local nature of the MAE found in the experiments here, the second-order motion detector responsible must operate prior to any mandatory grouping of pattern or ensemble information (Parkes, Lund, Angelucci, Solomon, & Morgan, 2001).
First-order motion adaptation that is crowded out of awareness produces a strong, spatially localized MAE (Aghdaee, 2005; Aghdaee & Zandvakili, 2005; Whitney, 2005, 2006). The similar local nature of the second-order MAE effect in the present study (Fig. 8) raises the question of whether the second-order stimuli here were truly free of luminance artifacts. Several lines of evidence suggest that our second-order Gabors were, in fact, free of luminance information. First, we used a dynamic random noise background (Smith & Ledgeway, 1997). Second, we used the minimum motion technique to establish psychophysical equiluminance (Anstis & Cavanagh, 1983; Lu & Sperling, 2001a; Nishida et al., 1997; Scott-Samuel & Georgeson, 1999). Finally, we conducted a control experiment (Experiment 2) that ruled out the possibility that a luminance artifact could have determined the results in the first experiment. Together, these lines of evidence suggest that the second-order motion was artifact-free and that the detectors responsible for the adaptation in our experiments were, for the most part, spatially local.
It remains an open question whether there are several distinct mechanisms that can independently process second-order motion, but there is a great deal of evidence already accumulated, in addition to the experiments presented here, that support this hypothesis. For example, second-order motion perception is modulated by attention (Allen & Ledgeway, 2003; Del Vecchio et al., 2001; Ho, 1998; Lu et al., 2000). Moreover, attentive tracking is sufficient to generate awareness of second-order motion (Cavanagh, 1992; Culham & Cavanagh, 1994), and a position tracking mechanism like this may be responsible for detecting second-order motion within a certain range of speeds and contrasts (Seiffert & Cavanagh, 1999) and could contribute to global motion percepts (Culham et al., 2000). Conversely, salience-based motion detection (Lu & Sperling, 1995a) or feature tracking (Del Viva & Morrone, 1998; Derrington et al., 2004) could be largely bottom up, or modulated by attention, but may not require the awareness of a stimulus; the role of awareness in these sorts of mechanisms (i.e., second-and putative third-order motion processes) requires further study. Additional passive mechanisms may directly code second-order motion (via separate dedicated detectors or through the first-order motion system; Benton & Johnston, 2001; Johnston & Clifford, 1995; Lu & Sperling, 2001b). All of these hypotheses can be coarsely grouped into bottom-up and top-down camps. It is very likely, based on the published results and the data reported here, that both classes of mechanism are capable of coding second-order motion, and that under normal circumstances both do (Smith & Ledgeway, 2001).
The mechanism that produces crowding, and the neural stage at which this happens, are debated (Blake et al., 2006; He et al., 1997, 1996, Hess, Dakin, & Kapoor, 2000; Intriligator & Cavanagh, 2001; Pelli, Palomares, & Majaj, 2004) and remain unknown. Nevertheless, we can be certain that regardless of the mechanism responsible for crowding, the second-order motion adaptation reported here must occur at a site prior to that which produces crowding. The fact that second-order motion perception is generally believed to arise at a level at or beyond V1 (Dumoulin, Baker, Hess, & Evans, 2003; Nishida, Sasaki, Murakami, Watanabe, & Tootell, 2003; Seiffert et al., 2003; Smith, Greenlee, Singh, Kraemer, & Hennig, 1998) does place a lower limit on the stage at which crowding occurs (at least the form of crowding that operates in the present experiments; it is entirely possible that there are multiple forms of crowding that operate at multiple levels in the visual hierarchy).
6. Conclusions
The experiments here demonstrate that although high-level motion processes may be sufficient to code second-order motion—the kind of camouflaged motion we may face more often than realize—these high level or top-down mechanisms that require awareness of features are not necessary. Even in the absence of awareness, second-order motion produces strong local motion adaptation that cannot be due to attention, awareness, or top-down processes.
