Abstract
Background
The ubiquitous, non-proteinaceous amino acid GABA (γ-aminobutyrate) accumulates in plants subjected to abiotic stresses such as chilling, O2 deficiency and elevated CO2. Recent evidence indicates that controlled atmosphere storage causes the accumulation of GABA in apple (Malus x domestica Borkh.) fruit, and now there is increasing interest in the biochemical mechanisms responsible for this phenomenon. Here, we investigated whether this phenomenon could be mediated via Ca2+/calmodulin (CaM) activation of glutamate decarboxylase (GAD) activity.
Results
GAD activity in cell-free extracts of apple fruit was stimulated by Ca2+/CaM at physiological pH, but not at the acidic pH optimum. Based on bioinformatics analysis of the apple genome, three apple GAD genes were identified and their expression determined in various apple organs, including fruit. Like recombinant Arabidopsis GAD1, the activity and spectral properties of recombinant MdGAD1 and MdGAD2 were regulated by Ca2+/CaM at physiological pH and both enzymes possessed a highly conserved CaM-binding domain that was autoinhibitory. In contrast, the activity and spectral properties of recombinant MdGAD3 were not affected by Ca2+/CaM and they were much less sensitive to pH than MdGAD1, MdGAD2 and Arabidopsis GAD1; furthermore, the C-terminal region neither bound CaM nor functioned as an autoinhibitory domain.
Conclusions
Plant GADs typically differ from microbial and animal GAD enzymes in possessing a C-terminal 30–50 amino acid residue CaM-binding domain. To date, rice GAD2 is the only exception to this generalization; notably, the C-terminal region of this enzyme still functions as an autoinhibitory domain. In the present study, apple fruit were found to contain two CaM-dependent GADs, as well as a novel CaM-independent GAD that does not possess a C-terminal autoinhibitory domain.
Keywords: Abiotic stress, Apple fruit, Biochemical regulation, Calmodulin, Controlled atmosphere storage, γ-Aminobutyrate, Glutamate decarboxylase, Recombinant protein
Background
The accumulation of γ-aminobutyrate (GABA) in plants subjected to abiotic stress is often attributed to the Ca2+/calmodulin (CaM)- or pH-mediated stimulation of glutamate decarboxylation, although polyamines may also contribute [1-3]. Plant glutamate decarboxylase (GAD) requires pyridoxal 5′-phosphate (PLP) as a co-factor, is specific for L-glutamate and maximally active at approximately pH 5.8, and exists as dimeric or hexameric complexes [1]. GAD activity in cell-free extracts prepared in the presence of protease inhibitors and partially purified by ammonium sulfate precipitation and ion-exchange chromatography is typically assayed as the production of 14CO2 from radiolabeled glutamate or GABA from unlabeled glutamate with or without the addition of CaM antagonists such as trifluoperazine (TFP). The activation of GAD activity by Ca2+/CaM is more dramatic at neutral pH than optimum pH.
The C-terminal CaM-binding domain (CaMBD) of GAD is highly variable and evidence exists for a C-terminal domain in rice GAD (OsGAD2) that does not bind CaM, but is autoinhibitory [1,4,5]. The extent of the Ca2+/CaM stimulation of activity can also vary widely among various recombinant GADs (5- to 60-fold) [6-10]. Possible explanations for this variability could be the final purity of the protein being tested, as well as the method used for purification. There is also evidence to suggest that a tag may influence yield, conformation and biological activity of the recombinant protein [11]; however, the time required to remove the motif may be detrimental to the activity of an unstable protein.
The accumulation of GABA in apple fruit stored under controlled atmosphere conditions was recently reported [12-14], and now there is increasing interest in the biochemical mechanisms responsible for this phenomenon [3,14]. Here, we report the identification of three GADs in apple fruit and demonstrate, via the use of suitable tagging/purification strategies, that two are CaM-dependent and one is CaM-independent.
Results
GAD activity in cell-free extracts from apple fruit
GAD activity in cell-free apple extracts at pH 5.5 in the absence or presence of Ca2+/CaM was similar with bis(2-hydroxyethyl)amino-tris(hydroxymethyl)methane (Bis-Tris–HCl) and pyridine-HCl buffers (data not shown). Thus, we used these two buffers to study GAD activity at several pHs between 5.0 and 7.0 (Table 1). The pH optimum was approximately 5.5 to 6.0, and at these pHs Ca2+/CaM stimulated activity by about 70% and TFP inhibited activity by 35-60% in the presence of Ca2+/CaM. At pH 6.5 and pH 7, the stimulation by Ca2+/CaM (440-670%) and the inhibition by TFP increased markedly. TFP did not affect activity in the absence of added Ca2+/CaM.
Table 1.
Effect of pH, Ca2+/CaM and TFP on GAD activity in cell-free apple extracts
| Treatment |
pH |
||||
|---|---|---|---|---|---|
| 5.0 | 5.5 | 6.0 | 6.5 | 7.0 | |
| |
nmol mg prot-1 min-1 |
||||
| +Ca2+/CaM -TFP |
7.48±0.58a1 |
14.69±2.92a |
12.91±0.74a |
3.66±1.18a |
0.54±0.21a |
| +Ca2+/CaM + TFP |
4.81±0.97b |
9.21±1.36a |
8.59±1.56b |
0.86±0.09b |
0.09±0.02b |
| -Ca2+/CaM -TFP |
3.73±0.56b |
9.91±2.21a |
8.63±0.33b |
1.03±0.09b |
0.11±0.06b |
| -Ca2+/CaM + TFP |
4.62±0.65b |
8.76±1.30a |
7.39±1.23b |
0.68±0.09b |
0.07±0.01b |
| % Ca2+/CaM stimulation2 | 62 | 68 | 75 | 440 | 670 |
1Data represent the mean ± SE of three independent preparations at each pH; data in a column and sharing the same letter are not significantly different according to Tukey’s test (P > 0.05).
2Calculated from activities at + Ca2+/CaM –TFP and -Ca2+/CaM + TFP.
Alignment of three putative apple GADs with previously characterized plant GADs
MdGAD1 (GenBank Acc. No. KC812242), MdGAD2 (GenBank Acc. No. KC812243) and MdGAD3 (GenBank Acc. No. KC812244), respectively, encode proteins of 503 (56.6 kDa), 501 (56.9 kDa) and 510 (57.0 kDa) amino acids, which are 64-75% identical to each other and 60-85% identical to several plant GADs that have been characterized experimentally (see the multiple sequence alignment, based on Clustal W [15], of PhGAD, AtGAD1, OsGAD1-2, and MdGAD1-3 in Additional file 1: Figure S1). The region of greatest variability in the sequences of these proteins is in the C-terminus, the location of the CaMBD [9,16,17]. The C-terminal segments of the three apple GADs are 3-27% identical to each other and 4-46% identical to those in GADs of the other plant species, although this region of MdGAD3 has only 3-6% identity. Closer examination and manual editing of the sequence alignment indicates that two conserved positively charged clusters of lysines flanking the CaMBD and a conserved tryptophan residue are involved in CaM binding [9,18] (Figure 1). Notably, OsGAD2 is missing the first lysine cluster and the tryptophan and does not bind CaM [5]. In general, both MdGAD1 and MdGAD2 contain most of the conserved features, but MdGAD3 contains none of these features.
Figure 1.
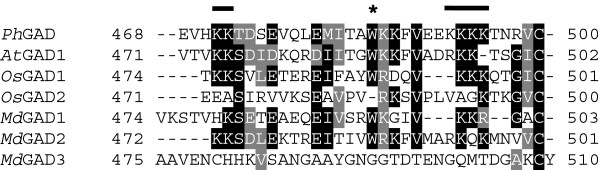
Multiple sequence alignment of the C-terminal segments of plant GADs. Multiple sequence alignment was created with Clustal W [15] and edited manually. The C-terminal CaMBDs of Petunia hybrida (PhGAD) [16], Arabidopsis thaliana (AtGAD1) [18] and Oryza sativa (OsGAD1) [5] have been characterized experimentally. Two conserved positively charged clusters of lysines that flank the CaMBD are marked with black lines [9]. The conserved tryptophan residue involved in CaM binding [16,17], marked with an asterisk, is not present in OsGAD2, which does not bind CaM [5]. Rice GAD numbering is taken from Akama and Takaiwa [5]: OsGAD1 (AB056060, LOC_Os08g36320.1); OsGAD2 (AB056061, LOC_Os04g37500.1). Identical residues are shown with a black background, and similar residues are shown with a grey background. All enzymes were identified as belonging to the aspartate aminotransferase superfamily (fold type I) of PLP-dependent enzymes by the NCBI CD-Search tool [18].
Expression of three putative apple GAD genes in various plant organs
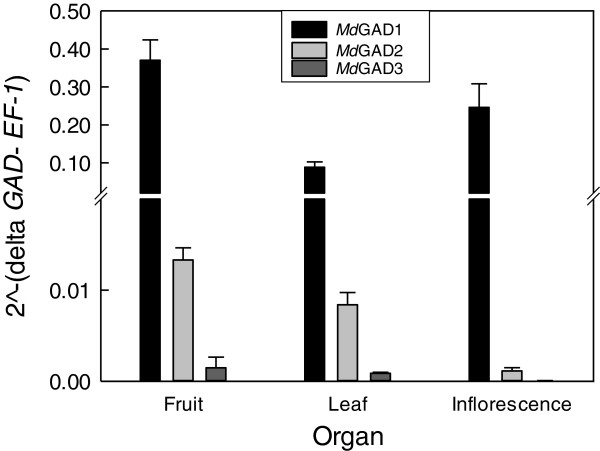
Transcripts for the three GAD genes were detectable in the fruit, leaf and inflorescence, but MdGAD1 was the most prominent, followed distantly by MdGAD2 and MdGAD3 (Figure 2). The relative proportions of MdGAD2 and MdGAD3, compared to MdGAD1, were highest in the fruit and lowest in the inflorescence.
Figure 2.
Relative expression of three apple GADs in various plant organs. While MdGAD3 was detectable, with the scale chosen for presentation, the low expression level is not evident. Data represent the mean ± SE of three biological replicates.
Impact of pH and Ca2+/CaM on the activity of various recombinant apple and Arabidopsis GADs
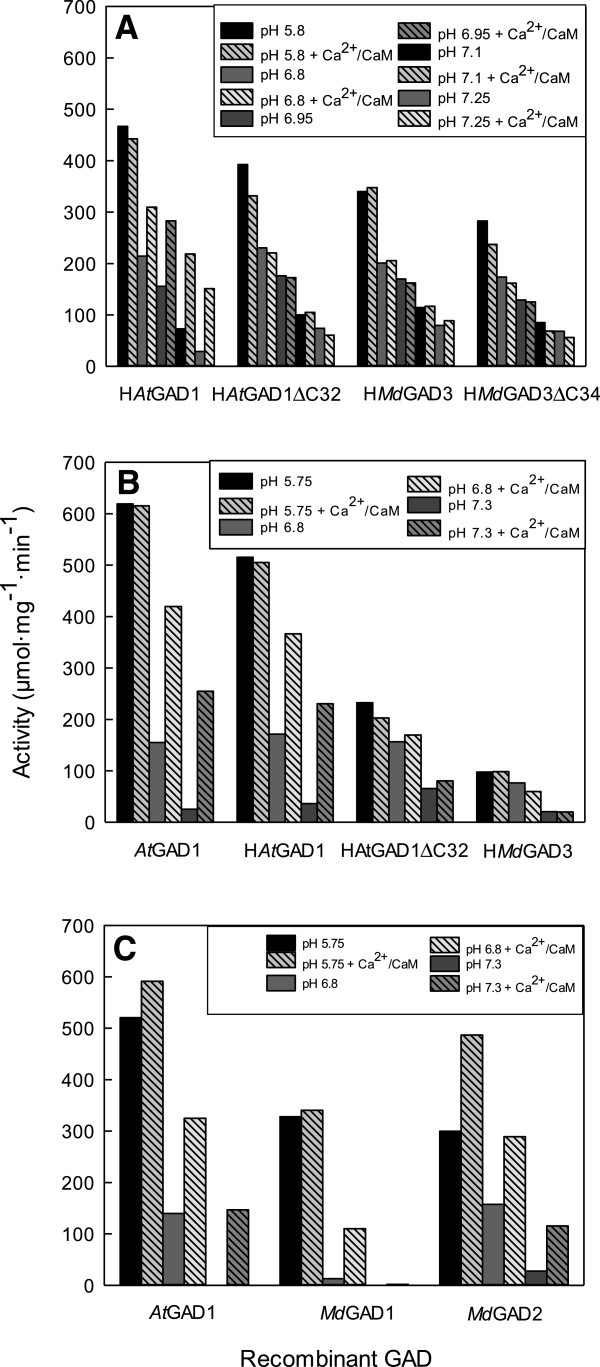
We investigated the impact of Ca2+/CaM on the performance (i.e., activity and spectral properties at various pHs) of recombinant full-length MdGAD1-3 and truncated MdGAD3ΔC34. Apple GAD activity was compared to the performance of the full-length AtGAD1 and in most cases a truncated version of the AtGAD1 lacking the C-terminal CaMBD (AtGAD1ΔC32), ensuring that the purification and assay of the enzymes were effective. Because AtGAD1ΔC32, MdGAD3 and MdGADΔC34 could not be purified by CaM-affinity chromatography, a 6-His motif was added (designated by the prefix H on the protein name), prompting us to also investigate the impact of the tag on GAD activity. Assays of GAD activity and determination of spectral properties were simultaneously conducted as soon as possible after the recombinant protein was extracted to minimize proteolysis that seemed to occur even in the presence of several popular protease inhibitors.