Acknowledgments
This work was supported by UC Davis.
Footnotes
Abbreviations used: MAE, motion aftereffect; PSE, point of subjective equality.
References
- Aghdaee SM. Adaptation to spiral motion in crowding condition. Perception. 2005;34(2):155–162. doi: 10.1068/p5298. [DOI] [PubMed] [Google Scholar]
- Aghdaee SM, Zandvakili A. Adaptation to spiral motion: global but not local motion detectors are modulated by attention. Vision Research. 2005;45(9):1099–1105. doi: 10.1016/j.visres.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Allen HA, Ledgeway T. Attentional modulation of threshold sensitivity to first-order motion and second-order motion patterns. Vision Research. 2003;43(27):2927–2936. doi: 10.1016/j.visres.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Anstis SM, Cavanagh P. A minimum motion technique for judging equiluminance. In: Mollon J, Sharpe RT, editors. Color vision: Physiology and psychophysics. London: Academic Press; 1983. pp. 155–166. [Google Scholar]
- Anstis SM, Reinhardt-Rutland AH. Interactions between motion aftereffects and induced movement. Vision Research. 1976;16(12):1391–1394. doi: 10.1016/0042-6989(76)90157-7. [DOI] [PubMed] [Google Scholar]
- Ashida H, Seiffert AE, Osaka N. Inefficient visual search for second-order motion. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 2001;18(9):2255–2266. doi: 10.1364/josaa.18.002255. [DOI] [PubMed] [Google Scholar]
- Ashida H, Susami K, Osaka N. Re-evaluation of local adaptation for motion aftereffect. Perception. 1996;25(9):1065–1072. doi: 10.1068/p251065. [DOI] [PubMed] [Google Scholar]
- Benton CP, Johnston A. A new approach to analysing texture-defined motion. Proceedings of Biological Science. 2001;268(1484):2435–2443. doi: 10.1098/rspb.2001.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton CP, Johnston A, McOwan PW. Computational modelling of interleaved first-and second-order motion sequences and translating 3f+4f beat patterns. Vision Research. 2000;40(9):1135–1142. doi: 10.1016/s0042-6989(00)00026-2. [DOI] [PubMed] [Google Scholar]
- Benton CP, Johnston A, McOwan PW, Victor JD. Computational modeling of non-Fourier motion: further evidence for a single luminance-based mechanism. Journal of the Optical Society of Ameica A, Optics, Image Science, and Vision. 2001;18(9):2204–2208. doi: 10.1364/josaa.18.002204. [DOI] [PubMed] [Google Scholar]
- Blake R, Tadin D, Sobel KV, Raissian TA, Chong SC. Strength of early visual adaptation depends on visual awareness. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(12):4783–4788. doi: 10.1073/pnas.0509634103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr DC, Ross J, Morrone MC. Seeing objects in motion. Proceedings of the Royal Society London, B Biological Science. 1986a;227(1247):249–265. doi: 10.1098/rspb.1986.0022. [DOI] [PubMed] [Google Scholar]
- Burr DC, Ross J, Morrone MC. Smooth and sampled motion. Vision Research. 1986b;26(4):643–652. doi: 10.1016/0042-6989(86)90012-x. [DOI] [PubMed] [Google Scholar]
- Cavanagh P. Attention-based motion perception. Science. 1992;257(5076):1563–1565. doi: 10.1126/science.1523411. [DOI] [PubMed] [Google Scholar]
- Cavanagh P, Mather G. Motion: the long and short of it. Spatial Vision. 1989;4(2–3):103–129. doi: 10.1163/156856889x00077. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A. Modulation of the motion aftereffect by selective attention. Nature. 1990;344(6261):60–62. doi: 10.1038/344060a0. [DOI] [PubMed] [Google Scholar]