Initially, the N-terminal His-tagged versions of MdGAD3, MdGAD3ΔC34, AtGAD1, and AtGAD1ΔC32 were compared. After purification via Ni2+- affinity chromatography, these recombinant proteins were thrombin-digested to remove the His-tag and concentrated for determining activity and spectral properties. Each of these preparations had a prominent band on a SDS-PAGE gel at the predicted molecular mass; only the preparation of HAtGAD1 appeared to contain a second prominent band, which was of a slightly lower molecular mass than the other and possibly a degradation product resulting from the sensitivity of the CAMBD to proteolytic activity (Additional file 2: Figure S2). The highest activities of the various GADs were evident at pH 5.8 and varied less than 1-fold (approximately 280 to 470 μmol mg prot-1 min-1), and there was no stimulation of these activities by added Ca2+/CaM (Figure 3A). Activities declined with increasing pH and for HAtGAD1 this was accompanied by 2- and 4-fold stimulations by Ca2+/CaM of activity at pHs 7.1 and 7.25, respectively. However, there was no stimulation of HAtGAD1ΔC32, HMdGAD3 and HMdGAD3ΔC34 activities by Ca2+/CaM at these pHs. Since both HAtGAD1 and HAtGAD1ΔC32 responded to Ca2+/CaM as predicted, these data support the notion that Ca2+/CaM could not bind to either HMdGAD3 or HMdGAD3ΔC34.
Figure 3.
Impact of pH and Ca2+/calmodulin on the activity of various recombinant apple and Arabidopsis GADs. Each panel (A-C) represents a separate experiment and shows results from recombinant proteins prepared simultaneously. The data represent the mean of three technical replicates for each treatment. All proteins, except those tagged with a 6-His motif (designated with the prefix H), were purified by calmodulin-affinity chromatography. While the activity of MdGAD1 was detectable at pH 7.3, with the scale chosen for presentation, the low activity is not evident in the figure. SDS-PAGE analysis of the expression and purification of these proteins is given in Additional file 2: Figure S2.
In a follow-up experiment, HMdGAD3 was compared to HAtGAD1, HAtGAD1ΔC32 and AtGAD1, with the first three recombinant proteins being purified by Ni2+-affinity chromatography and the fourth by CaM-affinity chromatography. Steps involving thrombin digestion and concentration were omitted. As above, preparations of purified HAtGAD1, as well as AtGAD1, had one main band at the predicted molecular mass on a SDS-PAGE gel, as well as a slightly smaller prominent band; the other GADs showed only one main band (Additional file 2: Figure S2). Activities reached 620 μmol mg prot-1 min-1 at pH 5, decreased with increasing pH, and appeared to be more variable than in the initial experiment (Figure 3B). Ca2+/CaM stimulated the activities of AtGAD1 and HAtGAD1 at pH 7.3 by 9- and 5-fold, respectively, but had no impact on the activities of HAtGADΔC32 and HMdGAD3. Therefore, the response of HAtGAD1, HAtGADΔC32 and HMdGAD3 to Ca2+/CaM was similar to that in the initial experiment. Notably, the impact of Ca2+/CaM was slightly greater with the AtGAD1, suggesting that the presence of the His tag influences the conformation of the protein. Overall, these results confirm that the C-terminus of AtGAD1 is autoinhibitory at neutral pH and that this autoinhibition can be relieved when bound to Ca2+/CaM. They also indicate that the C-terminus of MdGAD3 is not autoinhibitory and that it does not bind Ca2+/CaM.
Subsequently, MdGAD1 and MdGAD2 were compared to AtGAD1. All three recombinant proteins were purified by CaM-affinity chromatography and steps involving thrombin digestion and concentration were omitted (Figure 3C). Purified AtGAD1 had one prominent and one minor band, MdGAD1 had one major band, and MdGAD2 had two bands of equal prominence (Additional file 2: Figure S2). The higher molecular mass bands were equivalent to the predicted subunit molecular masses for the three proteins. The activity of AtGAD1 at pH 5 was similar to that in the previous experiment, but in this case the stimulation by Ca2+/CaM at pH 7.3 was 150-fold because the activity in the absence of Ca2+/CaM was very low (Figure 3C). The activities of MdGAD1 and MdGAD2 at pH 7.3 and 6.8, respectively, were stimulated by approximately 1- and 9-fold, and 3- and 1-fold. These results indicate that MdGAD1 and MdGAD2, like AtGAD1, have an autoinhibitory domain, but the pH optimum for MdGAD1 and MdGAD2 may differ somewhat from each other.
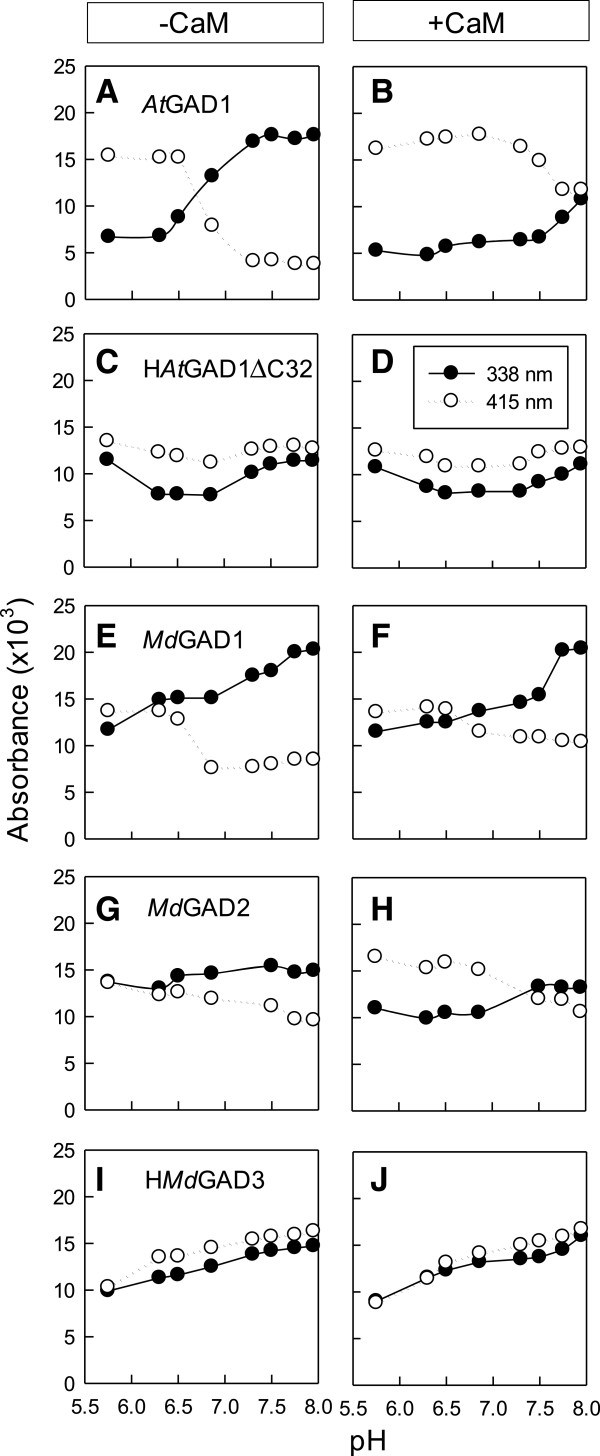
Impact of pH and Ca2+/CaM on the spectral properties of various recombinant apple and Arabidopsis GADs
Recently, Gut et al. [9] attributed absorption bands for AtGAD1 at 338 nm and 415 nm, respectively, to the enolimine and ketoenamine tautomers of the Schiff base of PLP and enzyme (internal aldimine) (Additional file 1: Figure S1). Here, the absorption bands were compared among AtGAD1, HAtGAD1ΔC32, MdGAD1, MdGAD2 and HMdGAD3 (Figure 4). As found previously for AtGAD1 [9], both bands were evident over the pH range of 4.75 to 7.95, the 415 nm band prevailed at low pH where the enzyme is most active and the 338 nm band prevailed at neutral and basic pHs, the absorption values at 338 nm or 415 nm displayed a sigmoidal curve with pH, and Ca2+/CaM essentially abolished the pH-dependence (Figure 4A and B). Also, the absorption bands for a truncated version of the protein lacking the CaMBD displayed a pH-independent pattern and was characterized by the prevalence of the 415 nm band over the entire pH range, regardless of whether Ca2+/CaM was present or not (Figure 4C and D); these characteristics are similar to those observed with a version of AtGAD1 in which Lys496 and Lys 497 in the CaMBD are replaced by alanine residues [9] (Figure 1). Here, the prevalence of the bands was similar in both MdGAD1 and MdGAD2 at low pH, but the 415 nm band decreased with increasing pH and the 338 nm band increased, although the change in levels was not as dramatic as with AtGAD1 and the sigmoidal relationship between absorbance and pH was less evident (Figure 4E and G). Nevertheless, Ca2+/CaM essentially abolished the pH dependence (Figure 4F and H). For HMdGAD3, the levels of the bands were similar, although they increased slightly over the pH range; Ca2+/CaM had no effect on this pattern (Figure 4I and J). Together, these data indicate that pH and Ca2+/CaM influence the spectral properties of MdGAD1 and MdGAD2 in a manner similar to that observed for AtGAD1, although the magnitude of the response is less dramatic, and that HMdGAD3, like HAtGAD1ΔC32, is essentially unaffected by pH and Ca2+/CaM.
Figure 4.
Impact of pH and Ca2+/calmodulin on the spectral properties of various recombinant apple and Arabidopsis GADs. These properties were determined on most of the purified proteins studied in Figure 3. The prefix H on some protein designations represents the 6-His motif. Absorbance at 338 nm and 415 nm, respectively, represents the enolimine and and ketoenamine forms of the internal aldimine. Each row of panels represents a different protein in the absence (A, C, E, G, I) and presence (B, D, F, H, J) of CaM.
Discussion
GAD activity in cell-free extracts of apple fruit was regulated by both pH and Ca2+/CaM
GAD enzymes are ubiquitous across all kingdoms, and in plants both transcript accumulation and activity may be induced during development and in response to abiotic and biotic stress perturbations [2,4]. In plants subjected to temperature stress, drought, salinity or mechanical handling, there is an increase in intracellular Ca2+ concentration [19], which would promote stimulation of GAD by Ca2+/CaM, whereas exposure to elevated CO2, O2 deficiency and wounding would influence GAD activity by cytosolic acidification in numerous plant tissues and species [19,20]. As GABA has been shown to accumulate in apple fruit during controlled atmosphere storage, and this level is higher during prolonged treatment with elevated CO2[12-14], we hypothesized that apple fruit GAD activity is stimulated by cytosolic acidification and/or Ca2+/CaM. GAD activity of cell-free extracts of apple fruit displayed optimal activity at pH 5.5 in the presence of PLP and saturating glutamate (Table 1); the final specific activity is comparable to in vitro activities of vegetative tissues of petunia, soybean, Arabidopsis and Maritime pine seedlings [1]. Apple fruit GAD activity was more dramatically stimulated by Ca2+/CaM at near physiological pH than at acidic pH (Table 1), indicating that it may be regulated in planta by both Ca2+/CaM and pH. As there was no effect of the CaM anatagonist, TFP, on in vitro GAD activity, it is suggested that GAD was not bound with endogenous CaM and the binding of Ca2+/CaM to GAD is tightly controlled by specific controlled atmosphere stress parameters. Overall, the biochemical properties of GAD activity in cell-free extracts of apple fruit provide support for the existence of a Ca2+/CaM-regulated GAD, as is typical of most plants [1]. Notably, three homologous GAD genes (Additional file 1: Figure S1), designated as MdGAD1, MdGAD2 and MdGAD3, were present in the apple plant (Figure 2). MdGAD1, like AtGAD2, was abundant and ubiquitous, whereas MdGAD2 and MdGAD3, like AtGAD3 and AtGAD4, were expressed at much lower levels and might be candidates for induction in fruit by the stresses (i.e., chilling, O2 deficiency and elevated CO2) imposed during controlled atmosphere storage (Figure 2) [2,3].
Recombinant GADs from apple fruit were both CaM-dependent and CaM-independent
With our tagging/purification strategies, we were able to confirm that recombinant AtGAD1 is activated at physiological pH by Ca2+/CaM whether or not the 6-His tag was retained during the assay of activity and spectral properties (Figures 3 and 4). However, the degree of activation appeared to be greatest with the recombinant form of the native protein, which could be attributed to the very low activity found in the absence of Ca2+/CaM. While this interpretation is complicated somewhat by the variable amount of a smaller contaminating protein, probably a degradation product, in the final protein preparation, these findings do suggest that interpretation of the impact of Ca2+/CaM on the conformation and biological activity of tagged recombinant plant GADs should be done with caution [11].