- Culham JC, Cavanagh P. Motion capture of luminance stimuli by equiluminous color gratings and by attentive tracking. Vision Research. 1994;34(20):2701–2706. doi: 10.1016/0042-6989(94)90226-7. [DOI] [PubMed] [Google Scholar]
- Culham JC, Verstraten FA, Ashida H, Cavanagh P. Independent aftereffects of attention and motion. Neuron. 2000;28(2):607–615. doi: 10.1016/s0896-6273(00)00137-9. [DOI] [PubMed] [Google Scholar]
- Del Vecchio AS, von GMW, Faubert J. Attentional selection of first-and second-order motion stimuli. Journal of Vision. 2001;1(3):88. [Google Scholar]
- Del Viva MM, Morrone MC. Motion analysis by feature tracking. Vision Research. 1998;38(22):3633–3653. doi: 10.1016/s0042-6989(98)00022-4. [DOI] [PubMed] [Google Scholar]
- Derrington AM, Allen HA, Delicato LS. Visual mechanisms of motion analysis and motion perception. Annual Review of Psychology. 2004;55:181–205. doi: 10.1146/annurev.psych.55.090902.141903. [DOI] [PubMed] [Google Scholar]
- Derrington AM, Badcock DR. Separate detectors for simple and complex grating patterns? Vision Research. 1985;25(12):1869–1878. doi: 10.1016/0042-6989(85)90010-0. [DOI] [PubMed] [Google Scholar]
- Dumoulin SO, Baker CL, Jr, Hess RF, Evans AC. Cortical specialization for processing first-and second-order motion. Cerebral Cortex. 2003;13(12):1375–1385. doi: 10.1093/cercor/bhg085. [DOI] [PubMed] [Google Scholar]
- Durant S, Johnston A. Temporal dependence of local motion induced shifts in perceived position. Vision Research. 2004;44(4):357–366. doi: 10.1016/j.visres.2003.09.022. [DOI] [PubMed] [Google Scholar]
- Finney DJ. Probit analysis. Cambridge: University Press; 1971. p. xv.p. 333. [Google Scholar]
- Georgiades MS, Harris JP. Attentional diversion during adaptation affects the velocity as well as the duration of motion after-effects. Proceedings of Biological Science. 2000;267(1461):2559–2565. doi: 10.1098/rspb.2000.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Cavanagh P, Intiligator J. Attentional resolution. Trends in Cognitive Sciences. 1997;1(3):115–121. doi: 10.1016/S1364-6613(97)89058-4. [DOI] [PubMed] [Google Scholar]
- He S, Cavanagh P, Intriligator J. Attentional resolution and the locus of visual awareness. Nature. 1996;383(6598):334–337. doi: 10.1038/383334a0. [DOI] [PubMed] [Google Scholar]
- Hess RF, Dakin SC, Kapoor N. The foveal ‘crowding’ effect: physics or physiology? Vision Research. 2000;40(4):365–370. doi: 10.1016/s0042-6989(99)00193-5. [DOI] [PubMed] [Google Scholar]
- Ho CE. Letter recognition reveals pathways of second-order and third-order motion. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(1):400–404. doi: 10.1073/pnas.95.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intriligator J, Cavanagh P. The spatial resolution of visual attention. Cognitive Psychology. 2001;43(3):171–216. doi: 10.1006/cogp.2001.0755. [DOI] [PubMed] [Google Scholar]
- Johnston A, Benton CP, McOwan PW. Induced motion at texture-defined motion boundaries. Proceedings of Biological Science. 1999;266(1436):2441–2450. doi: 10.1098/rspb.1999.0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston A, Clifford CW. A unified account of three apparent motion illusions. Vision Research. 1995;35(8):1109–1123. doi: 10.1016/0042-6989(94)00175-l. [DOI] [PubMed] [Google Scholar]