Here, His-tagged or untagged versions of the three apple GADs were expressed in Escherichia coli and purified to homogeneity or near homogeneity (Additional file 2: Figure S2). The predicted subunit Mr of the three recombinant apple proteins (56.6-57 kDa) is similar to the majority of plant GADs, which exist as hexamers of 43–62 kDa subunits [1]. The recombinant apple GADs displayed a similar pH profile as shown for GAD activity in the cell-free extracts of apple fruit (Table 1, Figure 3), as well as a similar pH optimum with the majority of plant GADs, and activities with Ca2+/CaM at physiological pH that are as high or higher than their maximal activities in the literature [1]. Unlike most plant GADs, MdGAD3 was not activated by Ca2+/CaM at near physiological pHs, even though the technique/assay used in this study was clearly able to demonstrate such a relationship (Table 1, Figures 3 and 4). Historically, the binding of CaM to the conserved C-terminal domain has been considered necessary to relieve autoinhibition of GAD at physiological pH [1]. However, OsGAD2 (Figure 1) was found to be CaM-independent and possess a C-terminal region that is autoinhibitory [5]. MdGAD3 is only the second reported plant GAD that cannot be purified by CaM-affinity chromatography and whose activity and spectral properties are not affected by Ca2+/CaM. Notably, MdGAD3 is the first plant GAD that possesses a C-terminal region that is not autoinhibitory (Figure 3).
Alignment of the C-terminal region of MdGAD1 and MdGAD2 with biochemically characterized plant GADs demonstrated that key CaMBD residues are conserved in the apple enzymes, and that these are absent in OsGAD2 (Figure 1). The well-conserved tryptophan and a pair of lysine residues at the C-terminal end are crucial for interactions with CaM and the formation of high molecular weight oligomers in PhGAD and AtGAD1 [9,16,21,22]. In comparison to plant GADs known to be stimulated by Ca2+/CaM, MdGAD3 is missing two positively charged flanking regions (Lys474-Lys475 and Lys496-Lys497) and the Trp488-Lys489-Lys490-Phe491-Tyr492 motif (Figure 1). Their substitution by non-conserved residues may explain the lack of Ca2+/CaM stimulation of the recombinant MdGAD3. Interestingly, almost all CaM-binding residues in plant GADs are conserved in MdGAD1 and MdGAD2; the main exceptions in which charge is not conserved are substitutions at Lys474 and Lys 490 in MdGAD1 and Lys497 in MdGAD2 (Figure 1).
Conclusions
Plant GADs typically differ from microbial and animal GAD enzymes in possessing a C-terminal 30–50 amino acid residue CaMBD [1,4,9]. To date, OsGAD2 is the only exception to this generalization; notably, the C-terminal region of this protein still functions as an autoinhibitory domain (5). In the present study, GAD activity in cell-free extracts of apple fruit could be stimulated by Ca2+/CaM at physiological pH. Based on bioinformatics analysis of the apple genome, we identified three apple GAD genes and then monitored their expression in various plant organs, including apple fruit. Like most plant GADs, the activity and spectral properties of recombinant MdGAD1 and MdGAD2 were regulated by both Ca2+/CaM and acidic pH and possessed a highly conserved CaMBD. In contrast, the activity and spectral properties of recombinant MdGAD3 were not affected by Ca2+/CaM and they were less sensitive to pH; furthermore, the C-terminal region neither bound CaM nor functioned as an autoinhibitory domain. These observations suggest that: (i) MdGAD3 is constitutively active, whereas OsGAD2 needs an activator other than Ca2+/CaM to be activated; and (ii) CaM–dependent and -independent MdGADs enzymes serve different roles in GABA production during the onset of physiological injury associated with controlled atmosphere storage conditions (12–14).
Methods
Preparation of cell-free extracts of apple fruit
'Empire’ apple (Malus × domestica Borkh.) fruit were purchased from a local supermarket and cell-free extracts were prepared essentially as described elsewhere [14,23]. A 10 to 60% (NH4)2SO4 cut was made and the resulting solution was desalted using an Econo-Pac 10DG column (Bio-Rad Laboratories) equilibrated with 100 mM Bis-Tris–HCl, pH 7.0, and 10% glycerol, and immediately assayed for GAD activity.
Identification and cloning of GADs from apple and Arabidopsis
The 502 amino acid (aa) Arabidopsis GAD1 (At5g17330) sequence was used to query the apple expressed sequence tag database at the National Center for Biotechnology Information [24]. Alignment of multiple sequences produced a single candidate that would produce a 503 aa product. This sequence was in turn used to query predicted peptides from the apple genome [25]. Five significant hits were obtained: MDP0000284588 on chromosome 1; MDP0000307719 on chromosome 16; and, MDP0000587459, MDP0000322533 and MDP0000388356 on chromosome 9. Closer inspection of MDP0000322533 and MDP0000388356, and their alternative gene sets MDP0000234887 and MDP0000201843, respectively, revealed that these two regions on chromosome 9 have identical nucleotide sequences. Several attempts were made to clone these open reading frames (ORFs) from fruit and leaf cDNA samples, but none were successful.
MDP0000284588 had five more exons than the candidate sequence obtained from the EST data so two sets of primers based on both sequences were designed to amplify the corresponding cDNA. Primers GAD1-FP and GAD1-RP, designed to amplify the sequence identified via EST data, produced the expected 1506 bp product from fruit cDNA, designated MdGAD1 (Additional file 1: Table S1 for all primer sequences used in this study). The primers CT-F32 and GAD1-RP, designed to amplify the longer version corresponding to MDP0000284588, did not produce a product from fruit or leaf cDNA samples. Comparing the MdGAD1 sequence to the apple genome permitted identification of the 5′ and 3′ untranslated regions (UTRs); primers (GAD1-FP and GAD1-RP) in these regions were used to amplify the entire ORF plus portions of the UTRs.
Primers CT-F33 and CT-R33, designed to amplify MDP0000587549, produced the expected 1506 bp product designated MdGAD2. 5′- and 3′-Rapid Amplification of cDNA Ends (RACE) were used to confirm that MdGAD2 was a full-length ORF. The SMART-RACE kit (Clontech, CA, USA) was used to prepare cDNA from total RNA according to the manufacturer’s instructions. The gene-specific primer CT-R37 was used for 5′-RACE with MdGAD2, and the primer CT-R38 was used for nested polymerase chain reaction (PCR). The gene-specific primer CT-F37 was used for 3′-RACE, and the primer CT-F38 was used for nested PCR.