- Lankheet MJ, Verstraten FA. Attentional modulation of adaptation to two-component transparent motion. Vision Research. 1995;35(10):1401–1412. doi: 10.1016/0042-6989(95)98720-t. [DOI] [PubMed] [Google Scholar]
- Ledgeway T, Smith AT. The duration of the motion aftereffect following adaptation to first-order and second-order motion. Perception. 1994a;23(10):1211–1219. doi: 10.1068/p231211. [DOI] [PubMed] [Google Scholar]
- Ledgeway T, Smith AT. Evidence for separate motion-detecting mechanisms for first-and second-order motion in human vision. Vision Research. 1994b;34(20):2727–2740. doi: 10.1016/0042-6989(94)90229-1. [DOI] [PubMed] [Google Scholar]
- Lu ZL, Liu CQ, Dosher BA. Attention mechanisms for multi-location first-and second-order motion perception. Vision Research. 2000;40(2):173–186. doi: 10.1016/s0042-6989(99)00172-8. [DOI] [PubMed] [Google Scholar]
- Lu ZL, Sperling G. Attention-generated apparent motion. Nature. 1995a;377(6546):237–239. doi: 10.1038/377237a0. [DOI] [PubMed] [Google Scholar]
- Lu ZL, Sperling G. The functional architecture of human visual motion perception. Vision Research. 1995b;35(19):2697–2722. doi: 10.1016/0042-6989(95)00025-u. [DOI] [PubMed] [Google Scholar]
- Lu ZL, Sperling G. Second-order reversed phi. Perception & Psychophysics. 1999;61(6):1075–1088. doi: 10.3758/bf03207615. [DOI] [PubMed] [Google Scholar]
- Lu ZL, Sperling G. Sensitive calibration and measurement procedures based on the amplification principle in motion perception. Vision Research. 2001a;41(18):2355–2374. doi: 10.1016/s0042-6989(01)00106-7. [DOI] [PubMed] [Google Scholar]
- Lu ZL, Sperling G. Three-systems theory of human visual motion perception: review and update. Journal of the Optical Society of Ameica A, Optics, Image Science, and Vision. 2001b;18(9):2331–2370. doi: 10.1364/josaa.18.002331. [DOI] [PubMed] [Google Scholar]
- Mather G, Verstraten F, Anstis S. The motion after-effect: A modern perspective. Cambridge, MA: MIT Press; 1998a. [Google Scholar]
- Mather G, Verstraten F, Anstis S. The motion aftereffect: A modern perspective. Cambridge, MA: MIT Press; 1998b. [DOI] [PubMed] [Google Scholar]
- McCarthy JE. Directional adaptation effects with contrast modulated stimuli. Vision Research. 1993;33(18):2653–2662. doi: 10.1016/0042-6989(93)90225-l. [DOI] [PubMed] [Google Scholar]
- McKee SP, Klein SA, Teller DY. Statistical properties of forced-choice psychometric functions: implications of probit analysis. Perception & Psychophysics. 1985;37(4):286–298. doi: 10.3758/bf03211350. [DOI] [PubMed] [Google Scholar]
- Muckli L, Kohler A, Kriegeskorte N, Singer W. Primary visual cortex activity along the apparent-motion trace reflects illusory perception. PLoS Biology. 2005;3(8):e265. doi: 10.1371/journal.pbio.0030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai I, Watanabe T. Differential effect of attention to translation and expansion on motion aftereffects (MAE) Vision Research. 2001;41(9):1107–1117. doi: 10.1016/s0042-6989(00)00308-4. [DOI] [PubMed] [Google Scholar]
- Nishida S. Spatiotemporal properties of motion perception for random-check contrast modulations. Vision Research. 1993;33(5–6):633–645. doi: 10.1016/0042-6989(93)90184-x. [DOI] [PubMed] [Google Scholar]
- Nishida S. Motion-based analysis of spatial patterns by the human visual system. Current Biology. 2004;14(10):830–839. doi: 10.1016/j.cub.2004.04.044. [DOI] [PubMed] [Google Scholar]
- Nishida S, Ashida H. A hierarchical structure of motion system revealed by interocular transfer of flicker motion aftereffects. Vision Research. 2000;40(3):265–278. doi: 10.1016/s0042-6989(99)00176-5. [DOI] [PubMed] [Google Scholar]
- Nishida S, Ashida H, Sato T. Complete interocular transfer of motion aftereffect with flickering test. Vision Research. 1994;34(20):2707–2716. doi: 10.1016/0042-6989(94)90227-5. [DOI] [PubMed] [Google Scholar]
- Nishida S, Edwards M, Sato T. Simultaneous motion contrast across space: involvement of second-order motion? Vision Research. 1997;37(2):199–214. doi: 10.1016/s0042-6989(96)00112-5. [DOI] [PubMed] [Google Scholar]
- Nishida S, Johnston A. Influence of motion signals on the perceived position of spatial pattern. Nature. 1999;397(6720):610–612. doi: 10.1038/17600. [DOI] [PubMed] [Google Scholar]
- Nishida S, Sasaki Y, Murakami I, Watanabe T, Tootell RB. Neuroimaging of direction-selective mechanisms for second-order motion. Journal of Neurophysiology. 2003;90(5):3242–3254. doi: 10.1152/jn.00693.2003. Epub 2003 Aug 3213. [DOI] [PubMed] [Google Scholar]
- Nishida S, Sato T. Motion aftereffect with flickering test patterns reveals higher stages of motion processing. Vision Research. 1995;35(4):477–490. doi: 10.1016/0042-6989(94)00144-b. [DOI] [PubMed] [Google Scholar]
- Parkes L, Lund J, Angelucci A, Solomon JA, Morgan M. Compulsory averaging of crowded orientation signals in human vision. Nature Neuroscience. 2001;4(7):739–744. doi: 10.1038/89532. [DOI] [PubMed] [Google Scholar]
- Patterson R, Donnelly M, Phinney RE, Nawrot M, Whiting A, Eyle T. Speed discrimination of stereoscopic (cyclopean) motion. Vision Research. 1997;37(7):871–878. doi: 10.1016/s0042-6989(96)00226-x. [DOI] [PubMed] [Google Scholar]
- Pelli DG, Palomares M, Majaj NJ. Crowding is unlike ordinary masking: distinguishing feature integration from detection. Journal of Vision. 2004;4(12):1136–1169. doi: 10.1167/4.12.12. [DOI] [PubMed] [Google Scholar]
- Reisbeck TE, Gegenfurtner KR. Velocity tuned mechanisms in human motion processing. Vision Research. 1999;39(19):3267–3285. doi: 10.1016/s0042-6989(99)00017-6. [DOI] [PubMed] [Google Scholar]
- Rezec A, Krekelberg B, Dobkins KR. Attention enhances adaptability: evidence from motion adaptation experiments. Vision Research. 2004;44(26):3035–3044. doi: 10.1016/j.visres.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Scott-Samuel NE, Georgeson MA. Does early non-linearity account for second-order motion? Vision Research. 1999;39(17):2853–2865. doi: 10.1016/s0042-6989(98)00316-2. [DOI] [PubMed] [Google Scholar]
- Seiffert AE, Cavanagh P. Position displacement, not velocity, is the cue to motion detection of second-order stimuli. Vision Research. 1998;38(22):3569–3582. doi: 10.1016/s0042-6989(98)00035-2. [DOI] [PubMed] [Google Scholar]
- Seiffert AE, Cavanagh P. Position-based motion perception for color and texture stimuli: effects of contrast and speed. Vision Research. 1999;39(25):4172–4185. doi: 10.1016/s0042-6989(99)00129-7. [DOI] [PubMed] [Google Scholar]
- Seiffert AE, Somers DC, Dale AM, Tootell RB. Functional MRI studies of human visual motion perception: texture, luminance, attention and after-effects. Cerebral Cortex. 2003;13(4):340–349. doi: 10.1093/cercor/13.4.340. [DOI] [PubMed] [Google Scholar]
- Shulman GL. Attentional effects of adaptation of rotary motion in the plane. Perception. 1993;22(8):947–961. doi: 10.1068/p220947. [DOI] [PubMed] [Google Scholar]