Primers CT-F34 and CT-R34, designed to amplify MDP0000307719, produced the expected 1533 bp product from a leaf cDNA sample only; it was designated MdGAD3. Comparing the MdGAD3 sequence to the apple genome permitted identification of the 5′ and 3′ UTRs; primers (CT-F44 and CT-R44) in these regions were used to amplify the entire ORF plus portions of the UTRs.
The AtGAD1 ORF was amplified with 5′ NdeI and 3′ BamHI restriction sites with primers AtGAD1-FP and AtGAD1-RP and sub-cloned into pET15b (Invitrogen, USA) to give pET15b-AtGAD1. The AtGAD1 ORF minus the 32 C-terminal residues that make up the CaMBD was amplified with 5′ NdeI and 3′ BamHI restriction sites with primers AtGAD1-FP and AtGAD1ΔCaMBD-RP; the resulting product was sub-cloned into pET15b to give pET15b-AtGAD1ΔCaMBD.
Two of the apple GADs were sub-cloned into pET15b after 5′ NdeI and 3′ BamHI restriction sites were added via PCR using primers aGL and aGR for MdGAD1, and primers CT-F39 and CT-R39 for MdGAD2. MdGAD3 was also cloned into pET15b after addition of XhoI restriction sites using primers CT-F60 and CT-R60. The primers CT-F61 and CT-R61 were used for site-directed mutagenesis to remove 32 residues from the C-terminus of the enzyme to give pET15b-MdGAD3ΔC.
Sequences encoding the His-tag and linker were deleted from the pET15b-AtGAD1, -MdGAD1, and –MdGAD2 constructs via site-directed mutagenesis using the primers CT-F67B and CT-R67B, CT-F68 and CT-R68, and CT-F69 and CT-R69, respectively.
Expression of apple GADs in various organs
RNA was isolated from 1 g of liquid N2-ground mature fruit that had been stored at 0°C under 5 kPa CO2 and 2 kPa O2 for 8 weeks post-harvest or liquid N2-ground fully expanded leaves essentially as described previously [14]. Tree branches were also collected in late winter from Simcoe, ON, and the cut ends were submerged in water and the containers placed into a growth chamber (22°C 16 h day/18°C 8 h night) for 10 days to induce flowering and leaf formation. Open flowers and then fully expanded leaves were harvested and stored at -80°C; all tissues were harvested from three separate branches.
Ribonucleic acid (RNA) integrity and quality was verified by formaldehyde gel electrophoresis and then the RNA was treated with DNase I using the TURBO DNA-free kit (Applied Biosystems, Austin, TX) according to the manufacturer’s instructions. One microgram total RNA was used for first-strand cDNA synthesis with Oligo(dT)20 and Superscript III (Invitrogen, Carlsbad, CA) at 50°C according to the manufacturer’s protocol. Primers used for quantitative real-time PCR (qPCR) were designed using Primer Express 3 software (Applied Biosystem) with 60°C melting temperature, 40% to 60% GC content, and 50 to 85 bp amplicon size range (Additional file 1: Table S2). Quantitative PCR was performed with an iQ5 Multicolor Real-Time PCR Detection system (BioRad Laboratories, Hercules, CA, USA), using SYBR Green supermix (BioRad Laboratories) to quantify cDNA synthesis. The final concentration of primers was adjusted to 0.2 μM, and the thermal profile of the qPCR reactions was 95°C for 2 min and 40 cycles of 95°C for 10 s, 55°C for 30 s, and 72°C for 15 s. Results of the qPCR were analyzed using the iQ5 2.1 optical system software. Relative expression and data analysis were determined using the 2-ΔCt method [26] and Elongation factor 1-alpha (NCBI GenBank ID MD0000294265) as the housekeeping gene. Two technical replicates were conducted for each biological replicate, and the average ± SE of three biological replicates was determined for each time point, which were arranged randomly in the controlled environment chamber.
Heterologous expression and purification of recombinant enzymes
All GAD constructs were expressed in Escherichia coli BL21 (DE3) cells as described by Gut et al.[9] with minor modifications. Briefly, cultures were induced with 0.4 mM isopropyl-β-D-thiogalactopyranoside at 20°C for 16 h. Harvested cells were resuspended in lysis buffer containing 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) pH 7.2, 150 mM NaCl, 1 mM DTT, 0.1 mM PLP, 1 mM PMSF, and SigmaFast protease inhibitor tablets (Sigma). Resuspended cells were lysed via three 30 s pulses at 80% power using a Sonic Dismembrator (Model FB-120, Fisher Scientific) and then centrifuged. The supernatant was passed through a 0.45 μM filter and CaCl2 was added to a final concentration of 10 mM prior to loading onto a column packed with Calmodulin Sepharose 4B (GE Healthcare, USA), which was pre-equilibrated with lysis buffer containing 2 mM CaCl2. Columns were washed with 20 bed volumes of lysis buffer containing 2 mM CaCl2 and proteins were eluted with lysis buffer containing 2 mM ethylene glycol-bis(2-aminoethylether)-N,N,N’,N’-tetraacetic acid. From 2 to 10 mg of purified recombinant GAD was obtained per liter of cell culture.
Several constructs were prepared with 6-His tags and the recombinant proteins were purified via Ni2+-resin. Cell culture and harvest were performed as reported above. Briefly, harvested cells were resuspended in lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, 1 mM PMSF, and SigmaFast protease inhibitor tablets, pH 8) and then lysed. Then lysate was centrifuged and the supernatant was filtered and loaded onto a column containing 0.25 mL His-Select Nickel Affinity Gel (Sigma-Aldrich, USA), which was pre-equilibrated with lysis buffer. The column was caped and the resin resuspended by inversion and then allowed to settle at 4°C for 5 min before draining the lysate from the column. The column was washed with 20 bed volumes each of lysis buffer, followed by wash buffer (50 mM NaH2PO4, 300 mM NaCl and 20 mM imidazole, 1 mM PMSF, pH 8). Protein was eluted in eight fractions, half a bed volume each, of elution buffer (50 mM NaH2PO4, 300 mM NaCl, 250 mM imidazole, 1 mM PMSF, pH 8). Fractions with high protein concentrations were pooled and used for further analysis.
The pET5a vector containing petunia CaM81 was a gift from Dr. Wayne Snedden. Recombinant CaM81 was produced in E. coli BL21 (DE3) cells as described previously [27], except that it was produced here without incorporation of 35S.
Protein concentrations were determined via the Bio-Rad Protein Assay [28]; all protein samples were stored at -80°C prior to electrophoresis. SDS-PAGE, staining with Coomassie Blue R-250, and western blotting were performed using standard protocols [14].
Thrombin cleavage of His-tagged GADs
Thrombin cleavage of the 6-His tag on several recombinant GADs was performed with restriction grade thrombin (1U/μl, Novagen, USA). Briefly, thrombin was diluted fifty times in 50 mM sodium citrate pH 6.5 containing 200 mM NaCl, 0.1% PEG-8000 and 50% glycerol. Eluted GAD protein (10 μg) was cleaved in a 50 μl reaction containing 5 μl 10× reaction buffer, and 1 μl diluted thrombin for 20 min at room temperature. Cleaved proteins were purified and concentrated using 0.5 ml Amicon Ultra Centrifugal Filters (EMD Millipore, USA) with a 50 kDa cutoff as per the manufacturer’s instructions.
In vitro assay of GAD activity
GAD activity of cell-free apple extracts was measured as the production of 14CO2 from radiolabeled glutamate essentially as described previously [23]. The CaM dependence of GAD activity was determined by adding 0.5 mM CaCl2 and 0.2 μM bovine CaM in the absence or presence of 100 μM TFP to the reaction mixture. An overlapping 100 mM buffer system was used (pyridine-HCl at pH 4.5, 5.0 and 5.5, and Bis-Tris HCl at pH 5.5, 6.0, 6.5 and 7.0) to determine the pH response of GAD activity. GAD activity at each pH is expressed as nmol 14CO2 produced per mg protein per minute, after correction for control assays conducted without extract. Data represent the mean ± SE of three separate apples. GAD activity in all assays was proportional to the amount of added cell-free extract and linear with time.
GAD activity of recombinant GAD was assayed in a final volume of 0.5 mL containing 100 mM Bis-Tris HCl at pH 5.8, 6.8, 6.95, 7.1 or 7.25 in the presence of 10% (v/v) glycerol, 1 mM DTT, 0.1 mM PLP, 0.2 mM PMSF at 30°C for 15 min. Reactions were performed with or without 1 mM CaCl2 and 0.2 μM recombinant petunia CaM81. One microgram of recombinant GAD was used in most reactions; the exception was those conducted at pH 6.95, 7.1, and 7.25 without Ca2+/CaM, which contained 2 μg. All reactions were initiated by adding glutamate to a final concentration of 1 mM and were terminated by adding sulfosalicylic acid to a final concentration of 31.1 mg ml-1. The pH was adjusted to 7.0 with 4 M NaOH. Reactions were passed through a 0.45 μm filter and diluted 20× prior to analysis of GABA and glutamate by reverse-phase high performance liquid chromatography after automatic derivatization with o-phthalaldehyde as described previously [29]. The mean of three technical replicates is presented for each experimental treatment; all experiments were conducted at least twice.
Assay of spectral properties
Spectral properties were determined as reported by Gut et al.[9].
Abbreviations
Bis-Tris–HCl: Bis(2-hydroxyethyl)amino-tris(hydroxymethyl)methane; CaM: Calmodulin; CaMBD: Calmodulin binding domain; cDNA: Complementary DNA; DNA: Deoxyribonucleic acid; DTT: Dithiothreitol; GABA: γ-aminobutyrate; GAD: Glutamate decarboxylase; HEPES: 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; 6-His: Hexahistidine; ORF: Open reading frame; PLP: Pyridoxal 5′-phosphate; PMSF: Phenylmethanesulfonyl fluoride; PVPP: Polyvinylpolypyrrolidone; qPCR: Quantitative polymerase chain reaction; RNA: Ribonucleic acid; SDS-PAGE: Sodium dodecyl sulphate polyacrylamide gel electrophoresis; TFP: Trifluoperazine; UTR: Untranslated region.
Authors’ contributions
BJS and GGB conceived the study, and CPT, AZ and JL performed the experiments and carried out the analysis. All authors contributed to design of experiments, interpretation of data and writing the manuscript, and read and approved the final manuscript.
Supplementary Material
Synthetic oligonucleotides used in this study. Table S2. Primers used for qPCR. Figure S1. Multiple sequence alignment of selected plant GADs.
SDS-PAGE analysis of expression and purification of various recombinant GAD proteins characterized in Figure 3A-C.
Contributor Information
Christopher P Trobacher, Email: ctrobach@uoguelph.ca.
Adel Zarei, Email: azarei@uoguelph.ca.
Jingyun Liu, Email: yundoushu@live.cn.
Shawn M Clark, Email: shawn.clark@nrc-cnrc.gc.ca.
Gale G Bozzo, Email: gbozzo@uoguelph.ca.
Barry J Shelp, Email: bshelp@uoguelph.ca.
Acknowledgements
The authors thank Dr. Gordon J. Hoover for performing the amino acid analysis and Dr. Wayne A. Snedden (Queen’s University, Kingston, ON K7L 3 N6, Canada) for the pET5a vector containing petunia CaM81. This research was supported by funds to: BJS and GGB from the Sciences and Engineering Research Council (NSERC) of Canada as a Strategic Project Grant and from the Ontario Ministry of Agriculture; BJS and GGB as a grant-in-aid from Rolm & Haas LLP; and, BJS as an NSERC Individual Discovery Grant. JL was supported by the Walter and Laura Scott Endowment from the University of Guelph.