- Smith AT. Correspondence-based and energy-based detection of second-order motion in human vision. Journal of the Optical Society of Ameica A, Optics, Image Science, and Vision. 1994;11(7):1940–1948. doi: 10.1364/josaa.11.001940. [DOI] [PubMed] [Google Scholar]
- Smith AT, Greenlee MW, Singh KD, Kraemer FM, Hennig J. The processing of first-and second-order motion in human visual cortex assessed by functional magnetic resonance imaging (fMRI) Journal of Neuroscience. 1998;18(10):3816–3830. doi: 10.1523/JNEUROSCI.18-10-03816.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AT, Ledgeway T. Separate detection of moving luminance and contrast modulations: fact or artifact? Vision Research. 1997;37(1):45–62. doi: 10.1016/s0042-6989(96)00147-2. [DOI] [PubMed] [Google Scholar]
- Smith AT, Ledgeway T. Motion detection in human vision: a unifying approach based on energy and features. Proceedings of the Royal Society of London, B. 2001;268(1479):1889–1899. doi: 10.1098/rspb.2001.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden RJ, Milne AB. Phantom motion after effects—evidence of detectors for the analysis of optic flow. Current Biology. 1997;7(10):717–722. doi: 10.1016/s0960-9822(06)00329-0. [DOI] [PubMed] [Google Scholar]
- Somers DC, Dale AM, Seiffert AE, Tootell RB. Functional MRI reveals spatially specific attentional modulation in human primary visual cortex. Proceedings of the National Academy of Scienes of the United States of America. 1999;96(4):1663–1668. doi: 10.1073/pnas.96.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Grunau MW, Bertone A, Pakneshan P. Attentional selection of motion states. Spatial Vision. 1998;11(4):329–347. doi: 10.1163/156856898x00068. [DOI] [PubMed] [Google Scholar]
- von Grünau M, Dube S. Comparing local and remote motion aftereffects. Spatial Vision. 1992;6(4):303–314. doi: 10.1163/156856892x00145. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Harner AM, Miyauchi S, Sasaki Y, Nielsen M, Palomo D, et al. Task-dependent influences of attention on the activation of human primary visual cortex. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(19):11489–11492. doi: 10.1073/pnas.95.19.11489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Sasaki Y, Miyauchi S, Putz B, Fujimaki N, Nielsen M, et al. Attention-regulated activity in human primary visual cortex. Journal of Neurophysiology. 1998;79(4):2218–2221. doi: 10.1152/jn.1998.79.4.2218. [DOI] [PubMed] [Google Scholar]
- Whitney D. Motion distorts perceived position without awareness of motion. Current Biology. 2005;15(9):R324–R326. doi: 10.1016/j.cub.2005.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney D. Contribution of bottom-up and top-down motion processes to perceived position. Journal of Experimental Psychology: Human Perception and Performance. 2006;32(6):1380–1391. doi: 10.1037/0096-1523.32.6.1380. [DOI] [PubMed] [Google Scholar]
- Whitney D, Cavanagh P. Motion distorts visual space: shifting the perceived position of remote stationary objects. Nature Neuroscience. 2000;3(9):954–959. doi: 10.1038/78878. [DOI] [PubMed] [Google Scholar]
- Whitney D, Cavanagh P. Motion adaptation shifts apparent position without the motion aftereffect. Perception & Psychophysics. 2003;65(7):1011–1018. doi: 10.3758/bf03194830. [DOI] [PubMed] [Google Scholar]
- Zeki S. The motion pathways of the visual cortex. In: Blakemore C, editor. Vision: Coding and efficiency. Cambridge: Cambridge University Press; 1990. pp. 321–345. [Google Scholar]