References
- Shelp BJ, Bozzo GG, Trobacher C, Chiu G, Bajwa VS. Strategies and tools for studying the metabolism and function of γ-aminobutyrate in plants: I. Pathway structure. Botany. 2012;90:651–668. doi: 10.1139/b2012-030. [DOI] [Google Scholar]
- Shelp BJ, Bozzo GG, Zarei A, Simpson JP, Trobacher CP, Allan WL. Strategies and tools for studying the metabolism and function of γ-aminobutyrate in plants: II. Integrated analysis. Botany. 2012;90:781–793. doi: 10.1139/b2012-041. [DOI] [Google Scholar]
- Shelp BJ, Bozzo GG, Trobacher CP, Zarei A, Deyman KL, Brikis CJ. Hypothesis/review: contribution of putrescine to 4-aminobutyrate (GABA) production in response to abiotic stress. Plant Sci. 2012;193–194:130–135. doi: 10.1016/j.plantsci.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Shelp BJ, Bown A, McClean MD. Metabolism and functions of gamma- aminobutyric acid. Trends Plant Sci. 2009;4:446–452. doi: 10.1016/s1360-1385(99)01486-7. [DOI] [PubMed] [Google Scholar]
- Akama K, Takaiwa F. C-terminal extension of rice glutamate decarboxylase (OsGAD2) functions as an autoinhibitory domain and overexpression of a truncated mutant results in the accumulation of extremely high levels of GABA in plant cells. J Exp Bot. 2007;58:2699–2707. doi: 10.1093/jxb/erm120. [DOI] [PubMed] [Google Scholar]
- Snedden WA, Koustia N, Baum G, Fromm H. Activation of a recombinant petunia glutamate decarboxylase by calcium/calmodulin or by a monoclonal antibody which recognizes the calmodulin binding domain. J Biol Chem. 1996;271:4148–4153. doi: 10.1074/jbc.271.8.4148. [DOI] [PubMed] [Google Scholar]
- Turano FJ, Fang TK. Characterization of two glutamate decarboxylase cDNA clones from Arabidopsis. Plant Physiol. 1998;117:1411–1421. doi: 10.1104/pp.117.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zik M, Arazi T, Snedden WA, Fromm H. Two isoforms of glutamate decarboxylase in Arabidopsis are regulated by calcium/calmodulin and differ in organ distribution. Plant Mol Biol. 1998;37:967–975. doi: 10.1023/A:1006047623263. [DOI] [PubMed] [Google Scholar]
- Gut H, Dominici P, Pilati S, Astegno A, Petoukhov MV, Svergun DI, Grütter MG, Capitani G. A common structural basis for pH- and calmodulin- mediated regulation in plant glutamate decarboxylase. J Mol Biol. 2009;392:334–351. doi: 10.1016/j.jmb.2009.06.080. [DOI] [PubMed] [Google Scholar]
- Yevtushenko DP, McLean MD, Peiris S, Van Cauwenberghe OR, Shelp BJ. Calcium/calmodulin activation of two divergent glutamate decarboxylases from tobacco. J Exp Bot. 2003;54:2001–2002. doi: 10.1093/jxb/erg210. [DOI] [PubMed] [Google Scholar]
- Arnau J, Laurtzen C, Petersen GE, Pedersen J. Current strategies for the use of affinity tags and tag removal for the purification of recombinant proteins. Protein Expr Purif. 2006;48:1–13. doi: 10.1016/j.pep.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Deewatthanawong R, Watkins CB. Accumulation of γ-aminobutyric acid in apple, strawberry and tomato fruit in response to postharvest treatments. Acta Hort. 2010;877:947–952. [Google Scholar]
- Lee J, Rudell DR, Davies PJ, Watkins CB. Metabolic changes in 1-methylcyclopropene (1-MCP)-treated 'Empire’ apple fruit during storage. Metabolomics. 2012;8:742–753. doi: 10.1007/s11306-011-0373-5. [DOI] [Google Scholar]
- Trobacher CP, Clark SM, Bozzo GG, Mullen RT, DeEll JR, Shelp BJ. Catabolism of GABA in apple fruit: Subcellular localization and biochemical characterization of two γ-aminobutyrate transaminases. Postharv Biol Technol. 2013;75:106–113. [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Arazi T, Baum G, Snedden WA, Shelp BJ, Fromm H. Molecular and biochemical analysis of calmodulin interactions with the calmodulin-binding domain of plant glutamate decarboxylase. Plant Physiol. 1995;108:551–561. doi: 10.1104/pp.108.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap KL, Yuan T, Mal TK, Vogel HJ, Ikura M. Structural basis for simultaneous binding of two carboxy-terminal peptides of plant glutamate decarboxylase to calmodulin. J Mol Biol. 2003;328:193–204. doi: 10.1016/S0022-2836(03)00271-7. [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer A, Lu S, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, Fong JH, Geer LY, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Jackson JD, Ke Z, Lanczycki CJ, Lu F, Marchler GH, Mullokandov M, Omelchenko MV, Robertson CL, Song JS, Thanki N, Yamashita RA, Zhang D, Zheng C, Bryant SH. CDD: a conserved domain database for the functional annotation of proteins. Nucleic Acids Res. 2011;39(D):225–229. doi: 10.1093/nar/gkq1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H, Knight MR. Abiotic stress signaling pathways. Trends Plant Sci. 2001;6:262–267. doi: 10.1016/S1360-1385(01)01946-X. [DOI] [PubMed] [Google Scholar]
- Kinnersley AM, Turano FJ. Gamma-aminobutyric acid (GABA) and plant responses to stress. CRC Crit Rev Plant Sci. 2000;19:479–509. doi: 10.1016/S0735-2689(01)80006-X. [DOI] [Google Scholar]
- Baum G, Lev-Yadun S, Fridmann Y, Arazi T, Katsnelson H, Zik M, Fromm H. Calmodulin binding to glutamate decarboxylase is required for regulation of glutamate and GABA metabolism and normal development in plants. EMBO J. 1996;15:2988–2996. [PMC free article] [PubMed] [Google Scholar]
- Zik M, Fridmann-Sirkis Y, Fromm H. C-terminal residues of plant glutamate decarboxylase are required for oligomerization of a high-molecular weight complex and for activation by calcium/calmodulin. Biochim Biophys Acta. 2006;1764:872–876. doi: 10.1016/j.bbapap.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Snedden WA, Arazi T, Fromm H, Shelp BJ. Calcium/calmodulin activation of soybean glutamate decarboxylase. Plant Physiol. 1995;108:543–549. doi: 10.1104/pp.108.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Biotechnology Information. http://www.ncbi.nlm.nih.gov/
- Velasco R, Zharkikh A, Affourtit J, Dhingra A, Cestaro A, Kalyanaraman A, Fontana P, Bhatnagar SK, Troggio M, Pruss D, Salvi S, Pindo M, Baldi P, Castelletti S, Cavaiuolo M, Coppola G, Costa F, Cova V, Dal Ri A, Goremykin V, Komjanc M, Longhi S, Magnago P, Malacarne G, Malnoy M, Micheletti D, Moretto M, Perazzolli M, Si-Ammour A, Vezzulli S. et al. The genome of the domesticated apple (Malus × domestica Borkh.) Nat Genet. 2010;42:833–839. doi: 10.1038/ng.654. http://www.rosaceae.org/ [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittigen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Fromm H, Chua N-H. Cloning of plant cDNAs encoding calmodulin-binding proteins using 35S-labeled recombinant calmodulin as a probe. Plant Mol Biol Rep. 1992;10:199–206. doi: 10.1007/BF02668347. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Allan WL, Shelp BJ. Fluctuations of γ-aminobutyrate, γ-hydroxybutyrate and related amino acids in Arabidopsis leaves as a function of the light–dark cycle, leaf age and N stress. Can J Bot. 2006;84:1339–1346. doi: 10.1139/b06-093. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Synthetic oligonucleotides used in this study. Table S2. Primers used for qPCR. Figure S1. Multiple sequence alignment of selected plant GADs.
SDS-PAGE analysis of expression and purification of various recombinant GAD proteins characterized in Figure 3A-C.